Results are provided for experimental studies of conditions for the formation of various types of M/A-constituent (structural component consisting of martensite and austenite) in the microstructure of low-carbon pipe steel specimens produced by rolling with thermomechanical treatment (controlled rolling and accelerated cooling). As a result of experiments different types of M/A constituent are obtained and the temperature-time conditions for their formation are determined. A research procedure is developed making it possible with maximum confidence to determine the type, size and volume fraction of M/A-constituent in the microstructure. Depending on cooling conditions and exposure temperature, two critical states of the M/A-constituent are identified: at high exposure temperature, predominantly twinned martensite is formed, and austenite is formed at a low temperature. At intermediate temperatures, various combinations of these phases are observed, while the fraction of martensite in the composition of M/A “islands” decreases with decreasing exposure temperature, but the fraction of austenite increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High-strength low-carbon steels manufactured by controlled rolling and accelerated cooling (CR + AC) form a complex microstructure consisting of a matrix represented by sorbite, ferrite, bainite, lath martensite, or a combination of them (depending the temperature for the end of accelerated cooling) and various secondary high-carbon phases and structural components, i.e., pearlite, high-carbon bainite, martensite, and residual austenite [1,2,3]. Use of rapid cooling after deformation makes it possible to suppress carbon diffusion to the extent that within the metal there is deformation of secondary phases, not containing cementite: residual austenite and high-carbon martensite or M/A constituent, i.e., a structure represented by a combination of martensite and residual austenite [4,5,6,7]. As a result within the structure of high-strength pipe steels, prepared by CR + AC technology there is always a content of some amount of M/A-constituent in the form of layers over boundaries between laths, or if the bainite matrix is granular in the form of “islands”. In this case the volume fraction of these structures in contemporary low-carbon microalloyed bainitic steels (type K60) is insignificant, normally not more than 2%. Nonetheless, there is interest is developing special technology for forming a significant amount of M/A-constituent, which will make it possible to obtain an additional tool for controlling steel properties. By means of forming M/A-constituent in a bainitic matrix it is possible to obtain a unique combination of mechanical properties [8]. Technologically formation of M/A-constituent may be achieved by using heat treatment in the rolling mill line (HOP-process) and also special accelerated cooling regimes.
Different types of M/A-constituent of a structure may have a different effect on steel mechanical properties and cold resistance. Formation of a large proportion of twinned martensite (TM) may lead to an increase in strength and a reduction in ductility, and presence of residual austenite as a result of a TRIP-effect, and the opposite may lead to an increase in relative and uniform elongation. Not only the M/A-constituent morphology has an important effect on properties, but also its fineness. It is well known that with presence of coarse M/A-“islands” there is a reduction in impact strength, since they become stress concentrators and facilitate crack propagation [9,10,11,12].
In world practice in order to identify M/A-constituent there is extensive use of color etching in LePera reagent [15], which makes it possible to separate with respect to color a matrix of ferrite, bainite, and M/A-constituent: ferrite is blue, bainite is brown, and austenite/martensite is white. From an image obtained it is possible to reveal only presence of M/A-constituent in a microstructure and to work out is volume faction. For reliable determination of the M/A-“islands” phase composition, the proportion in the structure and size, it is necessary to use in combination some research methods for study: transmission electron microscopy (TEM), X-ray diffractometry (XD), special procedures of color etching (LePera method and others). A method is also used of scanning electron microscopy (SEM), including use of analysis of diffraction of back-scattered electrons (EBSD). In this work in order to determine the composition and morphology of M/A-constituent comprehensive research was conducted using methods of color etching, TEM, and XD.
The aim of the work is to study the effect of temperature and time parameters for cooling and exposure on M/A-constituent formation within the structure of low-carbon steel during TMT, and also selection of procedures for unambiguous identification of secondary phases in these steels.
Research procedure. In order to conduct experiments workpieces were selected from industrial thick sheet steels of strength categories K60 and K65 prepared in the AO VMX 5000 mill by CR + AC regimes. The chemical composition of the test steels differed with respect to alloying elements: 0.06% C, 1.6% Mn for K60; 0.04% C, 1.7% Mn for K65, both steels were microalloyed with Nb, Ti (K65 contains in addition ≈ 0.3% molybdenum), both steels were deoxidized with Al, have a low sulfur and phosphorus content, and small amounts (≈ 0.2% of each) of silicon, nickel, and copper.
Preliminary study of the phase transformation kinetics showed that both test steels have presence of a bainitic region over a wide temperature range, which satisfies the experimental conditions: for steel K60 on average 150 ºC (temperature for the start of bainitic transformation (Tsb ) = 610 ºC, temperature for the end (Teb ) = 460 ºC), and for steel K65 the temperature range for bainitic transformation with a cooling rate of more than 10 ºC/sec comprises on average 170 ºC (Tsb = 610 ºC, Teb = 440 ºC).
Experiments were performed in a BAHR-805 deformation dilatometer on cylindrical specimens 5 mm diameter and 10 mm thick. They were fastened in quartz tubes with a circular inductor and heated in a vacuum by current of frequency 2 MHz to 1170 ºC. Then specimens were deformed in compression at 1050 and 850 ºC with a degree of deformation of 25% each, simulating the roughing and finishing stages of controlled rolling. After deformation specimens were subjected to controlled cooling.
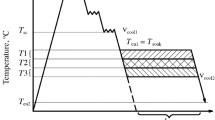
The effect was studied in specimens of the following parameters of post-deformation cooling on M/A-constituent composition and morphology: interrupted AC temperatures, temperatures and time for exposure between AC stages; cooling rate in the second stage (after exposure); interrupted AC temperatures after exposure. The deformation regime for all specimens was identical (indicated above). After treatment specimens were cooled to 820 ºC at a rate of 30 ºC/sec to the isothermal exposure temperature, which was varied within the limits of 600–450 ºC (with a step of 50 ºC), and its duration was also altered at 20, 40, and 60 sec, then specimens were cooled rapidly at a rate of 20 ºC/sec to 250 ºC. Specimens were studied additionally cooled after exposure with rates of 1.5–30 ºC/sec to 40–20 ºC. Two-stage AC was used in order to create favorable conditions for carbon redistribution with the aim of enriching of remaining areas of austenite with carbon.
The microstructure of the specimens obtained was studied by optical microscopy (OM) and transmission electron microscopy (TEM). Dilatometric specimens were cut in a longitudinal direction at a 1/4 through the thickness and cast in resin for preparation of metallographic microsections. Thin specimens were also cut at a 1/4 of the thickness in order to prepare foils and study metal by the TEM method.
Metallographic microsections were etched in 4% solution of nitric acid in alcohol and LePera reagent, and the microstructure was studied optically. Microsections etched in LePera reagent were photographed in bright white light at magnifications of × 400 and × 800 for collecting statistical data. Then these images were processed in an ImageExpert Pro3 program in order to evaluate the proportion and size of M/A “islands”. Preliminary treatment of images included avoiding an illumination gradient, a reduction in noise increasing sharpness and contrast in images, performed using Photoshop CS2 graphic editing. Then inversion and binarization of images was performed, and binarization criteria were selected in order to retain to a maximum information content of images, and they were corrected for each image in relation to the original level of photograph illumination and degree of specimen etching with reagent, which with an identical etching time depended strongly on the type of structure.
The type of M/A “islands” detected on etching with LePera reagent was determined by TEM for which specimens were prepared by a standard procedure using polishing in electrolyte based on orthophosphoric acid and chromium anhydride, and the foils prepared were studied in a JEM200-CX transmission electron microscope with an accelerating voltage of 120 kV. The proportion of residual austenite was determined by X-ray structural analysis (XD) in a Geigerflex diffractometer with a sharp focus tube from Philips. X-ray tube radiation with a cobalt anode was monochromatized with a graphite monochromator in the reflected beam. Before recording the surface layer of specimens was removed to a depth of not less than 10 μm by etching in hydrochloric acid. The volume fraction of austenite was calculated from the ratio of integral intensities of diffraction lines (111) for austenite and (110) for ferrite by a standard procedure.
Research results and discussion. Analysis of optical images of the microstructure of both steels obtained by etching in 4% nitric acid solution in alcohol showed that the microstructure of specimens constants of a ferrite matrix (polygonal, quasipolygonal, or bainitic) and a high-carbon phase, mainly represented by the M/A-constituent. A reduction in exposure temperature led to a change in the structure of both the ferrite matrix, and also secondary high-carbon phases. For both of the test steels (K60 and K65) exposure temperature had a more significant effect on secondary phase morphology (M/A-constituent). Typical changes in the structure of steel K60 are shown in Fig. 1a-c. With a reduction in exposure temperature ferrite grains comprising the matrix, change their shape, i.e., from similar to equiaxed to more extended, and in this case the structure became finer, which is a consequence of a reduction in temperature for the end of the first AC stage, At the same time, quite coarse (up to 8 μm) “islands” of high-carbon phase (martensite) etching dark (see Fig. 1a) with a reduced isothermal exposure temperature, change to finer light grey “islands” with residual austenite (see Fig. 1b). TEM data are provided below for studies confirming the secondary phase composition and changes are showed in the shape of matrix crystals.
In the course of studies by TEM a preferential type of matrix of the ferritic type and types of secondary phases in relation to isothermal exposure temperature were determined. With a reduction in Thol ferrite crystals, comprising the bainitic matrix, changed shape from equiaxed (Fig. 2a) to more extended, and with the lowest Texp approximated lath ferrite (Fig. 3c). There was also a change in morphology of the main secondary phases: coarse TM islands (with residual austenite over boundaries) were the main secondary phase with high Texp ≈ 600 ºC (Fig. 2b), and with reduced Texp there was a gradual increase in the number of “islands” of residual austenite in pure form, and the proportion of martensite in “islands” (TM + A) was reduced, and their size decreased (Fig. 4). With exposure at low temperatures of 500–450 ºC residual austenite (Fig. 3a, b) and cementite were detected in the structure, or over grains boundaries (Fig. 3c, d), or in the form of degenerated pearlite.
Studies by TEM revealed a common tendency of a change in the specimen microstructure of both steels, and therefore the exposure temperature was separated into three ranges (subsequently T1, T2, and T3) in relation to predominant type of secondary phases:
-
range T1 is high-temperature exposure (600–550 ºC). The matrix structure is represented by polygonal/quasipolygonal ferrite, secondary phases, i.e., predominantly coarse (with size up to 10 μm) “islands” consisting mainly of high-carbon TM with residual austenite over the periphery; in this case it is entirely correct to use the term M/A-phase”;
-
range T2 is exposure at 550–500 ºC (transition region), The matrix is represented by both equiaxed ferrite, and lath type ferrite; the secondary phases are approximately equal proportions observed as “islands” of twinned martensite with residual austenite (TM + A), and austenite (a) in pure form (Table 1);
-
range T3 is low-temperature exposure (at 500–450 ºC). The matrix is represented predominantly by bainitic ferrite of the lath type, and secondary phases are austenite in pure form (A) and a small amount of structural component containing cementite (pearlite, bainite) of cementite in pure form, over ferrite crystal boundaries.
On the basis of research results for structural changes within the steel during exposure may described as follows.
With a high exposure temperature the diffusion rate is quite fast and with an increase in exposure time there is an increase in carbon concentration in austenite islands and simultaneously there is a reduction in their volume as a result of occurrence of ferrite transformation in neighboring areas impoverished in carbon. As a result of this there is an increase in carbon concentration in quite coarse “islands” (up to 10 μm) to a level sufficient for obtaining high-carbon martensite during the second AC stage. Presence of and austenite “framing” of martensitic islands may be a result of enrichment of the surface of austenitic regions with carbon during ferrite transformation in neighboring metal areas. During performance of studies by the TEM method grains were often detected of almost dislocation-free ferrite in the vicinity of M/A “islands” and cementite was absent from the structure.
With low exposure temperatures due to the almost complete transformation of ferrite by a shear mechanism stable austenite is mainly detected in the form of layers between bainitic ferrite laths. Carbon concentration in austenite is high so that it is not converted into martensite in the second AC stage and remains stable at room temperature. However, with more prolonged exposure there may be austenite decomposition with precipitation of cementite particles, which is confirmed in studying specimens by the TEM method (see Fig. 3c, d).
With exposure in the medium temperature range ferrite transformation proceeds partly by a shear mechanism, volumes of austenite are fewer with interrupted exposure, and carbon concentration within them after exposure is quite high. After the second AC stage there may be formation of both pure austenite, and fine TM + A islands. With an increase in exposure duration, due to carbon access from neighboring regions, there is an increase in carbon concentration and a reduction in volume fraction and size of TM-“islands” with an increase in the proportion and size of pure austenite islands.
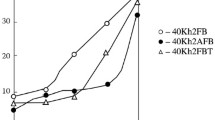
Results of determining the proportion of M/A-structure by calculation conducted in an ImageExpert Pro3 program (from optical photographs of the microstructure obtained with etching in LePera reagent) are shown in Fig. 5, where the data obtained are plotted for the dependence of M/A-constituent “islands” within structure on exposure temperature and time. It is seen that the effect of Texp on proportion of M/A-constituent in the microstructure (with exposure time of 20–60 sec) is definitive, whereas the exposure time the change in proportion of M/A-constituent depends weakly on the change in exposure time (within the limits studied). For both of the test steels the maximum proportion of M/A (≈ 5% for K60 and ≈ 5.5% for K65) was observed at 550 ºC, and with a further reduction in exposure temperature the proportion of M/A in the structure decreased sharply, and at 450 ºC already comprised less than 1% (see Fig. 5a, b). The proportion of M/A-constituent in steel K60) with a higher carbon content) with an exposure temperature in range T1 was significantly higher than for steel K65, which is mainly connected with a greater proportion within its structure of high-carbon twinned martensite. Then with a reduction in Texp and transition of secondary phases from TM to A, the overall M/A-“islands” proportion became approximately identical.
A change in average calculated diameter of M/A-“islands” ( Dav calculated for measurements in an ImageExpert Pro program) is shown in Fig. 6a in relation to exposure temperature. The relationship obtained is similar to that for proportion of M/A-“islands” in the metal structure. The effect is seen of chemical composition on curves: in steel K65 the island size is greater in the ranges T2 and T3, and at high temperature (in the range T1) in contrast the diameter of “islands” is larger in steel K60 containing more carbon. The coarsest islands (≈ 8 μm) are observed in the range T2 in steel K65 (Fig. 6b), i.e., it is martensite but the proportion of relatively coarse “islands” (2–8 μm) comprises not more than 10% of the overall amount of areas of M/A-constituent observed. It is seen that the average “island” diameter is small and even with exposure in ranges T2 and T1 (for steel K60, see Fig. 6a) comprises about 1 μm.
Results of experiments revealing the effect of temperature for dwell time in the second cooling stage after exposure at 600 ºC on the proportion and size of M/A-“islands” in the structure are shown in Fig. 7 for steel K60. It is seen that the temperature for the end of AC after exposure has a considerable effect on the proportion of M/A-“islands” of the TM + A type: with a reduction from 400 to 200 ºC the proportion of M/A-constituent increases by ≈ 2,5% (abs.). The diameter of M/A-“islands”, the same as in preceding cases, correlates with the volume fraction, i.e., Dav = 1.3μm is observed with their maximum proportion in the structure of ≈ 5% with a temperature for the end of AC of 200 ºC (see Fig. 7b). Cooling to room temperature does not lead to an increase in the proportion go M/A-constituent in the structure, and conversely in this case its proportion is at a maximum, i.e., ≈ 2% (see Fig. 7a).
The effect of cooling rate after isothermal exposure (v2) on the proportion and size of M/A-constituent in the structure of steel K60 is shown in Fig. 8. An increase in cooling rate from 5–10 to 20–30 ºC/sec led to significant increase (from ≈ 2 to ≈ 6%) proportion of M/A-structure type TM + A within the microstructure, and in this case the average island diameter varied from 1.3 to 1.6 μm in the range T at 600 ºC. With exposure in the T3 range a change in cooling rate did not have any significant effect on the reason for an initially lower proportion M/A-structure (see Fig. 8a, range T3, 600 ºC for steel K60). It is evident that slow cooling does not facilitate formation of a significant proportion of M/A-constituent within the structure of both steels, which is connected with the possibility of austenite decomposition during cooling at a slow rate.
Results of determining the proportion of residual austenite by the XD method and the proportion of M/A-constituent in the structure revealed by color etching are given in Table 2. It should be noted that the XD method makes it possible to determine only the overall proportion of austenite, and the metallographic method after etching in LePera reagent gives the general proportion of M/A-constituent without the possibility of separating them into twinned martensite and residual martensite. The color etching method also does not make it possible to consider entirely the proportion of fine (0.5 μm and less observed by the TEM method) sections of residual austenite in steels with low-temperature exposure. This explains the difference in significant proportion of M/A-constituent obtained by different methods (Table 2).
From data in Table 2 it is possible to estimate the effect of steel chemical composition on the proportion and type of M/A-constituent in the structure. More alloyed steel K65, containing more Mn and Mo additive, has a broader temperature range for existence of martensite within the secondary phase composition and lower tendency towards austenite decomposition with formation of cementite at low isothermal exposure temperatures. In this case in steel K60, containing more carbon, the proportion of M/A and residual austenite constituents (according to OM data and XD data) is higher in the ranges T1 and T2 that for steel K65, but austenite is less table and has a tendency towards decomposition with separation of cementite in the T3 temperature range.
Conclusion
The question of identifying M/A-constituent in the structure of high-strength pipe steels has been studied, and a procedure is proposed for determining the morphology of secondary phases, proportion of residual austenite, size, shape, and distribution of M/A-structure areas. The method of transmission electron microscopy (TEM) makes it possible to determine reliably the phase composition and morphology of M/A “islands”, but does not make it possible to provide quantitative evaluation of this constituent. The color etching method is suitable mainly for evaluating the volume fraction and size of residual coarse M/A “islands”. The X-ray diffraction method (XD) makes it possible to determine the proportion of austenite, including very fine areas, but does not make it possible to determine other phases. Therefore, combined study is required for structural steels by TEM, XD, and optical microscopy (OM) with etching in LePera reagent, and analysis of the images obtained using special software.
The effect of post-deformation cooling parameters has been studied: cooling rate and dwell time temperature for interrupted accelerated cooling temperature; exposure temperature and time; accelerated cooling parameters in the second stage (after exposure) on composition and morphology of M/A-constituent in the structure of low-carbon pipe steel of strength categories K60 and K65. It has been established that formation of the structure of a ferritic matrix and M/A-constituent is mainly affected by the temperature for completion of the first stage of accelerated cooling, determining exposure temperature. Exposure duration (in the test range 20–60 sec) does not have such a marked effect on proportion of M/A-constituent, and at the same time a short exposure does not give total occurrence of carbon redistribution processes, and too long exposure may lead to decomposition of austenite into ferrite and cementite. The effect cannot be overestimated of cooling rate and exposure temperature for cooling after conducting exposure: a slow cooling rate and high exposure temperature for cooling also leads to austenite decomposition into ferrite and cementite and reduces the proportion of M/A-constituent within the structure obtained.
For M/A-constituent in the form of “islands” or thin layers in a bainitic-ferrite matrix, depending on exposure temperature (with conditions for quite a low temperature for interrupted AC and quite a fast cooling rate) two boundary conditions have been revealed: with a high exposure temperature there is formation of predominantly twinned martensite, and with a low temperature there is pure residual austenite. With intermediate temperatures different combinations of these phases are observed, and in this case as there is a reduction in exposure temperature the proportion of martensite within the composition of islands decreases, but the proportion of austenite increases.
It has been established that in steel with the greatest austenite stability (K65) exposure temperature ranges facilitating formation of M/A “islands”, containing martensite and austenite, are broader. In this case according to XD data, in steel containing more carbon (K60) with comparable conditions the proportion of residual austenite is greater, but it is less stable and breaks down with formation of cementite phases.
Therefore, the experimental scheme developed has made it possible to determine conditions for microstructure formation, consisting of a matrix of bainitic ferrite and M/A-constituent of different morphology.
Temperate and time parameters have been determined for formation of a significant proportion of M/A-structure in steels K60 and K65 under conditions approaching the actual controlled rolling process with accelerated cooling. For both of the test steels the maximum proportion of M/A-constituent is of the order of 5– 5.5%, obtained with an isothermal exposure temperature of 550 ºC as a result of forming this component based on twinned martensite and residual austenite in pure form.
References
S. Zajac, V. Schwinn, and K.-H. Tacke, “Characterization and quantification of complex bainitic microstructures in high and ultrahigh strength line-pipe steels,” Proc. Intern. Symp. (San Sebastian, 2005), Materials Science Forum (2005).
V. Schwinn, P. Fluess, and D. Ormston, “Low carbon bainitic TMCP plate for structural and linepipe applications,” in: Recent Advances of Niobium Containing Materials in Europe, Verlag Stahleisen GmbH, Düsseldorf (2005).
M. Yu. Matrosov, I. V. Lyasotskii, A. A. Kichkina, et al., “Features and classification low-carbon low-alloy high-strength pipe steels structures,” Stal’, No. 1, 65–74 (2012).
V. Schwinn, P. Fluess, K.-H. Tacke, et al., “Bainitic steel plates for X100 and X120,” 4th Int. Conf. on Pipeline Technology (May 9–13, 2004. Ostend, Belgium) (2004).
D. Boyd, I. Yakubtsov, R. Zhang, et al., “Dual phase bainitic linepipe steels,” Pipelines for the 21st Century (August 21–24, 2005, Calgary, Alberta, Canada) (2005).
M. Diaz-Fuentes, A. Iza-Mendia, and I. Gutierrez, “Analysis of different acicular ferrite microstructures in low-carbon steels by electron backscattered diffraction,” Metall. Mater. Trans. A, 34A, No. 11, 2505–2516 (2003).
J. M. Reichert, T. Garcin, M. Militzer, et al., “Formation of martensite/austenite (M/A) in X80 linepipe steel,” 9th Int. Pipeline Conf. (2012), IPC2012–90465.
J. Shimamura, N. Ishikawa, S. Endo, et al., “Development of heavy wall X70 high strain linepipe steel,” Proc. of the 23 Int. Offshore and Polar Engineering (ISOPE) (June 30–July 5, 2013, Anchorage, Alaska, USA) (2013).
A. Cota and D. Santos, “Microstructural characterization of bainitic steel submitted to torsion testing and interrupted accelerated cooling,” Materials Characterization, 44, No. 3, 291–299 (2000).
F. Matsuda, et al., “Effect of M-A constituent on fracture behavior of 780 and 980 MPa class HSLA steels subjected to weld HAZ thermal cycles,” Trans. JWRI, 23, No. 2, 231–238 (1994).
F. Matsuda, et al., “Review of mechanical and metallurgical investigations of MA-constituent in welded joint in Japan,” Trans. of the JWRI (Japan Welding Research Institute), 24, No. 1, 1–24 (1995).
J. M. Reichert, W. J. Poole, M. Militzer, et al., “A new approach using EBSD to quantitatively distinguish complex transformation products along the HAZ of X80 linepipe steel,” Proc. 10th Int. Pipeline Conf. (2014), IPC’2014–33668.
F. S. LePera, “Improved etching technique to emphasize martensite and bainite in high-strength dual-phase steel,” J. Metall., 32, 38–39 (1980).
Author information
Authors and Affiliations
Corresponding author
Additional information
I. V. Lyasotskii is deceased
Translated from Metallurg, Vol. 62, No. 8, pp. 44–52, August, 2018.
Rights and permissions
About this article
Cite this article
Kichkina, A.A., Matrosov, M.Y., Éfron, L.I. et al. М/А-Constituent in Bainitic Low Carbon High Strength Steel Structure. Part 1. Metallurgist 62, 772–782 (2018). https://doi.org/10.1007/s11015-018-0719-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-018-0719-6