The paper presents the composition, physical operating principles, and metrological characteristics of GET 73-2022 Special State Primary Standard of the absorbed dose and absorbed dose rate to tissue-equivalent material of X-rays with a limiting photon energy of 10–60 keV. The functional capabilities of GET 73-2022 meeting the current needs of low-energy X-ray radiation therapy, which includes brachytherapy, are described. Absorbed dose and absorbed dose rate to tissue-equivalent material (water) are realized via the ionization method using primary measuring instruments, i.e., standard extrapolation ionization chambers: EK-R for realizing units in the fields of low-energy X-ray sources (X-ray tubes) and EK-I designed for work with radionuclide sources used in brachytherapy. Dosimetric measurements performed using GET 73-2022 comply with modern requirements for accuracy, as well as the realization and transfer range of the units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction. In modern medical practice, the quality of radiation therapy and its safety for the patient largely depend on the measurement accuracy of quantities characterizing ionizing radiation fields, as well as their effects on the human body. Requirements for the accuracy of measurements are strictly regulated in Russian and international documents [1, 2]. According to the list of measurements pertaining to state regulation in the area of measurement uniformity assurance,Footnote 1 the error in measuring a dose delivered to the patient during radiation therapy should not exceed ±5%. For these requirements to be met, it is necessary to use radiation sources and measuring instruments that are traceable to national standards.
Low-energy X-rays are used for surface and intracavitary irradiation, as well as intraoperatively to directly affect the tumor. One of the most promising and rapidly developing areas of radiation therapy using low-energy X-rays is contact radiation therapy (brachytherapy). The main advantage of brachytherapy consists in a sharp dose reduction with increasing distance from the radiation source, which allows healthy tissues to be spared while exposing the tumor to adequate radiation. Brachytherapy uses sealed radionuclide sources that can be temporarily or permanently implanted into the irradiated area. The use of permanently implanted iodine-125 microsources is effective in the treatment of certain types of early-stage eye cancer, brain tumors, and specifically prostate cancers.
The development of radiation therapy methods using low-energy X-rays prompted a great interest in determining the absorbed dose to tissue-equivalent material (water) at different depths within a given energy range. The existing recommendations on clinical dosimetry are based on air kerma measurements and absorbed dose calculations using conversion factors [1, 2]. It is possible to achieve a higher measurement accuracy for the absorbed dose to tissue-equivalent material (water) of low-energy X-rays by ensuring measurement traceability to corresponding primary standards.
In 1975, the D. I. Mendeleyev All-Russian Institute for Metrology (VNIIM) created GET 73-75 Special State Primary Standard of the absorbed dose of X-rays with the maximum photon energy of 3–9 fJ (20–60 keV) using the calorimetric measurement method. In 2019–2021, its functional capabilities and metrological characteristics were improved. As a result, GET 73-2022 Special State Primary Standard of the absorbed dose and absorbed dose rate to tissue-equivalent material of X-rays with a limiting photon energy of 10–60 keV was approved in 2022.Footnote 2
Composition of GET 73-2022. The special primary standard comprises the following technical means and auxiliary equipment:
-
KD-R standard calorimeter designed to realize absorbed dose to graphite under special conditions (included in GET 73-75);
-
EK-R standard extrapolation ionization chamber for realizing absorbed dose and absorbed dose rate to tissue-equivalent material (water) using low-energy X-ray sources (X-ray tubes);
-
EK-I standard extrapolation ionization chamber for realizing absorbed dose and absorbed dose rate to tissue-equivalent material (water) using radionuclide X-ray sources that are employed in brachytherapy;
-
an automated measurement system;
-
a transportable set on the basis of cavity ionization chambers and a solid-state phantom;
-
a set of radionuclide X-ray sources of variable composition.
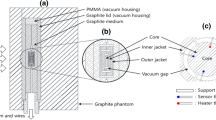
Realization of Quantity Units Using GET 73-2022. Absorbed dose and absorbed dose rate to tissue-equivalent material (water) is realized in GET 73-2022 via the ionization method. To this end, EK-R and EK-I standard extrapolation chambers are used (Fig. 1a, b).
An extrapolation chamber constitutes a variable-volume air cavity located between a stationary potential electrode (inlet window) and a mobile collecting electrode on the phantom (block of movable electrodes). The measuring volume of the chamber changes as the collecting electrode moves relative to the inlet window of the chamber. EK-R and EK-I extrapolation chambers are similar in terms of their design and main characteristics, while the main differences consist in the used materials and the range of available measuring volumes. The main parameters of EK-R and EK-I chambers are given in Table 1.
The method for measuring absorbed dose by means of an extrapolation chamber described in [3,4,5] is based on the following principle: with the increasing measuring volume of the chamber, the ionization charge increment is proportional to the absorbed dose in the differential air volume in the chamber. The absorbed dose to a phantom is determined in an infinitesimal air volume of the chamber (at a zero interelectrode distance). The transition to absorbed dose to tissue-equivalent material (water) is realized through correction factors.
For the given differential volumes of EK-R and EK-I standard chambers, the absorbed dose and absorbed dose rate to tissue-equivalent material (water) are determined as
where \({D}_{i}^{\mathrm{w}}\) and \({\dot{D}}_{i}^{\mathrm{w}}\), absorbed dose and absorbed dose rate to tissue-equivalent material (water); W, average energy required by an electron to form a pair of ions in the air; e, electron charge; ρ, air density in the chamber under normal conditions; C(xi, x0), correction factor ensuring the transition from air kerma in the measuring volume of the chamber to the absorbed dose to tissue-equivalent material (water) for the given chamber depth xi; Q(xi), Q(x0), I(xi), I(x0), average charge or current of the chamber, respectively, for the given chamber depth xi and the specified initial chamber depth x0 adjusted factoring in the differences of environmental conditions from those normally encountered and the recombination of charged particles in the chamber, as well as the radiation source decay (only for the EK-I chamber); kci, correction factors independent of the chamber depth. For both extrapolation chambers, the product of correction factors kci includes coefficients factoring in the initial ionization and the dependence of average ionization energy W on the radiation energy. Coefficients factoring in radiation attenuation by the source holder and the air layer between the radiation source and the inlet window are introduced only for the EK-I chamber.
The realized absorbed dose Dw and absorbed dose rate \({\dot{D}}_{i}^{\mathrm{w}}\) to tissue-equivalent material (water) are determined as the arithmetic means of corresponding values for nine differential chamber volumes selected depending on the X-ray radiation energy.
For the given chamber depth xi, the correction factor C(xi, x0) ensuring transition from air kerma in the measuring volume of the chamber to the absorbed dose to water takes the following form
where Vi and \(f\left({x}_{i}\right)\), chamber volume and the value of the function f for the given chamber depth xi; V0, and \(f\left({x}_{0}\right)\), chamber volume and the value of the function f for the specified initial chamber depth x0.
For the given chamber depth xi,
where A1, A2, t1, t2, and y0, parameters of the function f depending on the radiation energy and calculated according to the Monte Carlo computer simulation of interaction between radiation and the chamber under given conditions.
The simulation of EK-I and EK-R standard extrapolation chambers was performed using the egs_kerma application of the EGSnrc system [6]; the SpekCalc software was used to simulate the spectra of X-ray radiation [7].
In GET 73-2022, the absorbed dose to graphite is realized under special conditions using low-energy X-ray sources via the calorimetric method: measurement of thermal energy obtained using the measuring absorber of the calorimeter as a result of radiation energy conversion. For this purpose, a KD-R standard double differential calorimeter is employed (formerly a part of GET 73-75).
When realizing the absorbed dose to graphite by means of the KD-R calorimeter, the quasi-adiabatic operating mode involving electric calibration is used. The absorbed dose to graphite realized under special conditions is determined as follows
where Dg, absorbed dose to graphite under special conditions; Ic, calibration current intensity; Rh, heater resistance; meff, effective mass of the absorber; ΔUrad and ΔUel, changes in the unbalanced voltage of the Wheatstone bridge as a result of heating the measuring absorber of the calorimeter during irradiation and electrical calibration, respectively; τ, electrical calibration time (i.e., irradiation time); katt, correction factor factoring in radiation attenuation in the inlet window of the measuring absorber of the calorimeter; kc.rad/kc.el, ratio of correction factors factoring in the heat loss in the absorber of the calorimeter when heated by X-rays and the electric field.
The effective mass meff of the measuring absorber used in the KD-R standard calorimeter is calculated factoring in the energy of X-ray radiation affecting the calorimeter.
The absorbed dose to graphite realized under special conditions is determined as the arithmetic mean of 11 independent measurements.
Transfer of quantity units using GET 73-2022. The units are transferred directly from the standard to high-accuracy measuring instruments through working standards to other measuring instruments. Working standards and measuring instruments include measurement systems equipped with solid-state dosimeters, dosimeters having cavity and well ionization chambers, irradiation systems, etc.
The transportable set included in GET 73-2022 transfers the units of absorbed dose and absorbed dose rate to tissue-equivalent material (water) to standard X-ray radiation systems, standard dosimeters, and high-precision dosimeters equipped with well ionization chambers at their place of operation. The transportable set includes two cavity ionization chambers (TM23342 having a volume of 0.02 cm3 and TN23344 having a volume of 0.2 cm3), a solid plate phantom for work in the fields of low-energy X-ray sources, as well as a well ionization chamber TM33005 having a volume of 0.116 cm3 volume for measurements using radionuclide sources that are employed in brachytherapy.
Metrological characteristics of GET 73-2022. Table 2 presents realization ranges for the following characteristics: absorbed dose to graphite under special conditions, absorbed dose and absorbed dose rate to tissue-equivalent material (water), standard deviations S0 of the measurement result at n independent measurements, residual systematic error θ0, type A u0A and B u0B standard uncertainties, combined standard uncertainties u0c and expanded uncertainties U0 at a coverage factor of k = 2.
Conclusion. GET 73-2022 realizes absorbed dose and absorbed dose rate to tissue-equivalent material (water) along with absorbed dose to graphite under special conditions. The quantity units are realized both in the fields of low-energy X-ray sources and those of radionuclide sources used in brachytherapy.
Dosimetric measurements performed by means of GET 73-2022 are harmonized with the current international practice meeting the requirements for accuracy, as well as the realization and transfer range of the units. In terms of the main metrological characteristics and technical equipment, GET 73-2022 is in no way inferior to foreign analogs according to the results of realizing absorbed dose and absorbed dose rate to water from iodine-125 sources used in brachytherapy [4, 5].
GET 73-2022 provides a metrological basis for improving the accuracy of dosimetric measurements in medicine during low-energy X-ray radiation therapy, including brachytherapy employing low dose rate radionuclide sources. Conditions are created for conducting type approval tests, verification, and calibration of instruments measuring absorbed dose to water, including dosimeters equipped with well ionization chambers that are designed for measurements using radionuclide sources employed in brachytherapy.
Notes
Government Decree No. 1847 "On approval of Measurements Pertaining to State Regulation in the Area of Measurement Uniformity Assurance" (as of 16 November 2020).
Rosstandart order No. 379 "On approval of Special State Primary Standard of the absorbed dose and absorbed dose rate to tissue-equivalent material of X-rays with a limiting photon energy of 10–60 keV" (as of February 16, 2022).
References
International Atomic Energy Agency, Absorbed Dose Determination in External Beam Radiotherapy, Technical Reports Series, No. 398, IAEA, Vienna (2000).
R. Caswell et al., “ICRU Report 72, Dosimetry of beta rays and low-energy photons for brachytherapy with sealed sources,” J. ICRU, 4, No. 2, 5–8 (2004), https://doi.org/10.1093/jicru_ndh020.
T. Schneider, Phys. Med. Biol., 56, 3387–3402 (2011), https://doi.org/10.1088/0031-9155/56/11/013.
T. Schneider, Metrologia, 49, S198–S202 (2012), https://doi.org/10.1088/0026-1394/49/5/S198.
M. P. Toni et al., Metrologia, 49, S193–S197 (2012), https://doi.org/10.1088/0026-1394/49/5/S193.
I. Kawrakow, et al., The EGSnrc Code System: Monte Carlo Simulation of Electron and Photon Transport, Technical Report PIRS-701, National Research Council Canada (2017).
G. Poludniowski, et al., Phys. Med. Biol., 54, No. 433a (2009), https://doi.org/10.1088/0031-9155/54/19/N01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel’naya Tekhnika, No. 7, pp. 8–12, July 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Villevalde, A.Y., Oborin, A.V. Get 73-2022 Special State Primary Standard of the Absorbed Dose and Absorbed Dose Rate to Tissue-Equivalent Material of X-Rays with a Limiting Photon Energy of 10–60 keV. Meas Tech 65, 471–476 (2022). https://doi.org/10.1007/s11018-023-02106-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-023-02106-y