Abstract
A new process to remove carbon dioxide (CO2) from the atmosphere, by combining commercial industrial technologies with ocean liming and CO2 storage, is presented. The process aims to overcome the limiting factors of other negative emission technologies (cost and energy requirements, potential competition for land and freshwater) while simultaneously addressing the problem of ocean acidification. The overall proposed process is based on the following: (a) a gasifier where the biomass is converted to syngas; (b) a thermal steam reformer working at high temperature where the hydrocarbons and tar oils are converted to hydrogen (H2) and carbon monoxide (CO); (c) a kiln to produce Ca(OH)2 (slaked lime) from limestone by using the enthalpy of the hot syngas; (d) the spreading, by means of vessels, of the slaked lime into the seawater to achieve ocean liming; (e) the delivery of syngas to a water gas shift reactor producing CO2 and H2 that are then separated; (f) the final storage of all CO2 produced in the process; (g) the use of H2, being the valuable by-product of the whole process, for decarbonized energy production as well as for ammonia synthesis, offsetting part of the production cost, thus generating “low-cost” negative emissions. The mass and energy balances show that the total atmospheric CO2 removed by the process is 2.6 ton per ton of biomass used. By adding an estimated 0.43 ton avoided—thanks to the use of produced H2—the overall CO2 benefit of the process increases to 3.0 ton per ton of biomass. A preliminary cost analysis resulted in an average levelized cost of 98 $ per ton of CO2 removed; when considering the revenues from the produced energy, the cost falls to 64 $/tCO2. The higher efficiency in carbon removal obtained allows to reduce the amount of biomass required by BECCS (bioenergy with carbon capture and storage) to achieve negative emissions, and thanks to the valuable H2 produced it lowers the costs of CO2 removal from the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Despite all mitigation efforts to reduce carbon dioxide (CO2) emissions, atmospheric CO2 concentrations are still increasing at a high rate with mounting evidence that a huge amount of carbon will need to be removed from the atmosphere to reach the ambitious climate targets established in the Paris Agreement of limiting global temperature increase to well below 2 °C of pre-industrial levels (let alone to pursue all efforts to stay below + 1.5 °C). Most scenarios assessed in the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) (Clarke et al. 2014) rely on the use of negative emission technologies (NETs) to limit global warming to 2 °C cost-effectively. Many NETs have been proposed, such as bioenergy with carbon capture and storage (BECCS), afforestation and reforestation, land management to increase carbon in soils, enhanced weathering, direct air capture and carbon storage, ocean fertilization, and ocean alkalinization, and have been widely discussed in the literature (Minx et al. 2018; EASAC 2018). Although the current knowledge on NETs is still incomplete, many estimates of costs, potentials, and side effects are available (Fuss et al. 2018).
NETs still face difficulties in their deployment due to many different environmental, social, and economic reasons (Smith et al. 2016); in particular, the cost of the existing NETs and technologies for carbon capture and storage (CCS) is very high and is a substantial obstacle for their deployment at a large scale (Honegger and Reiner 2018). For this reason, the availability of affordable processes for the removal of CO2 will be a key factor for effectively limiting its increase in the atmosphere in the coming decades. According to Nemet et al. (2018), although the majority of technologies are still in the research and development stage, several gigatonnes (Gton) of removal for each individual NET are required in order to have an impact on Earth’s climate; innovation in NETs is thus crucial and consists in generating better performances (such as more carbon removed), fewer adverse side effects, and more societal acceptance, including lower costs.
The aim of this paper is to describe and assess the potential performances and costs of a new process (patent pending, IPN WO2018/134/775/A1) that allows the removal of CO2 from the atmosphere by combining available industrial technologies with ocean liming and CO2 storage. The process aims to overcome the limiting factors of NETs, such as cost and energy requirements (for direct air capture), logistics of spreading materials over large areas (for enhanced weather technologies), and potential competition for land and freshwater (for afforestation and BECCS). Furthermore, this process also allows for addressing oceans’ acidification—the “evil twin” of climate change.
Although a complete life-cycle assessment (LCA) of the process is currently in progress and is not reported in this paper, a detailed CO2, mass, and energy balance, highlighting its innovative characteristic and its overall performance, is described and commented. A cost analysis is also reported, and the main environmental challenges are discussed.
2 Description of the process
A schematic outline of the process is shown in Fig. 1 and is described in the following paragraphs.
2.1 Biomass gasification
The first step is the gasification (auto-thermal reforming, ATR) of biomass feedstocks. It is a proven industrial process (Sikarwar et al. 2017) that converts solid biomass into a synthesis gas (syngas) composed of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), nitrogen (N2), water vapor (H2O), and hydrocarbons (tars). The gas composition depends on the feedstock used, the gasifying agent, the type of gasifier, and the process parameters (Couto et al. 2013; Baláš et al. 2015). The process is performed at atmospheric or subatmospheric pressure, in order to promote the downstream calcination of limestone, as explained in detail by García-Labiano et al. (2002). The gasification could also be applied to other solid carbonaceous materials like coal, as discussed in Section 4.2; however, the use of biomass emphasizes the role of the process as a negative emission technology.
The syngas from the ATR is purified from the tars by means of a well-known thermal steam reforming process (also called thermal cracking), a widespread technology in the petrochemical sector (Milne et al. 1998); with this process, by controlling the temperature and residence time, almost all hydrocarbons are converted into H2, CO, and CO2. The resulting high temperature gas (~ 1000 °C) is mainly composed of H2, CO, CO2, H2O, and N2, whose relative amount depends mainly on the purity of the oxygen used as gasification agent. Ninety percent purity oxygen, produced by a vacuum pressure swing adsorption unit, has been assumed in the calculations, in order to reduce the overall cost for this medium-size modular gasification plant.
2.2 Calcination and production of slaked lime
The sensible heat of the high-temperature syngas leaving the cracking unit at about 1000 °C is used to provide the energy for the limestone calcination process (CO2APPS 2017). The hot syngas is sent to an updraft calcination kiln (calcinator) where the limestone is loaded counter-currently. The calcination reaction of the limestone is highly endothermal, and the rate at which it occurs depends on the temperature and on the CO2 partial pressure in the syngas (Stanmore and Gilot 2005). The CO2 produced during the calcination of the limestone increases the amount of CO2 of the syngas. The exact temperature of the syngas leaving the top of the calcinator (~ 280 °C) will be optimized for the downstream processes. The calcinator also allows the removal of all the acidic components in the syngas (Hu et al. 2006). The lime produced by the calcination process is further processed into calcium hydroxide (Ca(OH)2, also called slaked lime) by adding the proper amount of water; the heat generated by this very exothermal process is conveniently used within the process through a heat recovery steam generator (HRSG) to provide steam to the gasifier.
2.3 Ocean liming
Slaked lime is dissolved in seawater through a process named ocean liming (or ocean alkalinization) that adds alkalinity to seawater. Increasing the ocean’s alkalinity allows more atmospheric carbon to be absorbed by the ocean, which is stored in the seawater as bicarbonates. Simultaneously, it locally increases the CO2 buffering capacity of the ocean, thus contrasting the ocean acidification caused by the natural uptake of atmospheric carbon. The use of open ocean alkalinization as a mean to sequester atmospheric carbon and limit the decrease of pH at the ocean’s surface has been increasingly studied during the last decades. Many authors (Kheshgi 1995; Köhler et al. 2013; González and Ilyina 2016; Renforth and Henderson 2017) indicate a large potential of carbon removal by ocean alkalinization, as well as technological challenges (Renforth et al. 2013) and potential side effects, such as interferences with ocean ecological and biogeochemical functioning, that will be discussed in Section 4.1.
Slaked lime can be discharged within the wake of a ship (Keller et al. 2014; Renforth et al. 2013); a more complex, but potentially more effective, alternative based on the injection into the deep seawater currents that end into upwelling regions will be discussed in Section 4.2.
Alternatively, the calcium hydroxide can be used in the industrial sector (i.e., to capture CO2 from large point source flue gases (Hiltz et al. 2017)).
2.4 Water gas shift reforming and CO2 separation
The syngas with extra CO2 produced by the calcination reaction of the limestone will be fed to a water gas shift reforming (WGSR), where CO and H2O react generating H2 and CO2 (Reddy and Smirniotis 2015). The gas leaving the WGSR, composed by H2, CO2, H2O, and N2, is sent to a CO2 separation unit, based on pressure swing adsorption (PSA; Chou et al. 2013) or membranes (MTR 2017). The composition of the gas after CO2 separation is calculated using the technical specifications of the membrane technology and is composed mainly of H2 (87.8%mol), with minor amount of CH4 (6%mol), CO2 (5.2%mol), CO (0.5%mol), Argon (0.4%), and N2 (0.1%mol).
2.5 CO2 storage
The removed CO2 is compressed and conditioned for its further disposal in a permanent storage that could be a saline formation, a depleted oil, or gas reservoir (Aminu et al. 2017) or in glass capsules into the deep seabed (Caserini et al. 2017).
The geological storage of CO2 is currently at the demonstration stage, with more than 30 projects in operation or under construction around the world (GCCSI 2017). Although in principle, there is a large potential for available area across the globe to geologically store CO2 permanently (Fuss et al. 2018), and in a CCS project the storage phase is generally less expensive than the capture, this phase is the most critical one. This is because it generally takes a complex and time-consuming assessments in order to qualify a saline formation suitable for CO2 storage and because of the large up-front investment needed to secure storage capacity (IEA 2014). Furthermore, the storage potential has large regional variations with many regions having limited “practical” and “matched” capacity, as defined by the “Geologic CO2 storage capacity pyramid” (Dooley 2013; IEAGHG 2008).
Reservoirs used for enhanced oil recovery (EOR) and enhanced gas recovery (EGR) are the most promising CO2 deposits currently used and are a key business driver for CCS (GCCSI 2017).
Another technique to permanently store CO2, based on its compression in the liquid form inside glass capsules disposed into the deep seabed, has been recently proposed and evaluated by the authors of this paper (Caserini et al. 2017; Barreto et al. 2018). Although this option, called submarine carbon storage (SCS), is still at the research and development stage, it can represent an alternative to the conventional storage technologies, in particular for emission sources located in coastal areas where CO2 injection in geological formations is not an option. A detailed life cycle and cost assessment has been carried out by the authors, showing a 10% average penalty for CO2 storage and an average storage cost of 17 $/tCO2. This technology has been considered as the baseline option for CO2 storage of this process.
2.6 Hydrogen-based products
H2 and N2 obtained from the separator could be used in different ways. H2 can be directly fed to an internal combustion engine for electric energy production, generating revenues that will lower the overall cost of the process. Another very efficient option is to further process H2 and N2 into anhydrous ammonia (NH3) through a Haber Bosch reactor, generating valuable fuel for transportation which can be of great interest in particular for the marine sector (Zamfirescu and Dincer 2009; Barret 2015), as discussed in Section 4.2.3.
3 Results
3.1 Mass and energy flows
The flows of energy and materials of the whole process are shown in Fig. 2, based on 1 ton (metric ton, from now on referred as ton) of biomass processed in the gasifier (carbon content 44.6%, moisture 10%, lower heating value 17 GJ/t).
Based on average data from existing plants (Stork et al. 2014), the energy generated during the reforming of 1 ton of biomass allows the production of 0.93 ton of calcium oxide (CaO) in the calcinator, resulting in 1.23 ton of slaked lime that is then discharged into the ocean. The amount of limestone and water that should be provided is 1.67 ton and 0.3 ton, respectively.
The CO2 derived from the carbon content of biomass and from the limestone that is separated and sent to storage amounts to 2.02 ton, whereas 0.33 ton of flue gas, with an energy content of 12.8 GJ (39 GJ/t) and 88%v H2, is partly used in a cogeneration plant for the production of the energy required by the process, with the remaining provided to external users. In both cases, a 57% energy conversion efficiency is assumed (i.e., gas turbine GE Power 7HA.01/.02).
3.2 CO2 balance
The carbon balance of the process is described in Fig. 3, again based on 1 ton of biomass fed into the gasifier.
The amount of absorbed atmospheric CO2 from the biomass is 0.446 tons of C, but the net carbon removal according to a LCA approach will be lower because of the energy spent during the production chain (for biomass transport, fertilizers) and due to indirect land use change effects (Plevin et al. 2010).
The theoretical amount of CO2 taken up from the atmosphere after the discharge of the slake into the ocean is 2 mol per 1 mol of added CaO (i.e., 2 mol of alkalinity neutralize 2 mol of carbonic acid, with 2 mol of atmospheric carbon dioxide dissolving into the ocean to re-establish the equilibrium). In practice, because of the reaction between a small proportion of the hydroxyl and bicarbonate ions to form carbonate and water, only between 1.6 and 1.8 mol of carbon dioxide are actually absorbed (Renforth et al. 2013). Furthermore, the efficiency of the ocean liming process is also limited by the air-sea gas exchange (driven by wind speed and by the partial pressure difference between the ocean and the atmosphere) and by the actual availability of the alkaline water at the ocean’s surface. Thus, a conservative value of 1.4 mol of carbon dioxide absorbed per each mole of Ca(OH)2 added has been assumed (Keller et al. 2014). Thus, 1.23 Mtons Ca(OH)2 allow to absorb 1.03 Mtons of CO2 (1.03 = 1.23·1.4·44/74, where 44 and 74 are the molecular weight of CO2 and Ca(OH)2, respectively (g/mol)).
The carbon sent to the final storage, 0.55 ton of C, is the sum of the carbon taken from the atmosphere and of the carbon content of limestone (0.20 ton of C). The carbon emitted in the atmosphere during the combined production of heat and power required by the process (0.016 ton) was discounted because of the carbon impurities (CO2, CH4, CO) contained in the H2 stream.
The total amount of carbon directly removed from the atmosphere, 0.71 ton of C (2.6 tons of CO2), is calculated by summing the carbon taken from the atmosphere by the biomass and by the ocean, then subtracting the carbon emitted during the production of energy required by the process (Table 1). C1 should be considered in the assessment of direct carbon removal as done in many carbon balance of BECCS (Fajardy and Mac Dowell 2017; Laude et al. 2011; Tokimatsu et al. 2017), because the carbon taken from the atmosphere and stored in the biomass is what allows BECCS to produce negative emissions.
The carbon avoided by the electric energy that could be generated by the external use of H2 produced in the process (0.116 ton of C) can be estimated by considering the energy content of H2 provided to external users (9.8 GJ), a 57% power plant efficiency, and assuming an average CO2 emission factor for the electricity displaced of 400 kg CO2/MWh (average for OECD countries in 2015 (IEA 2017)), as well as the residual carbon in the flue gas used by the power plant.
Added to the direct carbon removal, the carbon benefit of the process increases to 0.83 ton of C, that is 3.0 tons of CO2 for 1 ton of biomass used as a base unit.
3.3 Economic assessment
A preliminary cost assessment of the process has been carried out and is presented here. The methodology applied is based on Rubin (2013), Roussanaly et al. (2013), Roussanaly et al. (2014), Renforth et al. (2013), and Caserini et al. (2017) and aims to assess the total cost of negative CO2 emission in terms of levelized CO2 cost (i.e., the total annual cost divided by the total amount of negative carbon dioxide, in US dollar) for a plant that processes 1 Mton of biomass per year.
Both capital expenditures (CAPEX) and operational expenditures (OPEX) are assessed. The CAPEX is split between bare erected costs (BEC); Engineering, procurement, and construction costs (EPCC); and contingencies. Details on BEC and on the fixed and variable OPEX costs evaluated are shown in Table 2.
The basic data used for CAPEX assessment are listed in Table 3.
The assessment of a future cost of slaked lime application and CO2 storage is challenging, since the literature is scarce and is based on theoretical assessments and few data on existing facilities.
Many alternatives have been considered for ocean liming, causing a variation in costs due to different types of application, energy, and capital investments. For example, a CAPEX of 14 M$ has been assumed for port infrastructure of handling and discharging slaked lime; an OPEX of 7 $/ton for slaked application, calculated considering the transportation of the Ca(OH)2 with a dry bulk 76,000 ton vessel with a daily cost of 14,750$/day (Vasilieou 2018) for a round trip of 14 days (loading and unloading time included); and the energy needed for diluting and injecting the Ca(OH)2 solution into the sea. All these costs are assumed for a 1 Mton of biomass per year.
For CO2 storage, the average cost of the submarine carbon storage technology assessed in Caserini et al. (2017) has been considered, with 120 M$ CAPEX and 6.6 $/ton of CO2 OPEX, the latter not including the cost for external supply of electricity and natural gas, that is internally generated by the process. This cost is similar to other estimated costs for CO2 storage in geological formations (IPCC 2005; ZEP 2011; Barreto et al. 2018), although these are more dependent on the storage plant size, thus on the biomass supply potential.
An interest rate of 5% and a time horizon of 25 years have been assumed for the calculation of the annual payment. The CAPEX amounts to 672 M$, with an annual payment of 48 M$. The hydrogen infrastructure is the most expensive, accounting for 42% of the total cost.
The variable operating cost is a function of the amount of biomass used, thus of the negative CO2 emissions. On the contrary, in the fixed costs, the number of workers is independent from the quantity of feedstock used.
The total OPEX results 206 M$/year (Table 4), with the cost for biomass accounting for a huge 49% of the total.
The total levelized cost is 253 M$/year, resulting in a CO2 removal cost of 98 $/ton of CO2 (the total amount of CO2 removed from the atmosphere is 2.6 Mton CO2/year).
For the assessment of the final cost, the revenues from the energy generated by the fuel gas produced should be taken as a credit. Considering a reference price of decarbonized gas of 9 $/GJ (market price of natural + carbon cost in EU-ETS), the production of 9.7 million GJ per year generates a total revenue of 88 M$/year, that is equivalent to 34 $/ton of CO2. The final actual price for a ton of CO2 removed from the atmosphere through the described process amounts then to 64 $/ton of CO2 (Table 5).
4 Discussion
As shown in Section 3.1, the proposed process is highly efficient in terms of carbon removal since for every ton of biomass used, it removes 2.6 tons of CO2 from the atmosphere. This is 58% higher than the removal obtained by the traditional BECCS, that is only due to the carbon absorbed from the atmosphere (1.64 ton of CO2 for the same biomass considered in the process, see Table 3). Furthermore, the CO2 emission avoided by the electric energy that can be generated by the external use of H2, 0.43 ton of CO2/ton of biomass (see Table 3), is only 13% lower than the one avoided in the same grid by the electricity generated by a biomass-fired power plant with CCS (0.5 ton of CO2/ton of biomass), assessed based on 28.4% efficiency with CO2 capture and compression proposed by Ali et al. (2017) and a further 1.7% losses due to transport and injection into geological storage (Kemper 2015).
The better performance of the proposed process derives from the exploitation of the sensible heat of syngas for slaked lime production, as well as from the high CO2 concentration in the flue gas exiting the calcination, which reduces the specific energy for its capture. Moreover, ocean liming is a highly efficient way to store carbon, in the form of bicarbonate ions.
From an economic point of view, the process can rely on different feedstocks (biomass or coal), along with cheap and largely available limestone, and could produce different valuable hydrogen-based final products that are already traded as commodities. Although carbon storage and ocean liming still need to address many technological challenges, as will be discussed in the following, the technological core of the process is based on proven commercial technologies; thus, it is easy to scale up.
One major part of the total negative emissions achieved comes from CO2 uptake by biomass, but the two other contributions—CO2 absorbed by ocean and avoided CO2 by fuel displaced by the H2 provided to external users—are of great importance for the carbon and economic balance of the process.
In other words, the higher efficiency in carbon removal obtained by combining calcination and biomass gasification allows to reduce the amount of biomass required for the removal of 1 ton of CO2, thus mitigating one of the main limitations of BECCS. In fact, a major discussion within the scientific community on the generation of negative emissions with BECCS is related to the actual availability of biomass and to the impacts on the ecosystems and the populations in upscaling the production at a large scale, as required for significant carbon removal (Honegger and Reiner 2018).
It is worth noting that the removal ratio of 3 tCO2/t biomass is the result of the carbon balance of the process, but the net saving is lower and should be assessed on a life-cycle basis by taking into account the amount of energy and materials required for biomass harvesting and transport, for construction and maintenance of the infrastructures (gasifier, calcinator, CO2 compression and storage, etc.), and for the extraction and processing of raw materials.
Although many authors have pointed out that the mitigation potential of bioenergy technologies is overestimated when biogenic-CO2 flows and indirect land use changes are excluded (i.e., Giuntoli et al. 2016), and specific studies based on the specific feedstock considered should be performed, this problem is related to any type of carbon removal process based on biomass.
The penalty for CO2 storage through glass capsules in the deep seabed has been assessed as 10% of the stored CO2 (Caserini et al. 2017); about 35% of this is related to the energy spent for capsule production that has been already considered as internal consumption in the present evaluation.
On the other hand, the carbon benefits from electric energy generated by the process considered in this evaluation (400 g CO2/kWh, OECD countries), when based on a LCA approach, will rise to 486 g CO2/kWh for Europe and 750 g CO2/kWh for the global level calculated according to ILCD 2011 method (v1.10) (EC, 2013).
4.1 Potential environmental implications
Most of the literature on the biological response to changes in seawater carbonate chemistry has focused on acidifying condition (Haigh et al. 2015). The absorption of rising levels of atmospheric CO2 by the ocean modifies its chemistry (Feely et al. 2004; Doney et al. 2012), altering ecological networks and their functions (Hoegh-Guldberg and Bruno 2010; Nagelkerken and Connell 2015). On the contrary, the ecological implications concerning the direction and magnitude of marine biota responses to artificial ocean alkalinization are not yet understood (González and Ilyina 2016). Experimental studies reveal that the increase of alkalinity could disrupt the acid-base balance of marine organisms (e.g., littoral crabs; Cripps et al. 2013). The marine biota, in fact, relies on pH to regulate ion transport; the energy they invest to maintain intra- and extracellular pH depends on ambient pH. Accordingly, the potential ecological impact of increasing ocean alkalinity relies on the variation in the pH level produced during the ocean liming process.
The variation of ocean alkalinity also influences the saturation state of carbonate minerals that are essentials for marine carbonate-producing organisms, such as the species that inhabit the tropical (e.g., reefs populated with benthic species such as coral shellfish and green macroalgae) and the pelagic ecosystems (e.g., components of planktonic ecosystem such as coccolithophores, foraminifera, pteropods/heteropods). Experimental studies have been conducted on the capability of some marine species to produce (Langer et al. 2006) and dissolute carbonate (Schneider et al. 2011) at elevated levels of alkalinity. However, more research is required to investigate the positive and negative response of marine organisms and ecosystems to the artificial ocean alkalinization.
An increase in alkalinity can also influence the carbon compensation depth (CCD—the depth at which the rate of dissolution of CaCO3 compensates for the rate of CaCO3 sedimentation (Ridgwell 2007; Renforth and Henderson 2017)), increasing the extent of sediment exposed to elevated saturation states, the calcification, and accordingly the net removal of CaCO3 from the ocean. Consequently, the potential environmental effects and the magnitude of the ocean liming impact depend on different factors such as the following: the variation in the pH level generated at the deployment site, the deployment depth, the applied spatial scale (local, regional, or global), and the ecosystems (coastal or pelagic) selected to apply the liming process. Given all the above, a better understanding of the ecological implication of ocean liming necessarily requires additional dedicated experimental studies.
4.2 Alternative scenarios of the process and applicability
Different alternatives within the process can be considered by changing the feedstock (i.e., using coal instead of biomass), the CO2 storage technology (CCS instead of SCS), the method for spreading the slaked lime, or the main by-product (i.e., ammonia instead of hydrogen).
4.2.1 Coal
If biomass is not available in the required amount, fossil fuels might be used without any significant modification of the process. The use of coal substantially reduces the advantages of the process in terms of carbon removal; however, the balance is still positive, with 1.1 tons of CO2 removed from the atmosphere per 1 ton of coal (in case of a lower heating value of 26.2 GJ/ton) and 0.94 ton as avoided emission—although the net advantage should be verified also through a LCA.
It is worth noting that the possibilities to use coal as feedstock are another important advantage of this process in comparison to the BECCS. The possible use of coal and limestone, two very abundant and cheap feedstocks available worldwide, allows to widen the potential applications of this process. In fact, in the base configuration, the actual availability of biomass is a limiting factor, due to the limitation in forested areas, to site-specific barriers, and to the competition for resources like land for food production and water (Kato and Yamagata 2014; Smith et al. 2016; Muratori et al. 2016; Muri 2018).
Desert areas, or countries with lack of sufficient amount of biomass and CO2 storage possibilities (suitable geological formations for CCS or deep ocean waters for SCS application), could also contribute significantly to implement a negative emission infrastructure. Among others, Egypt, Libya, Algeria, Morocco, Western Sahara, Mauritania, Senegal, Namibia, South Africa, Yemen, Oman, Somalia, Iran, Pakistan, India, Australia, Chile, Peru, Mexico, and California are places that seem appropriate for the deployment of the technology using fossil fuels and SCS. In those countries, the process could be conveniently integrated also with the possibility to use cheap solar electric energy for the process and for the glass production in the case of the SCS.
4.2.2 Alternative pathways for liming the ocean
Many authors have evaluated alternative ocean liming processes. Köhler et al. (2013) studied the addition of CaCO3 particles (1 μm) in the ocean; Harvey (2008) projected a fleet of 3000 ships to add 4 Gton/year of CaCO3. Keller et al. (2014) proposed the increase of Ca(OH)2 assuming instantaneous dissolution within the wake of a ship; Renforth et al. (2013) suggested a fleet of 100 ships to add 1 Gton/year of Ca(OH)2. Aside from the cost of energy, capital investment, and environment control, the differences between these proposals fall mainly in the solubility factor of the materials added.
In open ocean, the depth of the euphotic zone extends on average 200 m below the surface. Photosynthesis occurs in this zone due to the availability of light and nutrients. Consequently, this surface layer is characterized by a high level of primary productivity, hosting a high biodiversity of marine species and making it a very highly sensitive ecosystem (Costello and Chaudhary 2017). Due to this consideration, the maximum addition rate of Ca(OH)2 into the wake of a cruising ship will be severely limited by ecological considerations and by the number of ships needed.
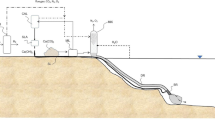
To overcome this limitation, an alternative solution is proposed to maximize the addition of Ca(OH)2 into seawater, while at the same time minimizing the environmental impact (i.e., rapid increase in pH) in surface waters. The alternative solution is the Ca(OH)2 direct injection into the deep, cold, and “corrosive” seawaters of main submarine currents that end, after a proper distance from the injection point, preferably into upwelling regions (Fig. 4). A small quantity of the OH− of the slaked lime solution injected inside the current will first neutralize the (H+) of the carbonic acid; the balance will react with the abundant (HCO3−) forming the vital CO32−; it is well-known that low CO32− in seawater imposes severe constraints to organisms that secrete CaCO3 to build their skeletal material. The adjusted “corrosive” upwelling waters will reach the ocean surface where the carbonate will be used by organisms and will react with the excess (H+) of the carbonic acid and thus promote the absorption of “new” CO2 from the atmosphere.
Deep currents move millions of cubic meters of water per second (Encyclopedia Britannica 2018) at a speed of a few kilometers per hour; they are cold and acidic, generally undersaturated in aragonite (Franco et al. 2018; Lachkar 2014; Hauri et al. 2016; Capone and Hutchins 2013). All such physical and chemical conditions look optimal to quickly dissolve the added Ca(OH)2 solution. A plume, rich in carbonates, will form inside the current at the injection point. At a proper distance from the injection point, the “neutralized” carbonated current will reach the euphotic zone with a minimum pH perturbation of the ocean surface.
The main ocean upwelling currents that could be exploited for the above-described option are the Humboldt, Canary, Benguela, California, and Somalia currents (Garcia-Reyes et al. 2015). Other currents, like the Gulf current, might also be evaluated for this purpose. In addition, site-specific environmental assessment studies should be performed in order to understand the ecological implication in the different sites. If this liming process would be applied, the discharge rate of Ca(OH)2 into the deep current can be much higher than the one into the wake of a ship, thus lowering the number of ships involved and their distance traveled. Because the injection point would be static, there will be no need for the ship to cruise for liming, thus saving operative costs.
4.2.3 NH3 for transportation
Another alternative of the process is to transform H2 into a valuable fuel for transportation like the anhydrous ammonia, which could be used in the marine sector (LRGL and UMAS 2017). In this case, there is a better economic rentability that will further decrease the cost of the carbon removal. The production of a commodity like anhydrous ammonia, which is transportable and storable, could be the best solution for plants located on remote coastlines and far from any H2 or electricity consumption site.
4.2.4 Alternative to CO2 storage
In addition to the storage of concentrated CO2 generated by the process using CCS or SCS, an alternative option could be the storage of CO2 in the form of bicarbonates, through the reaction of the enriched CO2 gas stream with additional limestone and water (i.e., seawater), as proposed in Rau (2011). Although the injection of this bicarbonate/alkalinity stream to the seawater could further increase the potential of the process for contrasting ocean acidification, further research is needed to overcome the main limitation of the Rau (2011) process (i.e., the large demand for carbonate mineral and water).
4.2.5 Combination with other processes for negative emission
The proposed process is in some aspects similar to the one proposed by Hanak et al. (2017), which utilizes the residual heat from a solid oxide fuel cell (SOFC) to calcine a carbonate material that can subsequently remove CO2 from the air. Although both methods use calcium oxides to uptake CO2 from the atmosphere (spreading calcined material in the seawater in order to produce calcium bicarbonate, or directly through the carbonation of the calcined material), the process presented here can use any feedstock (solid, liquid, and gaseous) in order to produce a hydrogen-rich fuel (H2, NH3 and with a proper methanation process even CH4). In contrast, the process by Hanak et al. (2017) uses a hydrogen-rich fuel (CH4) in order to produce electricity. The use of the Hanak et al. (2017) process downstream of the proposed process (feeding the SOFC with the hydrogen produced by the proposed process) deserves attention, but it is outside the scope of this paper.
5 Conclusions
In order to generate negative carbon emissions in the amount required to comply with the Paris Agreement, it is necessary to develop technologies that can be deployed worldwide using cheap and available feedstock and energy sources with the lowest investment and operational cost. The proposed technology aims at producing a valuable hydrogen-based commodity to partially offset the cost of generating negative emissions. With its capability to be operated also on coal, this path has a wider application than BECCS and can reduce its two main limitations (i.e., the biomass availability and its impacts on the ecosystems).
By producing metal hydroxides such as Ca(OH)2 and dissolving them in seawater to increase its alkalinity through ocean liming, the process also helps reduce the ongoing ocean acidification, thanks to the capture and storage of atmospheric CO2 in the seawater in the form of metal bicarbonates. Although industrial alternatives exist for the use of slaked lime (i.e., to capture CO2 from an industrial flue gas), for the massive quantities of negative emissions required in the next decades, it is necessary to exploit the large buffer power of the ocean.
An aspect that should be taken into account is that the addition of alkalinity to the oceans and the submarine carbon storage in glass capsules require an adequate regulatory framework. Some of the complexity relies in the fact that interventions on coastal waters would fall under each national administration, while open ocean addition is under international oversight (Renforth and Henderson 2017). The United Nations Convention on the Law of the Sea (UNCLOS), signed in 1982, is the legal framework for most matters related to seas and oceans. Other regulations, such as the 1996 London Protocol to the Convention on the Prevention of Marine Pollution by Dumping of Wastes, are important for permitting, monitoring, and the long-term liability of storage in oceans.
Although further research is needed to assess the feasibility of this technology as a large-scale negative emission option, as well as the related political, social, and environmental challenges, the results reported in this paper are promising and could be considered the path towards the unprecedented scale-up of carbon removal from the atmosphere required to avoid irreversible effects on the climate at the time scale of centuries to millennia and longer (Clark et al. 2016; Nemet et al. 2018).
References
Ali U, Font Palma C, Akram M, Agbonghae EO, Ingham DB, Pourkashanian M (2017) Comparative potential of natural gas, coal and biomass fired power plant with post - combustion CO2 capture and compression. Int J Greenh Gas Con 63:184–193
Aminu MD, Nabavi SA, Rochelle CA, Manovic V (2017) A review of developments in carbon dioxide storage. Appl Energy 208:1389–1419
Baláš M, Lisý M, Moskalik J, Skála Z (2015) Steam influence on biomass gasification process. Holist approach environ 6:127–132. https://hrcak.srce.hr/file/236398. Accessed 10 December 2018
Barret M (2015) Renewable synthetic fuels for transport, is ammonia also a friend? UCL Energy Institute, London
Barreto B, Caserini S, Dolci G, Grosso M (2018) CO2 submarine storage in glass containers: life cycle assessment and cost analysis of four case studies in the cement sector. International Conference on Negative CO2 Emissions, May 22–24, 2018, Göteborg, Sweden
Capone DG, Hutchins DA (2013) Microbial biogeochemistry of coastal upwelling regimes in a changing ocean. Nat Geosci 6:711–717
Caserini S, Dolci G, Azzellino A, Lanfredi C, Rigamonti L, Barreto B, Grosso M (2017) Evaluation of a new technology for carbon dioxide submarine storage in glass capsules. Int J Greenh Gas Con 60:140–155
Chou C, Chen F, Huang Y, Yang H (2013) Carbon dioxide capture and hydrogen purification from synthesis gas by pressure swing adsorption. Chem Eng Trans 32:1855–1860. www.aidic.it/cet/13/32/310.pdf. Accessed 10 December 2018
Clark PU, Shakun JD, Marcott SA, Mix AC, Eby M, Scott K, Anders L, Milne GA, Pfister PL, Santer BD, Scharag DP, Solomon S, Stocker TF, Strauss BH, Weaver AJ, Winkelman R, Archer D, Bard E, Goldner A, Lambeck K, Pierrehumbert RT, Plattner G (2016) Consequences of twenty-first-century policy for multi-millennial climate and sea-level change. Nat Clim Chang 6:360–369
Clarke L, Jiang K, Akimoto K, Babiker M, Blanford G, Fisher-Vanden K, Hourcade JC, Krey V, Kriegler E, Löschel A, McCollum D, Paltsev S, Rose S, Shukla PR, Tavoni M, van der Zwaan B, van Vuuren DP (2014) Assessing transformation pathways. In: Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
CO2APPS (2017) Patent PCT/IB2018/050336 19/1/2018 (patent pending)
Costello MJ, Chaudhary C (2017) Marine biodiversity, biogeography, deep-sea gradients, and conservation. Current Biology, Volume 27, Issue 13
Couto N, Rouboa A, Silva V, Monteiro E, Bouziane K (2013) Influence of the biomass gasification processes on the final composition of syngas. Energy Procedia 36:596–606
Cripps G, Widdicombe S, Spicer JI, Findlay HS (2013) Biological impacts of enhanced alkalinity in Carcinus maenas. Mar Pollut Bull 71:190–198
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4:11–37
Dooley J (2013) Estimating the supply and demand for deep geologic CO2 storage capacity over the course of the 21st century: a meta-analysis of the literature. Energy Procedia 37:5141–5150
EASAC (2018) Negative emission technologies: what role in meeting Paris Agreement targets? European Academies’ Science Advisory Council policy report 35, February. https://easac.eu/fileadmin/PDF_s/reports_statements/Negative_Carbon/EASAC_Report_on_Negative_Emission_Technologies.pdf. Accessed 10 December 2018
EC (2013) Recommendation 2013/179/EU of 9 April 2013 on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations. European Commission. Official Journal of the European Union L 124 - 4 May 2013.
Encyclopedia Britannica (2018) Ocean current. www.britannica.com/science/ocean-current. Accessed 10 December 2018
Fajardy M, Mac Dowell N (2017) Can BECCS deliver sustainable and resource efficient negative emissions? Energy Environ Sci 10:1389–1426
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305:362–366
Franco AC, Gruber N, Frölicher TL, Kropuenske Artman L (2018) Contrasting impact of future CO2 emissions scenarios on the extent of CaCO3 mineral undersaturation in the Humboldt current system. J Geophys Res Oceans
Fuss S, Lamb WF, Callaghan MW, Hilaire J, Creutzig F, Amann T, Beringer T, Garcia WO, Hartmann J, Khanna T, Luderer G, Nemet GF, Rogelj J, Smith P, Vicente JLV, Wilcox J, Dominguez MMZ, Minx JC (2018) Negative emissions – part 2: costs, potentials and side effects. Environ Res Lett 13:063002
García-Labiano F, Abad A, de Diego LF, Gayán P, Adánez J (2002) Calcination of calcium –based sorbents at pressure in a broad range of CO2 concentrations. Chem Eng Sci 57:2381–2393
Garcia-Reyes M, Sydeman WJ, Schoeman DS, Rykaczewski RR, Black BA, Smit AJ, Bograd SJ (2015) Under pressure: climate change, upwelling, and eastern boundary upwelling ecosystems. Front Mar Sci 2:1–10
GCCSI (2017) The Global Status of CCS: 2017. Global Status of CCS. Global Carbon Capture and Storage Institute. https://www.globalccsinstitute.com/sites/www.globalccsinstitute.com/files/uploads/global-status/1-0_4529_CCS_Global_Status_Book_layout-WAW_spreads.pdf. Accessed 10 December 2018
Giuntoli J, Agostini A, Caserini S, Lugato E, Baxter D, Marelli L (2016) Climate change impacts of power generation from residual biomass. Biomass Bionergy 89:146–158
González MF, Ilyina T (2016) Impacts of artificial ocean alkalinization on the carbon cycle and climate in Earth systems simulations. Geophys Res Lett 43:6493–6502
Haigh R, Ianson D, Holt CA, Neate HE, Edwards AM (2015) Effects of ocean acidification on temperate coastal marine ecosystems and fisheries in the Northeast Pacific. PLoS One 10(2):e0117533
Hanak DP, Jenkins BJ, Kruger T, Manovica V (2017) High-efficiency negative-carbon emission power generation from integrated solid-oxide fuel cell and calciner. Appl Energy 205:1189–1201
Harvey LDD (2008) Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions. J Geophys Res 113:C04028
Hauri C, Friedrich T, Timmermann A (2016) Abrupt onset and prolongation of aragonite undersaturation events in the Southern Ocean. Nat Clim Chang 6:172–176
Hiltz J, Heilbig M, Haaf M, Daikeler A, Ströhle J, Epple B (2017) Long-term pilot testing of the carbonate looping process in 1 MWth scale. Fuel 210:892–899
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world's marine ecosystems. Science 328:1523–1528
Honegger M, Reiner D (2018) The political economy of negative emissions technologies: consequences for international policy design. 18:3, 306–321
Hu Y, Watanabe M, Aida C, Horio M (2006) Capture of H2S by limestone under calcination conditions in a high-pressure fluidized-bed reactor. Chem Eng Sci 61(6):1854–1863
IEA (2014) CCS 2014 What lies in store for the CCS? International Energy Agency. https://www.iea.org/publications/insights/insightpublications/Insight_CCS2014_FINAL.pdf. Accessed 10 December 2018
IEA (2017) Energy and CO2 emissions in the OECD. International Energy Agency, Paris. https://www.iea.org/media/statistics/Energy_and_CO2_Emissions_in_the_OECD.pdf. Accessed 10 December 2018
IEAGHG (2008) A regional assessment of the potential for CO2 storage in the Indian subcontinent. International Energy Agency Greenhouse Gas R&D Programme Report 2/2008. http://hub.globalccsinstitute.com/sites/default/files/publications/95746/regional-assessment-potential-co2-storage-indian-subcontinent.pdf. Accessed 10 December 2018
IPCC (2005) Special report on carbon dioxide capture and storage. New York: Cambridge University Press. ISBN: 92-9169-1190-4. https://www.ipcc.ch/pdf/special-reports/srccs/srccs_wholereport.pdf. Accessed 10 December 2018
Kato E, Yamagata Y (2014) BECCS capability of dedicated bioenergy crops under a future land-use scenario targeting net negative carbon emissions. Earth’s Future 2:421–439
Keller DP, Feng EY, Oschilies A (2014) Potential climate engineering effectiveness and side effects during a high carbon dioxide-emission scenario. Nat Commun 5:3304
Kemper J (2015) Biomass and carbon dioxide capture and storage: a review. Int J Greenh Gas Con 40:401–430
Kheshgi HS (1995) Sequestering atmospheric carbon dioxide by increasing ocean alkalinity. Energy 20:915–922
Köhler P, Abrams JF, Völker C, Hauck J, Wolf-Gladrow DA (2013) Geoengineering impact of open ocean dissolution of olivine on atmospheric CO2, surface ocean pH and marine biology. Environ Res Lett 8:014009
Lachkar Z (2014) Effects of upwelling increase on ocean acidification in the California and Canary Current systems: acidification in upwelling systems. Geophys Res Lett 41:90–95
Langer G, Baumann K, Kläs J, Riesbesell U, Thoms S, Young J (2006) Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem Geophys Geosyst 7:Q09006
Laude A, Ricci O, Royer-Adnot J, Fabbri A (2011) CO2 capture and storage from a bioethanol plant: carbon and energy footprint and economic assessment. Int J Greenh Gas Con 5:1220–1231
LRGL and UMAS (2017) Zero-emission vessels 2030. How do we get there? Lloyd’s Register Group Limited and University Maritime Advisory Services. https://www.lr.org/en/insights/articles/zev-report-article/. Accessed 10 December 2018
Milne TA, Evans RJ, Abatzoglou N (1998) Biomass gasifier “tars”: their nature, formation, and conversion. National Renewable Energy Laboratory. Golden, Colorado. https://www.nrel.gov/docs/fy99osti/25357.pdf. Accessed 10 December 2018
Minx JC, Lamb WF, Callaghan MW, Fuss S, Hilaire J, Creutzig F, Amann T, Beringer T, Garcia WDO, Hartmann J, Khanna T, Lenzi D, Luderer G, Nemet GF, Rogelj J, Smith P, Vicente JLV, Wilcox J, Dominguez MDMZ (2018) Negative emissions-part 1 : research landscape and synthesis. Environ Res Lett 13:063001
MTR (2017) CO2 removal from syngas. Membrane Technology & Research. www.mtrinc.com/co2_removal_from_syngas.html. Accessed 10 December 2018
Muratori M, Calvin K, Wise M, Kyle P, Edmonds J (2016) Global economic consequences of deploying bioenergy with carbon capture and storage (BECCS). Environ Res Lett 11:95004
Muri H (2018) The role of large-scale BECCS in the pursuit of 1.5°C target: an Earth system model perspective. Environ Res Lett 13:044010
Nagelkerken I, Connell SD (2015) Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc Natl Acad Sci 112:13272–13277
Nemet GF, Callaghan MW, Creutzig F, Fuss S, Hartmann J, Jérôme H, Lamb WF, Minx JC, Rogers S, Smith P (2018) Negative emissions – part 3: innovation and upscaling. Environ Res Lett 13:063002
Ossino F (2018) 2018 Bank loan market outlook. Newfleet Asset Management
Plevin RJ, O’Hare M, Jones AD, Torn MS, Gibbs HK (2010) Greenhouse gas emissions from biofuels’ indirect land use change are uncertain but may be much greater than previously estimated. Environ Sci Technol 44:8015–8021
Rau G (2011) CO2 mitigation via capture and chemical conversion in seawater. Environ Sci Technol 45(3):1088–1092
Reddy GK, Smirniotis PG (2015) Chapter 1 – introduction about WGS Reaction. Water Gas Shift Reaction, Elsevier
Renforth P, Henderson G (2017) Assessing ocean alkalinity for carbon sequestration. Rev Geophys 55:636–674
Renforth P, Jenkins BG, Kruger T (2013) Engineering challenges of ocean liming. Energy 60:442–452
Ridgwell A (2007) Interpreting transient carbonate compensation depth changes by marine sediment core modeling. Paleoceanography 22
Roussanaly S, Jakobsen JP, Hognes EH, Brunsvold AL (2013) Benchmarking of CO2 transport technologies: part I—onshore pipeline and shipping between two onshore areas. Int J Greenh Gas Con 19:584–594
Roussanaly S, Brunsvold A, Skontorp E (2014) Benchmarking of CO2 transport technologies: part II – offshore pipeline and shipping to an offshore site. Int J Greenh Gas Con 28:283–299
Rubin ES (2013) A proposed methodology for CO2 capture and storage cost estimates. Int J Greenh Gas Con 17:488–503
Schneider K, Silverman J, Woolsey E, Eriksson H, Byrne M, Caldeira K (2011) Potential influence of sea cucumbers on coral reef CaCO3 budget: a case study at One Tree Reef. J Geophys Res 116:G04032
Sikarwar VS, Zhao M, Fennell PS, Shah N, Anthony EJ (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 61:189–248
Smith P, Davis SJ, Creutzig F, Fuss S, Minx J, Gabrielle B, Kato E, Jackson RB, Cowie A, Kriegler E, Van Vuuren DP, Rogelj J, Ciais P, Milne J, Canadell JG, McCollum D, Peters G, Andrew R, Krey V, Shrestha G, Friedlingstein P, Gassar T, Grübler A, Heidug WK, Jonas M, Jones CD, Kraxner F, Littleton E, Lowe J, Moreira JR, Nakicenovic N, Obersteiner M, Patwardhan A, Rogner M, Rubin E, Sharif A, Torvanger A, Yamagata Y, Edmonds J, Yongsung C (2016) Biophysical and economic limits to negative CO2 emissions. Nat Clim Chang 6:42–50
Stanmore BR, Gilot P (2005) Review – calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process Technol 86:1707–1743
Stork M, Meinderstma W, Overgaag M, Neelis M (2014) A competitive and efficient lime industry. Technical Report Ecofys. https://www.eula.eu/documents/competitive-and-efficient-lime-industry-cornerstone-sustainable-europe-lime-roadmap-1. Accessed 10 December 2018
Tokimatsu K, Yasuoka R, Nishio M (2017) Global zero emissions scenarios: the role of biomass energy with carbon capture and storage by forested land use. Appl Energy 185:1899–1906
US-DOE/NETL (2010) Assessment of hydrogen production with CO2 capture volume 1: baseline state-of-the-art plants. DOE/NETL-2010/1434. http://www.canadiancleanpowercoalition.com/pdf/SMR9%20-%20H2_Prod_Vol1_2010.pdf. Accessed 10 December 2018
Vasilieou V (2018) Intermodal research and evaluation. Weekly market report n.12. https://www.investinthefuture.gr/files/data/blog/Intermodal%20Report%20Week%2012%202018.pdf. Accessed 10 December 2018
Zamfirescu C, Dincer I (2009) Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process Technol 90:729–737
ZEP (Zero Emission Platform) (2011) The costs of CO2 capture, transport and storage- post-demonstration CCS in the EU. European Technology Platform for Zero Emission Fossil Fuel Power Plant. www.globalccsinstitute.com/publications/costs-co2-capture-transport-and-storage. Accessed 10 December 2018
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caserini, S., Barreto, B., Lanfredi, C. et al. Affordable CO2 negative emission through hydrogen from biomass, ocean liming, and CO2 storage. Mitig Adapt Strateg Glob Change 24, 1231–1248 (2019). https://doi.org/10.1007/s11027-018-9835-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11027-018-9835-7