Abstract
A new series of 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one derivatives VI–XIII were synthesized. Their chemical structures were confirmed by spectral and elemental analysis. The cytotoxic effect of the newly synthesized compounds was tested in vitro against human breast cancer cell line (MCF-7). Most of the tested compounds have shown promising cytotoxic activity. Compounds X and XIIIb exerted a powerful cytotoxic effect against MCF-7 with a very low IC50 (0.0015 and 0.0047 µmol/ml), while compounds VI, VII, VIII, XIIb, XI, XIIIc and IX exerted a moderate cytotoxic effect (IC50 0.01523, 0.0213, 0.031, 0.0478, 0.049, 0.068 and 0.079 µmol/ml respectively), compared to doxorubicin (0.0025 µmol/ml). Exploring their apoptotic effect; interestingly,all compounds activated apoptotic cascade in MCF-7. Compounds VI, XIIIb, XIIb, XI, XIIa, VII, V and VIII showed potent effect even much more than doxorubicin by 12.87–5.91 folds, while compounds XIIIc, IX, XIIIa, XIIc and X showed moderate increase in CASP3 activity by 4.96–3.22 folds relative to untreated cells more or less similar to doxorubicin (5.57 folds).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is considered one of the major health problems, with increasing incidence worldwide. Malignant tumors are ranked the third in developing countries after infectious-parasitic and cardiovascular diseases [1]. The worldwide burden of cancer rose to an estimated 14 million new cases per year. Cancer deaths are predicted to rise from an estimated 8.2 million annually to 13 million per year. Breast cancer incidence was (1.7 million, 11.9 % of the total) [2]. In cancer as well as many other serious diseases, the body loses control over apoptosis and angiogenesis where apoptosis is hindered and excessive angiogenesis occurs [1]. Programmed cell death “apoptosis” is one of the various cell death modalities regulating cell proliferation, as well as eliminating potentially harmful ones resulting from pathophysiological conditions. It was described as a major defense strategy that prevents cells from acquiring tumorigenic potential. This suicide mechanism is controlled by multiple and interrelated pathways ensuring that proteolytic initiators and executioners “Caspases” are triggered only in cells requiring termination [3]. Caspase-3 “CASP3” is a member of the cysteine protease family, which plays a crucial role in apoptotic pathways by cleaving a variety of key cellular proteins. Previous results suggest that lack of CASP3 can attenuate apoptosis in response to certain stimuli. CASP3 can be activated by diverse death-inducing signals, including chemotherapeutic agents [4].
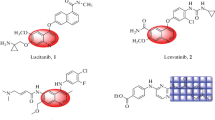
Discovering new chemotherapeutic agents has become one of the most challenging tasks in medicinal chemistry and remains critically important, especially the design and synthesis of new ones that can trigger apoptosis. One of the most important scaffolds in medicinal chemistry is quinazoline. It is well known that quinazoline derivatives have a wide range of biological activities such as anticancer [5–8], antibacterial [9], antiviral [10], antifungal [9], antihypertensive [11], anti-inflammatory [12], analgesic and COX-II inhibitors [13–16]. Several quinazoline derivatives have been approved by FDA as anticancer drugs such as Erlotinib, Lapatinib, Gefitinib and Caneratinib (Fig. 1). Literature survey revealed that 2,3-disubstituted quinazoline-4-one derivatives have good anticancer activity against MCF-7 tumor cell line [17–19]. Moreover, quinazoline derivatives bearing phenyl moiety at position 3 show potent activity against breast cancer [19, 20]. On the other hand many 6,8-dibromoquinazolin-4-one derivatives showed greater activity than doxorubicin against MCF-7 [5, 6]. In view of previous rationale and in continuation of our drug research program concerning synthesis of new safer and more biologically active quinazolin-4-one derivatives [5–7, 9, 13, 15, 16], our interest was to synthesize new series of 6,8 dibromoquinazolin-4-one compounds having various substituents at position 3 and p-chorophenyl moiety at position 2 aiming to obtain new quinazolin-4-one derivatives with high activity against breast cancer.
Results and discussion
Chemistry
The reaction of 3,5 dibromoanthranilic acid I and p-chlorobenzoylchloride II afforded the amide analog III which was refluxed in acetic anhydride to obtain 6,8-dibromo-2-(4-chlorophenyl)-4H-benzo[d] [1, 3] oxazin-4-one IV [21] (Scheme 1). Compound IV reacted with formamide to afford compound V [22] (Scheme 1). The reaction of urea, thiourea, aniline, p-toluidine, p-aminoacetophenone with benzoxazine derivative IV by fusion at high temperature afforded the corresponding quinazoline-4-one derivatives VI–X (Scheme 2). The benzoxazine derivative IV was also reacted with hydroxylamine hydrochloride in dry pyridine to afford compound XI (Scheme 2).
6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one V reacted with primary amines like aniline, p-toluidine, 2-amino pyridine in the presence of formamide and glacial acetic acid to give Mannich bases XII a–c (Scheme 3). On the other hand compound V react with secondary amine like piperidine, 2-methyl piperidine, morpholine in presence of paraformaldehyde to give the corresponding Mannich bases XIII a–c (Scheme 3).
Biological activity
In vitro cytotoxic activity
In this study a novel series of 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one derivatives were designed and synthesized (Schemes 1, 2, 3). All synthesized compounds were screened for their in vitro cytotoxic and growth inhibitory activities against MCF-7 cell line, in comparison with doxorubicin as reference drug. The cytotoxic activities of our tested compounds were expressed as IC50 values (the dose that reduces survival to 50 %) in µmol/ml. Growth curves showed that our tested compounds exhibit significant efficacy against MCF-7 with IC50 values range 0.0015–0.317 µmol/ml, compared to doxorubicin (0.0025 µmol/ml). As shown in Table 1: Compounds X and XIIIb exerted a powerful cytotoxic effect against MCF-7 with a very low IC50 (0.0015 and 0.0047 µmol/ml). Compounds VI, VII, VIII, XIIb, XI, XIIIc and IX exerted a moderate cytotoxic effect (IC50 0.01523, 0.0213, 0.031, 0.0478, 0.049, 0.068 and 0.079 µmol/ml respectively), while compounds XIIa, V, XIIc, and XIIIa exerted a week cytotoxic effect against MCF-7 (IC50 0.0956, 0.12823, 0.208 and 0.317 µmol/ml respectively), as compared to doxorubicin.
In vitro apoptotic effect
It is well established that the induction of apoptotic cascade is one of the main mechanisms of chemotherapy-induced cell death [6]. CASP3 can be activated by diverse death-inducing signals, including chemotherapeutic agents [4]. Previous study has shown that approximately 75 % of tumors as well as morphologically normal peritumoral tissue samples lacked CASP3 transcript and protein expression [4]. In addition, CASP3 mRNA levels in commercially available total RNA samples from breast (MCF-7) and cervical studied tumors were undetectable [4].
In order to determine whether the chemo sensitizing effect of our newly synthesized 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one derivatives is secondary to its ability to activate an apoptotic cascade, MCF-7 were treated with test compounds at their IC50 concentrations, for 6 h. The activity of CASP3 was measured using in vitro human CASP3 assay. Figure 2 represents enzyme activity expressed in folds relative to untreated cells.
All our tested compounds caused significant increase of CASP3 activity against MCF-7 compared to doxorubicin. Compounds VI, XIIIb, XIIb, XI, XIIa, VII, V and VIII increased CASP3 activity even more than doxorubicin by 12.87–5.91 folds, while compounds XIIIc, IX, XIIIa, XIIc and X showed moderate increase in CASP3 activity by 4.96–3.22 folds relative to untreated cells more or less similar to doxorubicin (5.57 folds).
The sensitivity of CASP3- deficient breast cancer (MCF-7) cells to undergo apoptosis in response to doxorubicin and other apoptotic stimuli could be augmented by reconstituting CASP3 expression. These results suggest that loss of CASP3 expression may represent an important cell survival mechanism in breast cancer patients [5].
SAR
Substitution of 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one moiety with 4-acetylphenyl group at position 3 gave the most potent anticancer activity against MCF-7 exhibited by compounds X.
It is interesting to note that a minor alteration in the molecular configuration of investigated compounds may have a pronounced effect on anticancer activity, As demonstrated hereby: compound XIIIb with “(2-methylpiperidin-1-yl) methyl” substitution at position 3 led to a potent cytotoxic effect against MCF-7, while substitution with “morpholinomethyl” give compound XIIIc with moderate cytotoxic activity, on the other hand substitution with “piperidin-1-ylmethyl” at the same position give compound XIIIa with low cytotoxic activity.
Introduction of “carboxamide, carbothioamide, phenyl, p-tolyl, hydroxyl and/or (p-tolylamino)methyl)” group at position 3 give compounds VI, VII, VIII, IX, XI and XIIb respectively which exerted a moderate cytotoxic effect against MCF-7.
On the other hand substitution with “(phenylamino) methyl” and/or “(pyridin-2-ylamino) methyl” at position 3 gave compounds XIIa and XIIc respectively with weak cytotoxic effect.
On discussing the SAR of apoptotic activity of our newly synthesized derivatives; substitution of 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one moiety at position 3 with “carboxamide, (2-methylpiperidin-1-yl) methyl, (p-tolylamino)methyl), hydroxyl, (phenylamino) methyl, carbothioamide, phenyl” give compounds VI, XIIIb, XIIb, XI, XIIa, VII and VIII respectively which increase CASP3 activity even more than doxorubicin by 12.87 to 5.91 folds.
Conclusion
A new series 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one derivatives VI–XIII were synthesized. All synthesized compounds were tested in vitro against human breast cancer cell line (MCF-7). Most of the tested compounds have shown promising cytotoxic effect. Substitution of 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one moiety with “4-acetylphenyl” and/or “(2-methylpiperidin-1-yl) methyl” at position 3 gave compounds X and XIIIb respectively which exert the most potent anticancer activity against MCF-7 with a very low IC50 (0.0015 and 0.0047 µmol/ml),compared to doxorubicin (0.0025 µmol/ml).
All compounds activated apoptotic cascade in MCF-7. Substitution of 6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one moiety at position 3 with “carboxamide, (2-methylpiperidin-1-yl) methyl, (p-tolylamino)methyl), hydroxyl, (phenylamino) methyl, carbothioamide, phenyl” give compounds VI, XIIIb, XIIb, XI, XIIa, VII and VIII respectively which increase CASP3 activity by 12.87 to 5.91 folds relative to untreated cells compared to doxorubicin (5.57 folds).
Experimental
Chemistry
All melting points are uncorrected, elemental analyses were carried out in the micro analytical unit of National Research Centre and Cairo University, Egypt. IR spectra were recorded on FT-IR spectrophotometer- Nexus 670-Nicolet (USA) and Perkin Elmer-9712 spectrophotometer. 1H NMR spectra were determined on a Varian-Gemini 300 MHz and Joel-Ex 270 MHz NMR spectrometer using TMS as an internal standard. 13C NMR (DMSO-d6) spectra were recorded at 100.62 MHz at the aforementioned research center in Cairo University. Mass spectra were determined on Finnigan Mat SSQ 7000, mode EI 70 eV (Thermo Inst. Sys. Inc. USA). Thin layer chromatography was carried out on silica gel 60 F254 (Merck) plates using chloroform/methanol (9:1) as an eluent system.
General procedure for the synthesis of 3(4H)-substituted 6,8-dibromo-2-(4-chlorophenyl)-4-oxoquinazoline (VI–X)
Equimolar amount of benzoxazine IV and different primary amino containing moieties like urea, thiourea, aniline, p-toluidine, p-aminoacetophenone were fused together at 200o C in an oil bath for 1 h. The mixture was cooled and methanol was added to the mixture. The separated solid was collected by filtration, washed with methanol, dried and crystallized from proper solvent to give the compounds VI–X respectively.
6,8-dibromo-2-(4-chlorophenyl)-4-oxoquinazoline-3(4H)-carboxamide (VI)
Crystallized from ethanol to give yellow crystals of compound VI, m.p. 230 °C, 85 % yield. Analysis calculated for C15H8Br2ClN3O2; Calcd.: %C, 39.38; H, 1.76; N, 9.18, Found: %C, 39.29; H, 1.8; N, 9.2. IR: υ max./cm−1 3300 (NH2),3050 (C–H aromatic), 1690 (C=O of quinazolinone), 1650 (C=O of amide), 1630 (C=N) and at 1585 (C=C). 1H-NMR (DMSO-d6, ppm): δ 7.2–8.1 (m, 6H, Ar–H) and at 10.5 (s, 2H, NH2, exchangeable with D2O). 13C NMR (DMSO-d6):169, 160, 155.9, 148, 136.3, 136, 133, 126.5, 129, 128.5, 124, 121.9. MS: m/z = 461
6,8-dibromo-2-(4-chlorophenyl)-4-oxoquinazoline-3(4H)-carbothioamide (VII)
Crystallized from ethanol to give yellow crystals of compound VII, m.p. 270 °C, 80 % yield. Analysis calculated for C15H8Br2ClN3OS; Calcd.: %C, 38.04; H, 1.70; N, 8.87, Found: %C, 38.12; H, 1.76; N, 8.94. IR: υ max./cm−1 3600 (NH2), 3100 (C–H aromatic), 1690 (C=O of quinazolinone), 1633 (C=N), 1590 (C=C) and at 1560 and 1320 (C=S). 1H-NMR (DMSO-d6, ppm): δ 7.5–8.3 (m, 6 H, Ar–H) and at 9.4 (s, 2H, NH2, exchangeable with D2O). 13C NMR (DMSO-d6):183, 171, 156, 155, 140, 136, 132, 130, 129. 127, 122, 114. MS: m/z = 477
6,8-dibromo-2-(4-chlorophenyl)-3-phenylquinazolin-4(3H)-one (VIII)
Crystallized from ethanol to give brown crystals of compound VIII, m.p. 140 °C, 80 % yield. Analysis calculated for C20H11Br2ClN2O; Calcd.: %C, 48.97; H, 2.26; N, 5.70, Found: %C, 48.95; H, 2.20; N,5.62. IR: υ max./cm−1 3110 (C–H aromatic), 1680 (C=O of quinazolinone), 1630 (C=N) and at 1600 (C=C). 1H-NMR (DMSO-d6, ppm): δ 7.1–8.2 (m, 11 H, Ar–H). 13C NMR (DMSO-d6):164, 160.5, 155, 140, 135, 131, 129, 128.9, 128.5, 128, 126.7, 122, 113. MS: m/z = 494
6,8-dibromo-2-(4-chlorophenyl)-3-p-tolylquinazolin-4(3H)-one (IX)
Crystallized from ethanol to give yellow crystals of compound IX, m.p. 110 °C, 75 % yield. Analysis calculated for C21H13Br2ClN2O; Calcd.: %C, 49.98; H, 2.60; N, 5.55, Found: %C, 49.90; H, 2.50; N,5.47. IR: υ max./cm−1 3100 (C–H aromatic), 1685 (C=O of quinazolinone), 1625 (C=N) and at 1590 (C=C). 1H-NMR (DMSO-d6, ppm): δ 2.30 (s, 3H, CH3), and at 7.2–8.1 (m, 10 H, Ar–H). 13C NMR (DMSO-d6):165, 161, 154.4, 139.4, 136.9, 135.7, 129.8, 129.5, 129.2, 128.9, 128.4, 122, 113, 21.4. MS: m/z = 508
3-(4-acetylphenyl)-6,8-dibromo-2-(4-chlorophenyl)quinazolin-4(3H)-one (X)
Crystallized from ethanol to give brown crystals of compound X, m.p. 180 °C, 70 % yield. Analysis calculated for C22H13Br2ClN2O2; Calcd.: %C, 40.07; H, 1.99; N, 4.25, Found: %C 40.03; H, 1.90; N, 4.20. IR: υ max./cm−1 3200 (C–H aromatic), 1710 (C=O of acetyl), 1690 (C=O of quinazolinone), 1635 (C=N) and at 1600 (C=C). 1H-NMR (DMSO-d6, ppm): δ 2.60 (3H, s, CH3) and at 7.5–8.3 (m, 10 H, Ar–H). 13C NMR (DMSO-d6):197, 164, 160.6, 154.3, 139.4, 137.1, 136.9, 136, 132, 129.5, 129, 128.9, 126.5, 125, 124, 123, 113, 26.7. MS: m/z = 536
6,8-dibromo-2-(4-chlorophenyl)-3-hydroxyquinazolin-4(3H)-one (XI)
A mixture of benzoxazine IV (0.01 mol) and hydroxylamine hydrochloride (0.01 mol) in dry pyridine (30 ml) was heated under reflux for 8 h and the reaction mixture was then concentrated to half its volume poured into crushed ice containing few drops HCl. The separated solid was filtered, washed with water and crystallized with ethanol m.p. 245 °C, 60 % yield. Analysis calculated for C14H7Br2ClN2O2; Calcd.: %C, 39.06; H, 1.64; N, 6.51, Found: %C 39.12; H, 1.70; N, 6.53. IR: υ max./cm−1 3400 (OH), 3050 (C–H aromatic), 1690 (C=O of quinazolinone), 1630 (C=N) and at 1600 (C=C). 1H-NMR (DMSO-d6, ppm): δ 7.4–8.2 (m, 6 H, Ar–H) and at 10.60 (s, 1H, OH). 13C NMR (DMSO-d6):165, 162, 154.3, 139, 135, 132, 129, 128.5, 126.9, 125, 122, 115. MS: m/z = 434
General procedure for the synthesis of 6,8-dibromo-2-(4-chlorophenyl)-3-((substituted)methyl)quinazolin-4(3H)-one (XIIa–c)
A mixture of V (0.01 mol) and respective primary amines like aniline, p-toluidine, 2-amino pyridine (0.01 mol) in 30 mL of glacial acetic acid, formaldehyde solution 30 % (0.05 mol) was added. The solution was refluxed for 8 h, the reaction mixture was cooled and immediately poured into crushed ice. The product obtained was filtered, washed with methanol and crystallized from proper solvent to give the compounds XIIa–c respectively.
6,8-dibromo-2-(4-chlorophenyl)-3-((phenylamino)methyl)quinazolin-4(3H)-one (XIIa)
Crystallized from ethanol to give white crystals of compound XIIa, m.p. 260 °C, 65 % yield. Analysis calculated for C21H14Br2ClN3O; Calcd.: %C, 48.54; H, 2.72; N, 8.09, Found: %C 48.43; H, 2.65; N, 8.03. IR: υ max./cm−1 3420 (N–H), 3100 (C–H aromatic), 1670 (C=O of quinazolinone), 1620 (C=N) and at 1590 (C=C). 1H-NMR (DMSO-d6, ppm): δ 4.70 (s, 2H, CH2), 7.5–8.3 (m, 11 H, Ar–H) and at 9.5 (s,1H, NH, exchangeable with D2O). 13C NMR (DMSO-d6):169, 162,155, 148, 139.4, 135, 131.9, 129.5, 129.2, 12809, 126.7, 125.2, 120.7, 122, 113, 60.5. MS: m/z = 523
6,8-dibromo-2-(4-chlorophenyl)-3-((p-tolylamino)methyl)quinazolin-4(3H)-one (XIIb)
Crystallized from ethanol to give white crystals of compound XIIb, m.p. 290 °C, 70 % yield. Analysis calculated for C22H16Br2ClN3O; Calcd.: %C, 49.52; H, 3.02; N, 7.87, Found: %C 49.59; H, 3.14; N, 7.9. IR: υ max./cm−1 3400 (N–H), 3100 (C–H aromatic), 1665 (C=O of quinazolinone), 1620 (C=N) and at 1595 (C=C). 1H-NMR (DMSO-d6, ppm): δ 2.30 (s, 3H, CH3), 4.60 (s, 2H, CH2), 7.3–8.1 (m, 10 H, Ar–H) and at 9.4 (s, 1H, NH, exchangeable with D2O). 13C NMR (DMSO-d6):162, 160, 153, 144, 139.7, 135.5, 131, 129.7, 129.4, 129, 128, 126.7, 125.1, 122, 113, 60.3. MS: m/z = 537
6,8-dibromo-2-(4-chlorophenyl)-3-((pyridin-2-ylamino)methyl)quinazolin-4(3H)-one (XIIc)
Crystallized from ethanol to give white crystals of compound XIIc, m.p. 255 °C, 65 % yield. Analysis calculated for C20H13Br2ClN4O; Calcd.: %C, 46.14; H, 2.52; N, 10.76, Found: %C 46.25; H,2.61; N,10.80. IR: υ max./cm−1 3450 (N–H), 3150 (C–H aromatic), 1675 (C=O of quinazolinone), 1630 (C=N) and at 1600 (C=C). 1H-NMR (DMSO-d6, ppm): δ 4.70 (s, 2H, CH2), 6.9–8.1 (m, 10 H, Ar–H) and at 9.9 (s, 1H, NH, exchangeable with D2O). 13C NMR (DMSO-d6):165, 162, 158, 155, 148, 139.5, 138.6, 135.7, 131.3, 129.2, 128.9, 125, 126, 122, 118, 115, 107.4, 60.7. MS: m/z = 524
General procedure for the synthesis of 6,8-dibromo-2-(4-chlorophenyl)-3-(substituted-1-ylmethyl)quinazolin-4(3H)-one (XIIIa–c)
A mixture of compound V (0.01 mol), paraformaldehyde (0.01 mol) and the appropriate secondary amine (0.01 mol) namely, piperidine, 2-methyl piperidine and/or morpholine in DMF (20 mL) was refluxed for 8 h. The excess solvent was evaporated under vacuum and the obtained residue was poured into crushed ice. The solid product was filtered off and washed with water to obtain the desired products XIIIa–c respectively.
6,8-dibromo-2-(4-chlorophenyl)-3-(piperidin-1-ylmethyl)quinazolin-4(3H)-one (XIIIa)
Crystallized from ethanol to give brown crystals of compound XIIIa, m.p. 285 °C, 60 % yield. Analysis calculated for C20H18Br2ClN3O; Calcd.: %C, 46.95; H, 3.55; N, 8.21, Found: %C 46.90; H, 3.46; N,8.10. IR: υ max./cm−1 3110 (C–H aromatic), 1670 (C=O of quinazolinone), 1630 (C=N) and at 1600 (C=C). 1H-NMR (DMSO-d6, ppm): δ 1.55 (m, 6H, 3CH2 of piperdine), 2.43 (m, 4H, CH2NCH2 of piperdine), 4.20 (s, 2H, CH2) and at 7.2–8.13 (m, 6H, aromatic-H). 13C NMR (DMSO-d6):161.6, 155, 139, 135.5, 131.3, 129.1, 128.9, 126, 125.2, 122, 113, 57.4, 51.7, 25.7, 24.5. MS: m/z = 515
6,8-dibromo-2-(4-chlorophenyl)-3-((2-methylpiperidin-1-yl)methyl)quinazolin-4(3H)-one (XIIIb)
Crystallized from ethanol to give yellow crystals of compound XIIIb, m.p. 170 °C, 60 % yield. Analysis calculated for C21H20Br2ClN3O; Calcd.: %C, 47.98; H, 3.83 N, 7.99, Found: %C 47.92; H, 3.76; N,7.91. IR: υ max./cm−1 3100 (C–H aromatic), 1660 (C=O of quinazolinone), 1610 (C=N) and at 1595 (C=C). 1H-NMR (DMSO-d6, ppm): δ 1.12 (s, 3H, CH3), 1.4 (m, 6H, 3CH2 of piperdine), 2.40 (m, 3H, CHNCH2 of piperdine), 3.90 (s, 2H, CH2) and at 7.4–8.3 (m, 6H, aromatic-H). 13C NMR (DMSO-d6):164, 161.9, 155, 139.6, 136, 132, 129.4, 128.8, 126, 125,122, 113 54.8, 54.2, 49.5, 33.9, 25.9, 23.3. MS: m/z = 529
6,8-dibromo-2-(4-chlorophenyl)-3-(morpholinomethyl)quinazolin-4(3H)-one (XIIIc)
Crystallized from ethanol to give yellow crystals of compound XIIIc, m.p. 235 °C, 60 % yield. Analysis calculated for C19H16Br2ClN3O2; Calcd.: %C, 44.43; H, 3.14 N, 8.18, Found: %C 44.32; H, 3.10; N,8.10. IR: υ max./cm−1 3150 (C–H aromatic), 1690 (C=O of quinazolinone), 1640 (C=N) and at 1600 (C=C). 1H-NMR (DMSO-d6, ppm): δ 2.5 (t, 4H, 2CH2 of morpholine), 3.6 (t, 4H, 2CH2 of morpholine), 4.25 (s, 2H, CH2) and at 7.4–8.2 (m, 6H, aromatic-H). 13C NMR (DMSO-d6): 167, 163, 156, 139.6, 136.4, 131.5, 129.4, 128.9, 126.7, 125.1,123,113, 66.4, 57.4, 50.7. MS: m/z = 517
Biological studies
Cytotoxicity
Cell line culture
Human breast cancer cell line (MCF-7) was grown in DMEM medium with: 10 % fetal bovine serum, penicillin100 units/ml and streptomycin100 µg/mL. Cell line was maintained at 37 °C in humidified 5 % CO2 atmosphere. Tested compounds and doxorubicin solutions were prepared in DMSO and stored at–20 °C. Control (untreated cells) was treated with DMSO alone (DMSO concentration was always less than 0.1 %.
Cell viability assay (SulfoRhodamine B assay)
To assess cellular proliferation, Sulforhodamine B assay was used according to the manufacturer’s instructions. Briefly, cells were grown in tissue culture flasks, and then harvested by treating the flasks with 0.025 % trypsin and 0.25 mM EDTA for 5 min. Once detached, cells were washed, counted and an aliquot (5 × 103 cells) was placed in each well of a 96-well cell culture plate in a total volume of 100 μl. Cells were allowed to attach overnight and then treated with or without increasing concentrations of tested compounds at serial concentrations of 100–6.25 µmol/ml (each repeated thrice). After treatment, cell medium was aspirated, and 100 mm3/well of 10 % trichloroacetic acid was added. After fixation at 4 °C and washing, 50 mm3 of 0.4 % (w/v) sulforhodamine B (SRB; Sigma-Aldrich) was added. Plates were incubated at room temperature for 30 min. Unbound SRB was removed with 1 % acetic acid. Bound SRB was solubilized with 100 mm3 of 10 mM Tris-base solution. Absorbance was measured using a precision microplate reader (StatFax 2100, USA) at 570 and 650 nm (background). Doxorubicin was used as reference drug in this study; positive control [6, 23]. Cell viability was calculated using sigmoidal concentration response curve fitting models (Sigmaplot software) and expressed as percentage of survival relative to untreated cells.
Apoptotic effect (Caspase-3 activity)
Human CASP3 assay is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of Caspase-3 activities of cell culture supernatants. Six hours after (MCF-7) cell lines treatment with tested compounds or doxorubicin, CASP3 activity was determined according to manufacturer’s protocol (Cat. KA1868, Ver01, Abnova). Absorbance was measured at 450 nm, using a precision microplate reader (StatFax 2100, USA). Cellular apoptosis was calculated using Sigma plot software (best-fit straight line) and expressed in folds relative to untreated cells (negative control).
References
A. AA. Hashim, O. El-Ahmady, H.M. Khaled, M.M. Elmazar, Z. Hassen, IJAPBC 3, 1048–1059 (2014)
World Cancer Report, 2014, http://www.esmo.org/Oncology-News/World-Cancer-Report-2014
M. Olsson, B. Zhivotovsky, Cell Death Differ. 18, 1441–1449 (2011)
E. Devarajan, A.A. Sahin, J.S. Chen, R.R. Krishnamurthy, N. Aggarwa, A.-M. Brun, A. Sapino, F. Zhang, D. Sharma, X.-H. Yang, A.D. Tora, K. Mehta, Oncogene 21, 8843–8851 (2002)
M.F. Ahmed, A. Belal, M. Youns, Med. Chem. Res. (2015). doi:10.1007/s00044-015-1357-1
M.F. Ahmed, M. Youns, Arch. Pharm. Chem. Life Sci. 346, 610–617 (2013)
M.F. Ahmed, M. Youns, A. Belal, Acta Pol. Pharm. 73(1), (2016) (in press)
H.M. Abdel-Rahman, M. Abdel-Aziz, J.C. Canzoneri, B.D. Gary, G.A. Piazza, Arch. Pharm. (Weinheim) 347(9), 650–657 (2014)
M.S. Mohamed, M.M. Kamel, E.M.M. Kassem, N. Botaleb, S. Abd El-moez, M.F. Ahmed, Eur. J. Med. Chem. 45, 3311–3319 (2010)
B. Pati, S. Banerjee, J. Adv. Pharm. Edu. Res. 3, 136–147 (2013)
L.M. Tsai, S.N. Yang, S.F. Lee, Y.A. Ding, J.W. Chern, J.M. Yang, J. Cardiovasc. Pharmacol. 38(6), 893–899 (2001)
A.M. Alafeefy, A.A. Kadi, O.A. Al-Deeb, K.E.H. El-Tahir, N.A. Al-jaber, Eur. J. Med. Chem. 45(11), 4947–4952 (2010)
S.M. Mosaad, M.K. Mohsen, M.M. Emad, N. Abotaleb, M.N. Salwa, F.A. Marwa, Acta Pol. Pharm. Drug Res. 66, 487–500 (2009)
K.M. Amin, M.M. Kamel, M.M. Anwar, M. Khedr, Y.M. Syam, Eur. J. Med. Chem. 45(6), 2117–2131 (2010)
S.M. Mosaad, M.K. Mohsen, M.M. Emad, N. Abotaleb, M.N. Salwa, F.A. Marwa, Acta Pol. Pharm. Drug Res. 67, 159–171 (2010)
S.M. Mosaad, M.K. Mohsen, M.M. Emad, N. Abotaleb, M. Khedr, F.A. Marwa, Acta Pol. Pharm. Drug Res. 68, 665–675 (2011)
N.M. Abdel Gawad, H.H. Georgey, R.M. Youssef, N.A. El-Sayed, Eur. J. Med. Chem. 45, 6058–6067 (2010)
M.N. Noolvi, H.M. Patel, V. Bhardwaj, A. Chauhan, Eur. J. Med. Chem. 46, 2327–2346 (2011)
M.N. Noolvi, H.M. Patel, Arab. J. Chem. 6, 35–48 (2013)
A.S. El-Azab, M.A. Al-Omar, A.A.-M. Abdel-Aziz, N.I. Abdel-Aziz, M.A.-A. El-Sayed, A.M. Aleisa, M.M.S. Ahmed, S.G. Abdel-Hamide, Eur. J. Med. Chem. 45, 4188–4198 (2010)
A.R.R. Rao, R.H. Bahekar, Ind. J. Chem. 38B, 434–439 (1999)
M.J. Mphahlele, H.K. Paumo, A.M. El-Nahas, M.M. El-Hendawy, Molecules 19, 795–818 (2014)
A.M. Alafeefy, S.I. Alqasoumib, A.E. Ashour, V. Masand, N.A. Al-Jaber, T.B. Hadda, M.A. Mohamed, Eur. J. Med. Chem. 53, 133–140 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, M.F., AA. Hashim, A. Design, synthesis of novel quinazolin-4-one derivatives and biological evaluation against human MCF-7 breast cancer cell line. Res Chem Intermed 42, 1777–1789 (2016). https://doi.org/10.1007/s11164-015-2117-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2117-z