This study determines the thermodynamic regularities of the alloying and modification processes of steel with natural and man-made materials in an arc electric-steelmaking furnace and a ladle-furnace unit. The possibilities of the wide application of manganese ores of different compositions for alloying steel with a manganese content of up to 1% are also presented. Alloying takes place without using standard manganese alloys. For alloying, vanadium slag is used; when added, the steel is microalloyed with vanadium using carbon as the main reducing agent, and barium and strontium are also reduced.
The use of these materials has been proven to improve the technical and economic indicators of the process of modifying steels and significantly improve the quality of the final metal products. Conclusions on the prospects for a significant expansion of the use of natural and man-made materials are drawn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Advanced technologies that ensure an improvement in the quality and increase in the output of metal products and a decrease in material consumption are a high priority in the development of steelmaking production under modern conditions.
Technologies and technological methods that enable obtaining the highest quality steel should currently be applied. At the same time, the qualitative characteristics of materials used should fully ensure the stability, efficiency, cost effectiveness, and environmental safety of refining and modification technologies.
Microalloying and modification are effective out-of-furnace steel treatment methods aimed at changing the properties of metal and non-metallic inclusions, leading, in aggregate, to the improvement of the operational properties of metal products as a result of the effective influence of chemically active metals (e.g., rare-earth metals and alkaline-earth metals) on the structure of the crystallizing material.
The out-of-furnace treatment of steel and cast iron with chemically active metals (e.g., rare-earth metals and alkaline-earth metals) is an integral part of modern technologies for the manufacturing of high-quality and competitive products.
Russia produces a large number of alloys and master alloys containing various combinations of modifying and microalloying elements.
However, obtaining ligatures containing alkaline-earth metals is an energy-, labor-, and material-intensive production. At the same time, the operations of smelting, casting, granulation, crushing, and packing of master alloys not only require a lot of labor but are also accompanied by metal losses. These industries are also environmentally harmful and explosive [1, 2].
The integrated involvement of primary and secondary raw materials in steelmaking production is currently urgent. The extraction of components from such materials, which are valuable alloying additives, enables to increase production efficiency while improving the product quality.
In the global practice of steel production, the trend of replacing ordinary carbon steels with economical high-strength microalloyed steels is still relevant. Vanadium, titanium, niobium, rare-earth metals, barium, and strontium are used as microalloying (modifying) additives.
Vanadium is currently the most demanded microalloying element. The production of vanadium-containing alloys is based on a complex multistage technology associated with high losses of vanadium and high labor and energy costs, resulting in the high cost and scarcity of alloys, which limits the possibility of their use [3, 4].
When alloying steel with vanadium, the main vanadium-containing materials are ferrovanadium, alloys, and master alloys directly obtained from vanadium slag, vanadium cast iron, converter vanadium slag, vanadium- containing metal product obtained through crushing and magnetic separation of vanadium slag, and metallized vanadium-containing pellets and exothermic briquettes, which are most often used in smelting low-alloyed steels.
The formation of vanadium carbonitrides, which have a high solubility in steels, enables to neutralize the negative effect of nitrogen and categorize it as microalloying element, which plays a decisive role in vanadiumalloyed steels to enhance their precipitation hardening. In addition, the recrystallized austenite grain size of vanadium- alloyed steels remains constant over a wide temperature range. Therefore, the properties of such steels are insensitive to changes in the rolling temperature. Compared with niobium and titanium as alloying elements, vanadium has certain advantages, namely, higher solubility of carbonitrides in austenite; the possibility of obtaining fine austenite grains without the inhibition of the recrystallization processes because the separation of vanadium phases occurs below the temperature of the end of rolling; the use of less costly high-temperature controlled rolling; much less susceptibility of vanadium-alloyed steels to hot cracking during bending and straightening of a continuously cast billet; and good strength and viscosity of vanadium steel, even with a high nitrogen content, in the heat-affected zone of welding with the correct selection of welding parameters.
Among man-made vanadium-containing materials, vanadium slag is the most widely used alloying component, without processing it into vanadium pentoxide and ferrovanadium. This condition significantly reduces the cost of alloying steel and increases the degree of vanadium utilization due to the reduction of losses associated with ferroalloy production [5].
However, in existing steel treatment technologies with vanadium slag, high-cost aluminum, silicon, and calcium are used as reducing agents. This leads to an increased process cost, increased content of aluminum and non-metallic inclusions in the metal, and unstable assimilation of the alloying element.
The most significant costs in metal production are the costs of deoxidation and alloying of steel and technological energy, and energy tariffs affects not only directly but also indirectly the price of ferroalloys. In addition, existing steel alloying methods, in particular with manganese, are associated with their large losses at all processing stages. For instance, up to 20% manganese is lost during its extraction, 20–25% is lost during enrichment, 20–25% is lost during the smelting of ferroalloys, and up to 25% is lost during steel alloying, that is, it does not exceed 50% throughout the recovery of manganese.
The efficiency of the deoxidation, alloying, and modification technologies of steel can be increased by changing the method of introducing alloying additives. In some cases, the direct alloying of steel in a furnace or in a ladle can be switched using ores, concentrates, and converter vanadium slags [6,7,8,9,10,11].
The development of a technology for furnace and out-of-furnace treatments of steel with natural and manmade materials will enable to purposefully control the physicochemical state of the metal melt and, accordingly, the properties of metal products, which is an urgent task.
The research stages include the calculation of possible compositions and determination of thermodynamic conditions necessary for the implementation of the reduction of alloying elements from oxide raw materials, determination of the boundaries of the concentration areas of the recovery processes, finding the parameters of the system at which the system optimal composition in equilibrium conditions is achieved, and approbation of the obtained process parameters under laboratory and industrial conditions.
When implementing thermodynamic modeling, the software package Terra [12] was used, which, based on the principle of maximum entropy, determines the equilibrium composition of a multicomponent, heterogeneous thermodynamic system for high-temperature conditions.
The process was examined by solving model problems to determine the conditions for the reduction of vanadium in systems, the input flow of which consists of a set of V–O–C–Fe–Si elements, represented by a set of substances lV2O5–nC– pFe2O3 –mSi, where l, n , p, and m are the number of V2O5, Fe2O3, carbon, and silicon moles, respectively. The initial composition of the system was formed by setting the values of the parameters l, n , p , and m. At stage 1, the calculation of the possible compositions that can be obtained from the vanadium reduction processes in a thermodynamic system consisting of V–O–C elements was performed by varying the number of carbon moles in the system, which also enabled to estimate the boundaries of the concentration regions of the reduction processes. The V–O–C model system was formed by setting the initial mixture composition with 1 mole of V2O5 oxide and n moles of carbon. Approximately 20 thermodynamically possible substances were formed from the elements for the selected temperature range. All substances were divided into significant and insignificant ones with a discrimination threshold of 10−4 mole/kg of the mixture, according to the concentration value in the final state. Condensed phases, consisting of C, V, VC, VO, V2O4, V2O3, and V2O5, and the gas phase, consisting of CO and CO2, turned out to be significant substances.
The calculation of equilibrium compositions in the V2O5 + nC system was performed at a temperature of 1873 K within the range of the parameter n variation from 0 to 10 mole.
Calculating possible compositions that can be formed as a result of the reduction processes of barium and strontium in thermodynamic systems consisting of the elements Ba–Al–O, Ba–Si–O, Ba–C–O, Sr–Al–O, Sr–Si–O, and Sr–C–O was performed by changing the amount of carbon, silicon, and aluminum, which enabled to estimate the boundaries of the concentration regions of the reduction processes. All substances that can be formed as a result of numerical modeling for a given elemental composition of the mixture for temperatures of 1673–2473 K by the value of the concentration in the final state were divided into significant and insignificant with a threshold of significance of 10−4 mole/kg of the mixture. The condensed phase, consisting of the atoms and molecules of Ba, C, Al, Si, Sr, BaO, SrO, SiO2, BaSiO3, SrSiO3, BaAl2O4, SrAl2O4, and SrCO3, and the gas phase, consisting of the atoms and molecules of Ba, Sr, BaO, CO, SiO2, Al2O3, and Al, were significant.
The metal reduction process was investigated by calculating the equilibrium compositions in systems, the input flow of which consists of a set of elements Me–O–C–Fe–Si–Al and is represented by a set of substances lMeO–nC–mSi–k Al– p Fe. Depending on the values of the parameters l, k , n , m, and p, one or another initial composition of the system was formed.
The nickel reduction process was considered in the three-component system Ni–O–C, represented by a set of substances lNiO–nC, where l is the number of NiO moles and n is the number of carbon moles. The initial composition of the system was formed by setting the values of the parameters l and n .
The calculation of possible compositions that can be obtained as a result of the processes of nickel reduction in a thermodynamic system consisting of the elements Ni–O–C and Ni–O–C–Fe was performed by varying the number of moles of carbon in the system, which enabled to estimate the boundaries of the concentration regions of the reduction processes. The Ni–O–C model system was formed by setting the initial mixture composition in the form of 1 mole of NiO oxide and n moles of carbon. The calculation was performed in the temperature range from 573 to 1873 K, corresponding to the temperatures of steel melting.
Experimental Technique
In laboratory tests, steel was smelted in a laboratory arc furnace with a capacity of 10 kg. In the course of the laboratory research, a method for introducing nickel oxide into an electric arc furnace was experimentally determined and tested. Pellets with diameters of 20–30 mm were made from nickel concentrate obtained during the enrichment of polymetallic manganese ores, with a fraction of less than 0.5 mm and coke breeze. The pellets were loaded into the furnace in two ways: in charge and during the recovery period on the metal mirror before the introduction of the slag.
The calculation of the amount of pellets was performed based on nickel in steel, equal to 1%.
Experimental smelting was performed according to the classical two-slag technology, where a charge consisting of scrap metal, pellets, coke, and lime necessary for the formation of slag was loaded into the furnace. When using pellets from nickel concentrate, the amount of coke in the charge was increased, taking into account the amount of carbon required for the reduction of nickel from oxide and carbon waste. The pellets were loaded close to the slopes. When smelting steel, the metal scrap composed of 0.275 wt.% C, 0.267 wt.% Si, 0.423 wt.% Mn, 0.175 wt.% Cr, 0.1 wt.% Ni, 0.027 wt.% S, 0.028 wt.% P, and the rest of Fe was used.
Table 1 presents the composition of the charge materials.
After the completion of loading, the electrodes were lowered, and an electric arc was ignited. The melting time was 20 min.
By the end of melting, slag was formed in the furnace, a sample of which was taken for chemical analysis. To ensure the decarburization reaction, iron ore was added in portions. After a 5 min holding, the slag was drained. Samples of metal and slag were taken for chemical analysis.
After removing the oxidizing slag, preliminary deoxidation with ferrosilicon was performed based on obtaining a silicon content in the metal within the limits specified for the finished steel. After the addition of deoxidizers, the slag-forming materials (lime and quartzite, approximately 3% of the metal mass) were charged in the furnace. After the slag-forming materials were melted and the liquid slag was formed, it was deoxidized with coke powder and ground ferrosilicon.
Then, a sample of metal and slag was taken for chemical analysis.
The tapping of metal and slag was performed into a ladle. Then, the ingot obtained was analyzed by cutting it into three equal parts.
Industrial tests of the direct alloying technology of steel with nickel were performed during steel smelting in DSP-40. For direct alloying, pellets consisting of nickel concentrate and coke in a stoichiometric ratio were made. The experimental smelting metal, melted via the direct alloying technology, was certified according to the scheme adopted at the enterprise.
When determining the optimal parameters of microalloying steel with vanadium, a technological scheme was simulated, where the metal was released from a 100 ton electric arc furnace into a ladle, where a slagforming mixture of lime and vanadium-containing slag was added. Deoxidizers and alloying agents were added at the ladle-furnace unit during further processing of the melt; that is, the main reducing agent of vanadium was metal carbon. Slag was formed from slag-forming vanadium-containing slag and furnace slag that entered the ladle during tapping. In the calculations, it was assumed that the amount of lime added at the outlet was 0.7 ton, and the amount of electric-furnace slag entering the ladle during tapping was 0.1 ton.
To confirm the results of the thermodynamic modeling of alloying steel with vanadium from converter vanadium slag, a series of experimental melts was performed in a resistance furnace with a coal heater at a temperature of 1873 K. The starting materials consisted of carbon steel composed of 0.27% C, 0.49% Mn, 0.02% Si, and 0.02% V, as well as converter vanadium slag, reducing agents, and lime. Crystalline silicon and graphite were used as reducing agents. Briquettes were formed from pre-crushed and thoroughly mixed converter vanadium slag and reducing agents. Liquid glass was used as a binder.
A muffle furnace was used to calcine the manganese carbonate ore in an oxidizing atmosphere. The phase and chemical composition of ores, concentrates, heat treatment products, alloys, and slags was determined using chemical, spectral, and X-ray phase analyses. When studying the kinetics of the reduction of alloying elements from oxide raw materials, we performed continuous weighing of samples placed in a Tamman furnace, and the temperature was recorded with a tungsten-rhenium thermocouple VR 5/20. The reduction of manganese, nickel, and vanadium from the oxide melt was controlled by taking samples of slag and metal during the process.
The derivatographic method was used to study the conditions of the formation of solid solutions of calcium and manganese oxides and the results of the synthesis of the marokite and monophase materials. The metal quality was controlled by mechanical properties, micro- and macrostructures, and non-metallic inclusions, and the content of gases was determined using the standard methods. To reveal the presence of Ba and Sr in the steel, the structure of the samples was analyzed by the method of extract replicas using transmission electron diffraction microscopy.
A laboratory hydraulic press was used for briquetting the ore material, and a plate granulator was used for pelletizing the material.
Industrial tests of the technology of deoxidation and alloying of steel in a ladle with manganese using mixtures of various compositions were performed when smelting low-alloy carbon and low- and medium-carbon manganese steels in 25 ton arc furnaces.
Mixtures of the following compositions were used:
-
(I)
alloy of the Al–Mn–Si–Fe–C system (39.2%); heat treatment product of the carbonate manganese ore (39.2%), dolomite (19.6%), and binder (2%)
-
(II)
FS45 (42%); heat treatment product of the carbonate manganese ore (42%), dolomite (12%), and binder (4%)
-
(III)
FS75 (17.4%); heat treatment product of the carbonate manganese ore (43%), dolomite (11.5%), ash from thermal power plants (23.2%) and water (4.9%)
The mixtures were placed into a ladle during tapping. The steel temperature before tapping was 1883 K–1893 K, which is close to the upper limit recommended by the manufacturing specification. The briquettes were completely dissolved during tapping. When using briquettes and pellets of various compositions using oxide manganese-containing materials, 78%–88% manganese were extracted.
The results of the thermodynamic modification of steel with barium and strontium were implemented in the melting of steel grades 25G2S, G13, and 35HGSL in the electric arc furnace DSP-25. Melting was performed using a two-slag technology. The barium–strontium-containing material was a barium–strontium modifier BSK-2, which was added at the end of the recovery period together with a deoxidizing mixture (ferrosilicon powder and breeze coke). Part of the modifier (25% of the total amount) was added into the ladle during tapping. Before tapping, the slag was additionally deoxidized with granulated aluminum, and the metal in the ladle was deoxidized with aluminum ingots (approximately 1 kg/ton).
The metal was poured into ingots weighing 7.5 ton, rolled on a square 100, and rolled onto reinforcement No. 14.
The mechanical properties of steel were monitored according to standard methods in the mechanical testing laboratory of the central plant laboratory of Evraz ZSMK. For steel 25G2S, additional tests for impact toughness were performed at temperatures of + 20°C, 0°C, − 20°C, − 40°C, − 60°C, and − 70°C. For comparison, four melts of 25G2S steel, melted in a 25 ton arc furnace without treatment with the BSK-2 modifier, were subjected to the same tests. Impact toughness tests were performed at temperatures of + 20°C and − 60°C for steel grade G13 and at temperatures of + 20°C and − 40°C for steel 35HGSL.
To study the interaction process of slag melts containing barium and strontium compounds with a metal melt, laboratory studies were performed in a resistance furnace with a coal heater in an alundum crucible. The prepared samples were studied using metallographic analysis.
The following materials were used to process steel in a furnace and ladle:
-
Carbonate manganese ore with 26–31% manganese, 8–11% CaO, 1–3% MgO, 2–7% Fe2O3, and 8–17% SiO2,
-
Oxide manganese ore with 36–45 wt.% Mn, 5–9 wt.% Fe, 14–18 wt.% SiO2, 1–4 wt.% CaO, and 0.05–0.09 wt.% P,
-
Manganese concentrate with 55–64 wt.% Mn, 0.1–2.31 wt.% SiO2, < 0.55 wt.% Fe, < 0.073 wt.% S, and < 0.005 wt.% P,
-
Vanadium slag with 16.0 wt.% V2O5 , 5.0 wt.% TiO2 , 10.0 wt.% MnO, and 25.0 wt.% Fegen,
-
Barium–strontium modifier BSK-2 with 13.0–19.0 wt.% BaO, 3.5–7.5 wt.% SrO, 17.5–25.5 wt.% CaO, 19.8–29.8 wt.% SiO2, 0.7–1.1 wt.% MgO, 2.5–3.5 wt.% K2O, 1.0–2.0 wt.% Na2O, 1.5–6.5 wt.% Fe2O3, 0.0–0.4 wt.% MnO, 1.9–3.9 wt.% Al2O3, 0.7–1.1 wt.% TiO2 , and 16.0–20.0 wt.% CO2,
-
Nickel concentrate with 45.0 wt.% Ni, 2.3 wt.% Mn, 1.4 wt.% Fe, 0.5 wt.% Co, 0.1 wt.% Cu, < 0.015 wt.% P, traces of SiO2, and 2.82 of loss on ignition,
-
Alloy of the Al–Mn–Si–Fe–C system with 7 wt.% Al, 25.5 wt.% Si, and 27 wt.% Mn,
-
Ferrosilicon grade FS-45,
-
Ferrosilicon grade FS-75,
-
Thermal power plant ash with 8.88 wt.% Al2O3, 23.98 wt.% SiO2, 0.56 wt.% TiO2, 45.85 wt.% CaO, 4.98 wt.% MgO, 6.32 wt.% FeO, 8.18 wt.% Fe2O3, and 1.82 of loss on ignition,
-
Coke with 88.2 wt.% C, 10.65 wt.% ash, 1.44 wt.% volatiles, and 5.0 wt.% moisture,
-
Coke ash with 48.9 wt.% SiO2, 24.6 wt.% Al2O3, 5.7 wt.% CaO + MgO, 13.6 wt.% Fe2O3, and 0.67 wt.% P2O5.
Results and Discussion
An analysis of the advantages and disadvantages of the direct alloying of electric steel with manganese shows that the prospects of a particular technology are determined by such technical and economic indicators as the duration of smelting and the consumption of the reducing agent. Therefore, in the research process, the task was to maximize the replacement of silicon as the main reducing agent of manganese, with a cheap reducing agent, i.e., carbon, and to obtain a stable extraction of manganese from ores. The simplest direct alloying process of steel was performed by adding oxide raw materials to the furnace. Oxide manganese ore was used as a manganese ore raw material.
The study of the carbon-thermal reduction of manganese in the MnO2–C system in the absence of iron revealed that the reduction of manganese begins at temperatures above 1723 K with a carbon consumption of more than 1.5 mole. The evaporation process of manganese simultaneously began. At a temperature of 1723 K with an excess of carbon, the system contained manganese carbide Mn7C3, which disappeared with increasing temperature. The complete reduction of manganese occurred at a carbon consumption of 2 mole.
Calculations in the MnO2 –Si system showed that the reduction of manganese by silicon is possible over the entire specified temperature range. The research results are presented in Fig. 1, which illustrates that the complete reduction of manganese occurs at a silicon consumption of 1 mole. This value corresponds to the maximum manganese content of 47% in the system, which decreases with the increase in the consumption of the reducing agent due to dilution with excess silicon.
Thus, any of the considered reducing agents or their combination in certain ratios can be used as a reducing agent when using oxide manganese-containing materials for melting alloys and processing steels. Naturally, for the purposeful use of silicon in steel processing, a thorough preliminary deoxidation of the metal and slag is required to reduce the overall oxidation of the metal–slag system.
The physicochemical analysis of the processes revealed that, under the conditions of arc steelmaking furnaces, silicon is a real reducing agent of manganese from the oxide melt. To save silicon, the reduction of manganese should start with carbon, adding it as coke onto the surface of the oxide manganese-containing melt.
The creation of favorable thermodynamic and kinetic conditions for direct alloying with manganese in an electric arc furnace is facilitated by a thorough removal of the oxidation period slag, with the use of manganese ores with a manganese content higher than 30%, and with a low content of silicon oxide and phosphorus.
According to practical data, the amount of coke breeze additives should be within the range of 8–16% of the mass of an oxide manganese-containing material.
Thus, after the removal of the oxidizing slag, the oxide manganese-containing material was added into the furnace. After its melting, coke was added. After 10–15 min of exposure, a siliceous-reducing agent was added.
To determine the parameters of the technological process of MnO reduction with silicon in an electric arc furnace, a mathematical model was developed for the direct alloying of steel in an arc furnace [13]. In the course of the mathematical modeling, the effects of the amount and time of lime adding, the basicity of the slag, and the amount of manganese ore added on the completeness of the reduction of manganese from the oxide melt were studied, and the process optimal duration was determined. The temperature range for the addition of the silicon-reducing agent into the furnace was determined experimentally. The relative simplicity of smelting ordinary steels with Mn content of 0.4–0.9% with the addition of manganese ore up to 10 kg/ton did not raise difficulties for getting into a given chemical composition, and a stable assimilation of Mn at the level of 90–95% is ensured. The use of carbon-silico-thermal reduction enabled to increase the useful application of silicon up to 83–85%.
Smelting with the direct alloying of manganese steels with the Mn content of 1–2% requires strict adherence to technology. The stability of the results obtained in this case was influenced by the nature of the preliminary course of the melting, the timeliness of the charge melting, and the slag and temperature regimes of the oxidation period. Table 2 presents the technical and economic indicators for steel smelting using traditional technology and the technology of steel direct alloying.
To determine the purity of the metal produced via the direct alloying technology, a microstructural analysis of over 50 metal samples was performed. The difference in the composition and type of metal inclusions, smelted according to the traditional technology with the deoxidation and alloying of steel with standard ferroalloys and direct alloying technology, was not found.
For the deoxidation and alloying of steel with manganese in a ladle, the simplest option is to use selfmelting briquettes consisting of an oxide material and a reducing agent [6].
The reaction completeness in the briquette depends on the size of the contact surface of the initial substances, namely, the size of the materials and the concentration of the main elements in them, temperature, and the separation rate of the reaction products, such as the formation of a low-melting slag at a given temperature and a free-running slag.
Manganese can be reduced by aluminum, silicon, or carbon.
In laboratory conditions, the influence of various factors on the process of metallothermal reduction of manganese by silicon was studied. Standard silicomanganese and nonstandard alloys with different contents of silicon and aluminum were used as reducing agents. Lime and dolomite were used as fluxing agents.
The use of an alloy containing aluminum in addition to silicon as a reducing agent improved the performance of the manganese reduction process. With the combined reduction with aluminum and silicon in a given ratio, low-melting slags were formed, which increased the reaction rate in briquettes, allowed a complete restoration of the leading element, and reduced the consumption of fluxing agents. The high rate of the process and good separation of metal and slag was facilitated by 15–20% Al2O3 in the slag.
The ratio between the consumption of the roasted concentrate and the consumption of the reducing agent in the briquettes should be (0.7–0.8)/1.0. With such a ratio between the components of the briquette, the extraction of manganese into the metal formed in the briquette was 90–95% and was 85–90% from the concentrate included in the briquette. The manganese content in the slag formed in the briquette, with a residual silicon content in the metal droplets of 15–20%, decreased to 7–10% and below, and the slag ratio after the completion of processes in the briquette was only 0.70–0.75 instead of 2.0–2.5 for the conventional smelting of low-carbon ferromanganese.
To increase the extraction of manganese from carbonate manganese ores, the material obtained by a long roasting of carbonate ore in an oxidizing atmosphere, the so-called product of the thermal treatment of carbonate manganese ore, was used as an oxidizing agent. The chemical composition of the thermal treatment products of carbonate ores is presented in Table 3.
The roasting of carbonate manganese ore to obtain a product of the thermal treatment of carbonate manganese ore, suitable for obtaining moisture-resistant briquettes for the direct alloying of steel with manganese, must be performed in an oxidizing atmosphere for 1 h at 850°C –950°C. Cooling to 500°C –600°C must also be performed in an oxidizing atmosphere. In this case, low-melting manganese oxides were formed in the ore, which accelerated the formation of calcium ferrites and manganites. The product of the thermal treatment of carbonate ore did not contain free bases and was represented by 55–75 wt.% (Ca, Mg)(Mn, Fe)2O4, 5–25 wt.% Mn2O3 + Fe2O3, 10–30 wt.% silica, and other oxides.
The study on the influence of the particle size distribution of the starting materials on the indicators of the process of manganese reduction from oxides with direct alloying revealed that it is necessary to limit the amount of materials with a fraction larger than 1 mm in the mixtures.
It has been experimentally established that lignosulfonate concentrate and liquid glass can be used as binders for pelletizing mixtures for the direct alloying of steel with manganese in a ladle. Boron oxide is especially effective as a binder. In this case, even briquettes with lime retain their strength in air for two weeks. When using thermal power plant ash as a fluxing agent, it is advisable to use water to moisten the charge. Oxides CaO, SiO2, and Al2O3 present in the ash interact with water and form strong compounds, i.e., 2CaO·SiO2 · 4H2O, 3CaO·Al2O3 · 6H2O, and SiO2 · 2H2O. These compounds are astringent. The resulting briquettes and pellets are firm enough for transportation.
Industrial tests of the technology of deoxidation and alloying of steel in a ladle with manganese using mixtures of various compositions were performed during its smelting in 25 t arc furnaces.
The mixtures of the following compositions were used:
-
(I)
Alloy of the Al–Mn–Si–Fe–C system (39.2%); heat treatment product of carbonate manganese ore (39.2%), dolomite (19.6%), and binder (2%)
-
(II)
FS45 (42%); heat treatment product of carbonate manganese ore (42%), dolomite (12%), and binder (4%)
-
(III)
FS75 (17.4%); heat treatment product of carbonate manganese ore (43%), dolomite (11.5%), ash from thermal power plants (23.2%), and water (4.9%)
The technology of direct alloying was used to melt steel grades 40, 45, and 20 (briquettes of compositions I and II) and 45L and 35L (pellets of composition III). Table 4 presents the technological characteristics of some melts using briquettes of composition I.
The mixtures were placed into a ladle during tapping. The steel temperature before tapping was 1883 K–1893 K, which is close to the upper limit recommended by the manufacturing specification. The briquettes were completely dissolved during tapping. When using briquettes and pellets of various compositions using oxide manganese-containing materials, the extraction of manganese was 78–88%.
The assimilation of manganese in traditional melting melts is approximately 70.5%. However, if we take into account that when smelting ferromanganese and silicomanganese, approximately 80% of Mn is extracted from the ore, then the end-to-end utilization rate of manganese does not exceed 60%. Consequently, the use of oxide manganese-containing materials for deoxidation and alloying of steel in a ladle according to the technology developed provides a high throughout recovery of manganese and enables to eliminate almost completely the use of standard manganese ferroalloys when smelting steels with a manganese content of up to 1% with Mn content in steel of 0.35–0.65%.
When studying the quality of steel smelted using the technology of direct alloying with manganesecontaining oxide materials in a ladle, the metal, in terms of its macrostructure, content of gases, non-metallic inclusions, and mechanical properties, meets all the requirements of GOSTR 58228-2018 and does not differ from steel smelted using conventional technology.
For the direct alloying of steel in a ladle, a product synthesized from high-quality manganese concentrate obtained by the authors’ technology of chemical enrichment of poor high-phosphorous manganese and polymetallic manganese-containing ores and ferromanganese nodules was also used [14]. Manganese concentrates have a low SiO2 content (≤ 0.5%). At a low silica content, slag formation decreases, this results in increased losses of manganese during its reduction. Marokite (Ca,Mg)Mn2O4 and manganite (Ca,Mg)MnO3 of calcium and magnesium were obtained from the concentrate of chemical enrichment and dolomite via hightemperature synthesis [15]. The use of this material in briquettes for alloying steel with manganese in a ladle enabled to obtain 90% extraction of manganese in steel.
At the complex enrichment of polymetallic manganese ores, along with the high-quality manganese concentrate, concentrates of non-ferrous metals were obtained, particularly nickel concentrate, which can also be used for direct alloying [16].
The thermodynamic modeling results showed that nickel from oxide can be completely reduced at a temperature of 1073 K in the Ni–O–C system and at a temperature of 1873 K in the Ni–O–C–Fe system (Fig. 2).
The results of kinetic studies and X-ray phase analysis reveal that at temperatures of 1173 K–1473 K, nickel from oxide is almost completely reduced within 20–30 min, whereas at a temperature of 1073 K, nickel is reduced from oxide over a longer period of time (70 min). Thus, under the conditions of steel melting in an electric arc furnace in the temperature range of 1173 K–1473 K during the melting period, it is almost completely possible to reduce nickel from its oxide with solid carbon within 20–30 min.
In the course of laboratory studies, the optimal method of placing nickel oxide into an electric arc furnace was experimentally determined. In series of 1–3 melts, pellets made from nickel concentrate were used, and in series 4–6, pellets made from nickel concentrate and coke were used. The results of experimental melts of alloying steel using nickel-containing pellets show that the extraction of nickel from the concentrate when they are placed into the filling was 92–95% and 75–78% when they are placed into the recovery period on the metal mirror before the slag is added. The results of the experimental melts of alloying steel using nickel-containing pellets showed that the recovery of nickel from the concentrate was 92–95% according to variant I and 75–78% according to variant II.
The decrease in the extraction of nickel when it is added at the beginning of the recovery period is apparently due to its partial evaporation; when it enters the arc zone, nickel is reduced and can partially evaporate because it has a relatively low boiling point.
Based on the experimental study results and regularities in the thermodynamic modeling of the nickel reduction process from concentrates during steel smelting in an electric arc furnace, a technology for the direct alloying of chromium–nickel stainless steel 08(12)X18H10T was developed. Smelting was performed using by remelting alloyed waste with complete oxidation. The charge included pellets made from nickel concentrate and coke. The metal of the experimental melts, melted via the direct alloying technology, was certified according to the scheme adopted at the enterprise, and no deviations from the requirements were revealed.
The carbosilicothermal reduction of vanadium from oxide systems is discussed in detail in [17, 18].
The thermodynamic modeling of alloying steel with vanadium during the treatment with converter vanadium slag was performed for the processes occurring during steel tapping from an arc furnace and subsequent processing at a ladle-furnace unit at a temperature of 1873 K and different carbon content in steel, as well as different consumptions of converter vanadium slag, furnace slag, and slag-forming agents. The effect of the carbon content in steel at the outlet from the furnace, the consumption of the converter vanadium slag and slag-forming agents added, and the mass of furnace slag entering the ladle on the recovery of vanadium from the converter vanadium slag was analyzed.
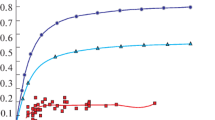
Figure 3 presents the results of thermodynamic modeling, presented by the dependences of the vanadium content in the metal on the carbon content in the steel at the outlet and the consumption of converter vanadium slag. These dependences indicate that they have extrema at a carbon content in steel up to 0.4%. The calculated data showed that at a converter slag consumption of up to 8 kg/ton of steel, the vanadium content in the metal is at least 0.04% for high-carbon and medium- and low-carbon steels. For medium-carbon steel, the vanadium content in the metal of no more than 0.09% can be obtained at a converter vanadium slag consumption of up to 20 kg/ton of steel.
The thermodynamic calculations enabled to determine the theoretical vanadium recovery factor from converter vanadium slag during steel tapping and ladle slag formation. The dependences of this coefficient on the carbon content in steel and the amount of vanadium slag are presented in Fig. 4.
During tapping, the vanadium recovery factor was 0.5 at a vanadium converter slag consumption of 10 kg/ton of steel, which enabled to obtain a vanadium content of 0.04% in the steel. For medium- and highcarbon steel at this slag consumption, the recovery factor is more than 0.9.
To assess the final results of the out-of-furnace steel treatment, the thermodynamic modeling of the vanadium reduction process from converter vanadium slag was performed, taking into account the reducing agents added (breeze coke carbon and ferrosilicium silicon).
The calculations were performed for the additional conditions, namely, medium-carbon steel, breeze coke consumption of 0 to 2 kg/ton of steel, consumption of converter vanadium slag of 4 to 16 kg/ton of steel, and amount of furnace slag of 10 kg/ton of steel. The results showed that in the specified range of slag consumption at a breeze coke consumption of more than 0.6 kg/ton of steel, the vanadium content in the metal does not change, whereas the vanadium recovery factor exceeds 0.9 at any slag consumption.
An analysis of the dependences obtained with the combined use of breeze coke and ferrosilicon showed that the vanadium content in steel at a ferrosilicon consumption of more than 1 kg/ton of steel does not depend on the carbon content in the steel and the breeze coke consumption. When the consumption of ferrosilicon is less than 1 kg/ton of steel, this indicator depends on the carbon in the steel and almost does not depend on the consumption of breeze coke for medium- and high-carbon steels. This is due to the fact that in the case of high-carbon steel, the mass of carbon added with the metal at the outlet is greater than the mass of carbon added with the breeze coke. Joint reduction with carbon and silicon is more efficient than reduction with a single reducing agent.
The analysis of the metal obtained as a result of the experimental melts revealed a stable vanadium content in the samples. The vanadium recovery factor in all samples was close to unity.
The results of the thermodynamic modeling and laboratory studies of the process of alloying steel with vanadium from converter vanadium slag enabled to develop a technology for metal production according to the scheme of smelting of an intermediate product in an electric arc furnace, out-of-furnace treatment at a ladlefurnace unit, casting at a section continuous casting machine. Industrial tests of the technology of alloying steel in the conditions of the electric-furnace melting shop were performed.
The results of the industrial tests were compared with the results of the thermodynamic modeling by the content of vanadium in the metal after treatment (Fig. 5). The results of the chemical analysis of vanadium content in metal after treatment at the ladle-furnace unit are close to the calculated data, which indicates the sufficient accuracy of the thermodynamic calculations performed.
The analysis of the industrial testing results showed that the recovery of vanadium from the vanadiumcontaining slag occurs in two stages:
-
(1)
With recovery during tapping in a ladle during the formation of slag from the slag-forming mixture
-
(2)
With additional reduction of vanadium during out-of-furnace treatment of steel at the ladle-furnace unit
The stage comparison results indicate that the most part of vanadium (70–90%) enters the metal during tapping. The best performance was obtained on a furnace with a siphon steel tapping system. The high drop height of the metal jet and significant mixing of the metal and slag in the ladle provide better kinetic conditions for the vanadium reduction process as compared to the siphon tapping system. Thus, at stage 1, the recovery of vanadium is determined by the degree of mixing of the metal and slag. The indicators obtained are close to the data of the thermodynamic calculations performed.
Metal processing at the ladle-furnace unit ensures a complete extraction of vanadium. The throughput coefficient of vanadium recovery, averaged over all melts, is close to 100%.
A stable process of extracting vanadium from the converter vanadium slag can be implemented within the range of technological parameters that exist in the production of steel under real conditions for each individual grade.
When developing technological methods for modifying steel with barium and strontium, it is required to take into account, on the one hand, the high melting point of barium–strontium-containing master alloys and, on the other, their low density. In this regard, the task of developing a technology for modifying cast iron and steel with a barium–strontium modifier obtained from complex ores containing barium and strontium, excluding the stages of production of barium–strontium-containing master alloys, is urgent.
The X-ray phase analysis showed that the main compounds that make up the modifier are baritocalcite BaCa(CO3)2, calcite CaCO3, calcitostrontianite CaSr(CO3)2, impurities of dolomite MgCO3, and siderite FeCO3.
To study the phase and structural transformations occurring in the ore mineral components of the barium–strontium modifier, differential thermal analysis (DTA) was used during heating.
The DTA results reveal that up to a temperature of 1223 K, dissociation of dolomite, calcite, baritocalcite, and calciostrontianite occurs. Hence, it is of interest to study the behavior of barium and strontium oxide compounds at temperatures of steelmaking processes (1873 K–1923 K).
Modification is usually performed at the final stage of production, either at the outlet from the furnace to the ladle, during the out-of-furnace treatment of steel in the ladle, or directly during casting. At different stages of the out-of-furnace treatment, carbon, silicon, and aluminum can be considered reducing agents. The analysis of the theoretical calculation results showed that carbon at temperatures characteristic of steelmaking processes (1873 K–1923 K) almost does not reduce barium and strontium from their oxides; silicon can reduce barium by approximately 60%, but it poorly reduces strontium (only 15%); when using aluminum, the degree of reduction of barium and strontium from oxides reaches 70% and 50%, respectively; and the temperature in the range of 1873 K–2073 K has an insignificant effect on the degree of recovery of the elements (Fig. 6).
The analysis of the thermodynamic modeling results presented by the authors of [13, 19, 20] shows that the barium and strontium from their oxides are reduced more fully when aluminum is used as a reducing agent.
For the effective use of aluminum, a thorough preliminary deoxidation of the metal and slag is required to reduce the oxidizing potential of the metal-slag system.
When analyzing the thermodynamic modeling results, it should be noted that under the conditions of the out-of-furnace treatment when modifying steel with barium–strontium-containing materials and using siliconcontaining ferroalloys for slag deoxidation, certain reduction of barium occurs, and strontium is almost not reduced, especially because the consumption of silicon for reduction is limited by the level of its grade content.
Thus, the modification of steel with a natural material is thermodynamically reasonable. The effect of modification with barium can manifest itself in steels deoxidized only with silicon, and for modification with strontium or joint modification with barium and strontium, aluminum should be used as a deoxidizing agent.
It is not possible to compare the results of using the modifier under industrial conditions with thermodynamic calculations due to the unavailability of methods for the analytical determination of the content of barium and strontium in the metal. This assessment can be performed using indirect data through the study of the effect of treatment with a modifier on the properties of the finished metal. The data of mechanical tests and metallographic studies are presented in Table 5.
An increase in the consumption of carbonatite from 4.0–4.7 to 7.7 kg/ton leads to an additional increase in the impact toughness (by 2.57–3.02 times) in the range of the investigated test temperatures of − 60°C and − 70°C.
The data of the metallographic analysis show that the treatment of steel with carbonatite reduces the contamination of steel with non-metallic inclusions and ensures a high level of their globularization. At the same time, the grinding of the ferrite-pearlite structure of steel is recorded due to the elimination of rough areas of silicon– manganese liquation formations.
The averaged results of the mechanical tests of the 35HGSL steel showed an increase in the structural strength of steel by 20–50% after the treatment with a barium–strontium modifier, characterized by the values of yield strength, resistance, and impact toughness at positive and negative temperatures (Fig. 7).
The analysis of the industrial test results reveals that the barium–strontium modifier affects actively the microstructure and non-metallic inclusions and increases the stock of structural strength of steels, estimated by the values of the yield strength, resistance, and impact toughness at positive and negative temperatures. In terms of technology, the production of steels with the specified properties is not difficult.
The analysis of the results showed that the metal treatment with barium and strontium compounds does not have a significant effect on the formation of non-metallic inclusions, but it affects the structure formation. The sample, during the smelting of which, no barium and strontium compounds were added to the slag-forming mixture, had a Widmanstätt structure characteristic of steels with low mechanical properties, and the samples treated with slag-forming mixtures containing barium and strontium had a structure represented by lamellar perlite with ferrite precipitates along grain boundaries and individual precipitates of ferrite inside pearlite grains, typical for steels with high mechanical properties.
The chemical composition analysis results did not show the presence of barium and strontium in the samples, presumably due to their low content. To reveal the presence of barium and strontium in steel, the samples were examined via scanning electron microscopy. Table 6 presents the research results.
Table 6 shows that barium and strontium are differentiated along the structural components of the metal matrix. The preferred locations for barium and strontium are the bulk of the pearlite and ferrite grains.
To detect the localization of barium and strontium in steel (solid solution or inclusions of second phases), the steel structure was analyzed using extract replicas through transmission electron diffraction microscopy.
The analysis of the results reveals that the particles extracted onto the replica are small in size (50–500 nm). At the same time, the analysis of the micro-electron diffraction pattern shows that, along with iron oxides and carbides, the studied samples contain barium and strontium compounds C2BaO4, SrFeO2.97, Ba2Fe6O11, BaSrFe4O8, Ba3Fe2O6, and Ba2Fe14O22 (Fig. 8).
Studies have confirmed that barium and strontium are actively involved in the formation of structural components at the time of crystallization. The presence of barium and strontium in the bulk of the grain indicates the interaction of these elements with the metal melt with a certain effect on the structure formation of the metal matrix. The presence of complex compounds, which include barium and strontium, indicates the interaction of elements with non-metallic inclusions. The revealed amounts of the compounds confirm the opinion that the interaction of barium and strontium compounds occurs at the nanoscale.
Conclusions
Natural and man-made materials have certain metallurgical properties that must be taken into account when developing a technology for their use. Thus, the process of direct alloying with manganese ores, concentrates, and converter vanadium slags studied in this work requires strict adherence to the operational procedure. The results obtained are influenced by the nature of the preliminary melting course, timeliness of the charge melting, and slag and temperature regimes of the oxidation period. With direct alloying with the listed materials, the contact surface area (size of the materials and concentration of the main elements in them), temperature, and separation rate of the reaction products (rate of formation of fusible and free-running slags) must be taken into account.
The study on the physicochemical processes occurring during the out-of-furnace treatment of melts using thermodynamic analysis and modeling, as well as by conducting high-temperature experimental studies, enables to identify control conditions for optimizing energy consumption, minimizing the cost of expensive components while improving the quality of the materials obtained, which enables to obtain an economic effect due to lower cost of materials. The results reveal that during tapping, the vanadium recovery factor is 0.5 at a vanadium converter slag consumption of 10 kg/ton of steel, which provides a vanadium content of 0.04% in the steel. For medium- and high-carbon steel at this slag consumption, the recovery factor is more than 0.9. In addition, the calculation results show that joint reduction with carbon and silicon is more efficient than reduction with a single reducing agent.
The modification of cast iron and steel with a barium–strontium modifier obtained from complex ores containing barium and strontium, excluding the stages of production of barium–strontium-containing master alloys, is thermodynamically substantiated. The effect of modification with barium can manifest itself in steels deoxidized only with silicon, and for modification with strontium or joint modification with barium and strontium, aluminum is required as a deoxidizing agent. The barium–strontium modifier affects actively the microstructure and non-metallic inclusions and provides a predetermined increase in the performance characteristics of the finished product. For example, an increase in the consumption of carbonatite during the smelting of 35HGSL steel from 4.0–4.7 to 7.7 kg/ton led to an additional increase in impact strength (by a factor of 2.57–3.02). After the treatment of 35HGSL steel with a barium–strontium modifier, the structural strength factor of the steel is increased by 20–50%. The expansion of the use of natural and man-made materials in the production of ironcarbon melts can be recommended.
References
I. V. Ryabchikov, et al., Ferroalloys with Rare Earth and Alkaline Earth Metals [in Russian], Metallurgiya, Moscow (1983).
V. I. Zhuchkov and S. V. Lukin, Technology of Ferroalloys with Alkaline Earth Metals [in Russian], Metallurgiya (1990).
V. P. Zayko, V. I. Zhuchkov, L. I. Leontiev, et al., Technology of Vanadium-Containing Ferroalloys [in Russian], IKC Akademkniga, Moscow (2004).
L. A. Smirnov, L. M. Panfilova, and B. Z. Belenky, “Problems of expanding the production of vanadium-containing steels in Russia,” Stal’, No. 6, 108–115 (2005).
V. A. Rovnushkin, Technological Aspects of Ladle Alloying of Steels Using Vanadium Slag, Proceedings of Congress II of steelmakers, Moscow, 250–251 (1994).
O. S. Bobkova, “On the mechanism of fusion of oxide materials and the reduction of metals from oxide melts,” Stal’, No. 1, 23–27 (1991).
P. Ding, Q. J. Liu, and W. H. Pang, “A review of manganese ore beneficiation situation and development,” Appl. Mech. Mater., 380–384, 4431–4433 (2013).
O. I. Nokhrina and V. I. Dmitrienko, “Reduction of manganese from its oxides by silicon in direct furnace alloying of steel,” Steel Transl., 34, No. 6, 34–37 (2004).
O. I. Nokhrina, V. P. Komshukov, and V. I. Dmitrienko, “Developing a technology for the direct alloying of steel with manganese in an electric-arc furnace,” Metallurgist, 48, No. 5–6, 264–265 (2004).
O. I. Nohrina, I. D. Rogihina, M. A. Golodova, and D. V. Valuev, “Resource-saving technologies in production cold-resistant steels,” Key Eng. Mater., 839 KEM, 93–98 (2020).
T. Kang, Y. Liu, Y. Huang, J. Dong, Q. Huang, and Y. Li, “Synthesis and dephosphorization of iron manganese composite oxide by acid leaching on iron manganese ore,” Adv. Mater. Res., 554–556, 489–493 (2012).
M. A. Golodova, V. I. Dmitrienko, I. D. Rozhikhina, and I. A. Rybenko, “Reduction of vanadium in elementary systems,” Steel Transl., 40, No. 4, 310–313 (2010).
G. V. Galevsky, V. V. Rudneva, A. K. Garbuzova, and D. V. Valuev, “Titanium carbide: nanotechnology, properties, application,” IOP Conference Series: Materials Science and Engineering, 91, 1–5 (2015).
O. I. Nokhrina, I. D. Rozhikhina, I. E Khodosov, and I. E. Proshunin, “Production and use of concentrates from polymetallic manganese ore,” Steel Transl., 45, No. 5, 295–300 (2015).
R. Langeborg and T. Siwecki, Scand J. Metallurg, 28, 186–241 (1999).
O. I. Nokhrina, I. D. Rozhikhina, P. D. Kravchenko, O. Yu. Kichigina, and M. S. Kostyuk, Patent 2583224, IPC S22V47/00, Method of Chemical Enrichment of Polymetallic Manganese-Containing Ores, Appl. 12/01/2015; Publ. 10/05/2016, Bul. 2016, No. 13.
M. A. Golodova, V. I. Dmitrienko, I. D. Rozhikhina, O. I. Nokhrina, and I. A. Rybenko, “Investigation of the conditions for the reduction of vanadium and iron from converter vanadium slag,” Izv. Vyssh. Ucheb. Zav. Chern. Metallurg., No. 4, 3–5 (2011).
O. I. Nokhrina, I. D. Rozhikhina, V. I. Dmitrienko, M. A. Golodova, and Yu. A. Efimenko, “Carbon-silicothermal reduction of elements from converter vanadium slag oxides,” Izv. Vyssh. Ucheb. Zav. Chern. Metallurg., No. 2, 27–30 (2014).
V. I. Dmitrienko, I. D. Rozhikhina, O. I. Nokhrina, R. S. Aizatulov, and M. A. Platonov, “Investigation of the reduction of barium and strontium as applied to the conditions of out-of-furnace treatment of steel,” Izv. Vyssh. Ucheb. Zav. Chern. Metallurg., No. 4, 27–29 (2012).
I. D. Rozhikhina, M. A. Platonov, I. S. Sulimova, V. I. Dmitrienko, and O. I. Nokhrina, “Metallurgical properties and phase transformations of the barium-strontium modifier,” IOP Conf. Series: Materials Science and Engineering, 127, 012050 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 12, pp. 65–69, December, 2021. Russian DOI https://doi.org/10.52351/00260827_2021_12_65.
Rights and permissions
About this article
Cite this article
Nokhrina, O.I., Gizatulin, R.A., Golodova, M.A. et al. Alloying and Modification of Iron-Carbon Melts with Natural and Man-Made Materials. Metallurgist 65, 1429–1448 (2022). https://doi.org/10.1007/s11015-022-01289-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-022-01289-z