Shaimerden oxidized zinc ore containing more than 21% wt.% zinc is studied in this work. When the first research on hydrometallurgical processing of this ore was conducted the authors of this research determined the following parameters for ore leaching: the size of ore reduction, sulfuric acid concentration, temperature, and leaching duration. These tests serve as a basis for zinc recovery from the given ore with a size of – 2.5 mm and a regime is recommended providing zinc recovery into solution at the level 93.5%. However, the production regime proposed for sulfuric-acid leaching of this zinc ore requires further development as this process is characterized by inadequate production, high energy consumption for heating leaching pulp, and considerable sulfuric acid consumption. In order to avoid these disadvantages the authors develop a production regime of four-stage sulfuric-acid leaching of Shaimerden ore – 1.0 mm in size. The regime developed in this article provides zinc recovery from ore into solution at the level 94.65% compared with the regime of sulfuric-acid leaching developed previously. At the same time, consumption of sulfuric acid for leaching is reduced by 22%, the leaching period is six times shorter, and energy consumed for heating leaching pulp is lower by approximately a factor of three.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Currently quite a large number of rich oxidized zinc ore deposits are known in the world. However, only a small part of these deposits is involved in hydrometallurgical treatment, which limits the raw material base for zinc production.
Oxidized zinc ore deposits, which are currently under industrial treatment, are provided in Table 1. For industrial processing of these ores Waelz treatment or sulfuric acid leaching are used. More detailed information about industrial processing of these ores has been provided in [1,2,3,4].
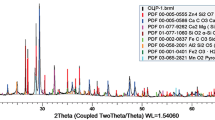
In order to develop the raw material base for zinc the authors of the work have selected for a study object oxidized zinc ore of the Shaimerden deposit (Kostanai region, North Kazakhstan) containing calamine (Zn4[Si2O7 ](OH)2 ·H2O) and smithsonite (ZnCO3). The starting materials used was that employed in experimental research [23] aimed at studying the possibility of sulfuric acid leaching of zinc from ore of this Kazakhstan deposit. The object studied in [23] was a sample of ore from the Shaimerden deposit, containing, %: 21.30 Zn, 0.55 Pb, 0.027 Cu, 3.9 Al, 2.75 Fe, 7.4 CaO, 30.0 SiO2. This deposit has been worked by a quarry method since the middle of the 1990s. Zinc reserves in this deposit are estimated at 1.26 million tons [4].
Ore of this deposit is treated in a Waelz process of the Ridder Metallurgical Complex TOO Kaztsink (Ridder, west-Kazakhstan region) where the main raw material is sphalerite concentrate obtained from local ores. Waelz zinc oxide extracted in this process is subjected to sulfuric acid leaching within the scope of traditional technology. However, as follows from results of exploratory studies direct use of this ore in the sulfuric acid leaching stage, including use of the expensive Waelz process, is more economically expedient.
In [23] a sulfuric acid regime was selected for repulping leaching of ore with sulfuric acid solution of concentration 180–200 g/liter followed by washing the insoluble residue with hot water on a filter (an ore sample weighing 250 g was used in studies). This regime provided zinc extraction from ore into solution at the level of 93.5%. The treatment regime selected involves: use in this process of ore with a size of – 2.5 mm, a leaching temperature of 70–95°C for 4 h; specific sulfuric acid solution consumption for ore selective dissolution of 0.72 g per 1 g of ore. Therefore, sulfuric acid leaching of oxidized zinc ore containing not less than 20% zinc and represented by zinc minerals that are opened up by acid, provides thorough zinc extraction from ore into sulfate solution at the level of 93.5%. In addition, complete extraction of zinc from sulfide ore into sphalerite concentrate and then from this concentrate into zinc sulfate solution in contemporary zinc technology hydrometallurgy comprises ≈ 75%.
However, the production regime provided above for sulfuric acid leaching of oxidized zinc ore from this deposit requires development since is has inadequate productivity, high energy consumption for leaching pulp heating, and high sulfuric acid consumption.
The authors in [25,26,27] also studied the possibility of sulfuric acid leaching of zinc from ore of this Kazakhstan deposit. In particular, in [25] ore of different fractions (including – 1 mm) was subjected to sulfuric acid leaching for 60 min at 60°C. However, this discontinuous and low-temperature leaching regime only made it possible to extract from ore onto sulfate solution less than 81% zinc.
It may be suggested that stage-wise leaching of ore is limited by the rate of sulfuric acid molecule diffusion into the depth of calamine particles through a layer of silica formed upon them considering its low solubility. Therefore, subsequently it was expedient to study the possibility of leaching the test ore in several stages with the aim of renewing the calamine reaction surface in each stage.
In subsequent studies the authors attempted to develop a production regime of stepwise sulfuric acid leaching of the test oxidized zinc ore free from the disadvantages indicated above. The Shaimerden deposit was used during performance of experimental studies ore, refined in a ball mill to a size of –1 mm of the following composition, %: 21.07 Zn, 0.55 Pb, 0.012 Cu, 3.0 Al, 1.56 Fe, 18.6 CaO, 20.7 SiO2.
In order to shorten the leaching duration and reduce expenditure on leaching pulp heating, and sulfuric acid consumption in this process experiments were conducted using sulfuric acid solution of different concentrations. A study was also made of the effect of number of leaching stages on zinc extraction into solution. Results of these studies made it possible to optimize the leaching stages for the test ore and stage-wise concentration of sulfuric acid solution.
Four series of tests (three tests in each) using single-, two, three- and four stage sulfuric acid leaching (see Fig. 1) were performed in the work. Experiments were conducted in chemical vessels with a volume of 0.5 dm3.
During the single-stage process refined ore in an amount of 25 g was charged into 01 dm3 of sulfuric acid solution of concentration 98 g/dm3. The vessel with the pulp obtained was installed on an electric plate after which ore leaching was conducted with pulp mixing by a magnetic stirrer at 40°C for 10 min (stirrer rotation rate was 220 rpm, temperature recorded with a alcohol thermometer with a measurement error ± 1.0°C). At the end of leaching the pulp was filtered in a funnel 13 cm in diameter through a “red ribbon” filter. The zinc sulfate solution obtained was analyzed for zinc content by a spectral method. Insoluble residue (cake) remaining after filtration was dried in a drying cabinet at 105°C to constant weight and the zinc content was analyzed by a spectral method (an ICP-MSAgilent 5700 cx mass spectrometer with an inductively coupled plasma produced by Agilent Technologies, USA, was used).
Two- and three-stage leaching differed with respect to the single-stage method solely by the fact that the moist cake from the preceding stage of the process was supplied to the next stage. In the this case in the second and third leaching stages sulfuric acid solution of concentration 28 and 14 g/dm3 respectively was used.
In a four-stage leaching process moist cake from the third stage of the process was subjected to repulping (washing) in distilled water with a volume 0.2 dm3 at room temperature for 10 min. The original sulfuric acid concentration in the liquid phase for pulp of the forth leaching stage was of the order to 1 g/dm3, which is explained by use of acid cake in this stage,
Results obtained in this work are provided in Tables 2 and 3. As is seen from the data provided, the process studied of ore four-stage leaching provided zinc extraction into solution at the level of 94.65% (see Table 2).
According to Table 3 (tests 1–3) the final zinc sulfate solution had an inadequate concentration of this compound (40–45 g/dm3 in terms of zinc); for classical zinc hydrometallurgy with processing of sphalerite the solution should contain more than 100 g/dm3 of zinc [28], for use of this of this solution during electrolysis. In addition, in the first ore leaching stage (tests 1–3) silica solubility, formed as a result of calamine with sulfuric acid, is low under conditions of this stage of the process (1.3–1.7 g/dm3 in terms of silicon) and answers the specifications of zinc hydrometallurgy.
Comparable characteristics are given in Table 4 for leaching the test ore and washing cake by the process proposed in this work, and also by the method proposed in [23].
As is seen from data provided in Table 4 due to the method developed the duration of the ore leaching process (30 + 10 = 40 min) is reduced by more than a factor of six compared with the traditional method (more than 240 min). This situation makes the method developed much more productive. Also, the new process occurs at half the known temperature (25–40°C against 70–95°C). In spite of the fact that in the method proposed the pulp volume is greater by approximately a factor of four than in the known method, this shorter duration and lower temperature for the new method made it possible to reduce by approximately a factor of three the energy consumed for leaching pulp heating and washing. In addition, the new method compared with known procedures provides a reduction in sulfuric acid consumption by 22% (from 0.72 to 0.56 g/g ore). Therefore, the method proposed based on renewing the calamine reaction surface during ore leaching makes it possible to avoid the disadvantages indicated above for the known method.
In addition, as is well known, the method developed is specified by a low zinc content in finished sulfate solution with a ore leaching stage, comprising correspondingly 50–60 g/dm3 [23] and 40–45 g/dm3 (see Tables 2 and 3, tests 1–3).
In order to increase the zinc content in final sulfate solution from the ore leaching stage it is possible to recommend a counter four-stage leaching regime. An additional increase in zinc concentration in the final solution to that required for zinc electrolysis (more than 100 g/liter) may be provided by using a final solution strengthened with respect to zinc for leaching sphalerite cinder within the scope of classical sphalerite treatment technology. The recommended four-stage counter process will make it possible to reduce water consumption considerably for ore leaching (from 3 dm3 to 1 dm3 for 250 g of ore), and therefore to reduce an addition energy consumed for leaching pulp heating, and also to reduce sulfuric acid consumption.
Conclusions
-
1.
The new method of sulfuric acid four-stage leaching of rich oxide zinc ores compared with classical treatment of sulfide zinc ores will make it possible to increase the thorough extraction of zinc from ore into sulfate solution from ≈ 75.00 to ≈ 94.65%.
-
2.
The method proposed is more effective compared with the well-known method [23], since it will make it possible to reduce sulfuric acid consumption by 22%, duration by a factor of six, and temperature of the process by approximately a factor of two, and also to reduce the energy expended in leaching pulp heating by approximately a factor of three.
Work was carried out with financial support of a Kazakhstan Republic Ministry of Education and science grant No. 0538/GS4.
References
A. Poltaeva and A. Myagkova, Development of a Rational method for Leaching Iranian Zinc Oxidized Ore [in Russian], VNIItsvetmet, Ust’-Kamenogorsk (1972).
Skorpion Zinc. Contemporary Data for Zinc Refining, Information Center [Electronic Source]. Access Regime: http://jgnoinski@skorpionzinc.com.na.
L. A.Kazanbaev, P. A. Kozlov, and V. L. Kubasov, Zinc Hydrometallurgy. Leaching Processes [in Russian], Ruda i Metally, Moscow (2007).
E. E. Palenova and E. V. Belogub, Mineralogy of Nodular Oxidized Zinc Ores of the Shaimerdin Deposit [in Russian], Mineral. Muzei, St. Petersburg (2008).
Metallurgical Bulletin. Information-Analytical Journal [Electronic Source], Access Regime: https://www.metalbulletin.ru.
A. A. Lykasov, G. M. Ryss, and V. N. Vlasov, Zinc Metallurgy [in Russian], Izd. Tsentr YuUrGU, Chelyabinsk (2009).
Yu. P. Romanteev, A. N. Fedorov, and S. V. Bystrov, Zinc and Cadmium Metallurgy [in Ruussian], MiSIS, Moscow (2006).
I. G. Antropova, Physicochemical and Production Bases of Sulfidized Firing of Oxidized Lead-Zinc Ore in a Heated Water Vapor Atmosphere, Author’s ref. Diss. Cand. Techn. Sci., Ulan Ude (2005).
A. P. Snurnikov, Comprehensive Use of Mineral Resources in Ferrous Metallurgy [in Russian], Metallurgiya, Moscow (1986).
Mei Yang, Wending Xiao, Xiang Yang and Patrick Zhang, “Processing mineralogy study on lead and zinc oxide ore in Sichuan,” Metals,93. No. 6, 1–7 (2016).
G. Borg, “A review of supergene nonsulphide zinc (SNSZ) deposits-the 2014 update,” in: S. M. Archibald and S. J., Piercey (editors), Current Perspectives of Zinc Deposits, Irish Assoc. for Economic Geology: Dublin (2015).
Dong-sheng He, Yun Chen, Ping Xiang, Zheng-jun Yu, and J.H. Potgieter, “Study on the pre-treatment of oxidized zinc ore prior to flotation,” Int. J. of Minerals, Metallurgy and Materials,25. Nо. 2, 117–120 (2018).
Mineral Catalog. Information Center [Electronic Src.]. Access Regime: http://www.catalogmineralov.ru/mineral/hemimorphite.html.
New Methods for Treating Zinc-Containing Raw Material Abroad [in Russian], Proizvod. Tyazh. Tsvet. Met. Obzor Inform, TsNIIÉITsM, Moscow (1984).
B. F. Kulikov, V. V. Zuev, I. A. Vainshenker, and G. A. Mitenkov, Mineralogical; Handbook for Enrichment Technology [in Russian], 2nd. Ed, Nedra, Leningrad (1985).
Procedural Recommendations for Use of Classification of Deposit Reserves and Prediction of Solid Useful Mineral Resources. Lead and Zinc Ores [in Russian], FGU GKZ, Moscow (2007).
Zinc Minerals. Information Center [Electronic source]. Access regime: https://ru.wikipedia.org.
V. P. Zhivopistsev and E. A. Seleznova, Zinc Analytical Chemistry [in Russian], Nauka, Moscow (1975).
North Podesiya. Economic Minerals [Electronic Source]. Access Regime: http://geo-nature.ru/severnayarodeziya-poleznyeiskopaemye/.
USSR Economic Minerals. Mineral Resources, Lead and Zinc Deposits, Stratiform Deposits [Electronic Source]. Access Regime:http://posmotrel.net/karatau2011/minres.html.
Mining Encyclopaedia within the Greater Soviet Encyclopaedia. Information Center [Electronic Source]. Access Regime: http://posmotrel.net/karatau2011/minres.html.
P. A. Kozlov, Waelz Process [in Russian], Rudy i Metally, Moscow (2002).
Zh. K. Temesheva, Physicochemical Study of Leaching Zinc-Containing Ores of the Shaimerden Deposit, Diss. Master Metallurgy, VKGTU, Ust’-Kamenogorsk (2015).
A. A. Abramov, Oxidized Ore Treatment [in Russian], Nauka, Moscow (1985).
R. A. Ramazanova, N. V. Seraya, R. A. Bykov, S. V. Mamyachenkov, and O. S, Anisimova, “Features of leaching oxidized zinc ore of the Shaimerden deposit,” Metallurg.,60, No. 6, 78–82 (2016).
R. A. Ramazanova, N. V. Seraya, R. A. Bykov, S. V. Mamyachenkov, and O. S. Anisimova, “Features of Shaimerden deposit Oxidized zinc ore leaching,” Metallurgist,60. No. 5-6, 629–634 (2016).
R. A. Bykov, R. A. Ramazanova, E. Yu. Van, N. V. Seraya, and S. V. Mamyachenkov, KR Patent 2062. Method for Treating Oxidized Zinc Ore, Publ. 03.15.2017.
R. A. Ramazanova, N. V. Seraya, and R. A. Bykov, Problem of Processing Low-Grade Oxidized and Mixed Zinc Ores, Proc. Internat. Sci.-Pract. Conf. Almaty (2014).
Yu. N. Matveev and V. S. Strizhko, Technology of Nonferrous Metallurgical Production [in Russian], Metallurgiya, Moscow (9186).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 64, No. 2, pp. 73–78, February, 2020.
Rights and permissions
About this article
Cite this article
Ramazanova, R.А., Seraya, N.V., Samoilov, V.I. et al. New Method of Rich Oxidized Zinc Ore Sulfuric Acid Leaching. Metallurgist 64, 169–175 (2020). https://doi.org/10.1007/s11015-020-00977-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-020-00977-y