The possibility is demonstrated of adsorption extraction of vanadium compounds from acid media with modified aluminum silicate. Very fine layered aluminum silicate modified with surfactant cations (didecyldimethyl ammonium chloride) were used for extracting vanadium compounds. Sorbent modification proceeds as a result of fastening didecyldimethyl ammonium chloride molecules in the space between sorbent layers. As a result of modification, the sorbent acquires the property of selective absorption of vanadium compounds from solution. In order that the sorbent is not compacted and passes solution without hindrance, it is applied to quartz sand with a particle size of 1–3 mm treated with polyacrylamide. Vanadium compound adsorption proceeds most completely at pH 2.8–3.4, which is connected with the existence in the solution of various polyoxo compounds. The possibility is established of repeated sorbent use in adsorption and desorption cycles. The constant volume content of sorbent is 0.64 mmole/g, the dynamic exchange capacity is 0.38 mmole/g, and the static exchange capacity is 0.26 mmole/g. The solution after the desorption cycle is used for preparing pure vanadium pentoxide by hydrolytic precipitation under conditions of achieving the required concentration. Results of extracting vanadium compounds from model solutions correspond to vanadium adsorption on colloidal modified aluminum silicate from Chusovskoi Metallurgical Plant (ChMZ) washing solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The main industrial source of preparing vanadium is titanium magnetite ore containing vanadium as an impurity. During preparation of vanadium in hydrometallurgical production, it is necessary to separate it selectively from associated metal ions that pass into solution with acid breakdown of ore raw material. Vanadium in solution depending on medium concentration and pH forms many compounds [1,2,3].
Adsorption methods are most widespread in order to extract vanadium from solution [4, 5]. The selective adsorbents used normally are ion exchange materials of organic and inorganic nature. With the use of ionite FIBAN AK 22, with a linear solution passage rate of 5 m/h the maximum capacity is achieved with respect to vanadium compounds of 93 mg/g (0.51 mmole/g) [6].
Recently, work has appeared for use of different natural aluminosilicates as adsorbents [7]. Among them in the opinion of authors there is most interest in very fine modified aluminosilicates.
Data are provided in [9] for extraction of vanadium compounds from acid technogenic solutions by adsorption coagulation methods using natural montmorillonite (MM) modified with surfactant cations didecyldimethyl ammonium chloride (DDAC). For this, it was necessary to study adsorption of vanadium compounds in an inert charge with the sorbent indicated.
Experimental procedure. The natural very fine aluminosilicates used were bentonites of the Cherkasskii and Zyryanovskii deposits containing up to 95–96% MM of composition (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O (Table 1).
Modification of MM was accomplished in two stages. The first stage is treatment of bentonite with calcined soda with the aim of replacing calcium and magnesium ions by sodium ions, (sodium form of MM). Then, 50 g of MM were dissolved in 1 dm3 of distilled water and set aside for 24 h. Certain amounts of cationic surfactants were added to 5% aqueous MM solution with constant mixing. During adsorbent washing after modification, desorption of the modifier was absent, which points to the strength of its fastening within the adsorbent structure. Very fine MM systems were obtained with particle sizes of 50–60 nm and pore size of 5–6 nm. Adsorption of vanadium compounds was performed in model solutions of vanadium prepared using vanadium pentoxide (chemically pure) and sulfuric acid (especially pure). Adjustment of solution pH was carried out with ammoniacal water (28%).
On loading an adsorption column, washing out of sorbent and its compaction in the course of adsorption and desorption operation were excluded. For this purpose, the adsorption column was previously filled with quartz sand (d = 1–3 mm) treated with polyacrylamide. The very fine modified MM was added to the column in weight ratio with sand of 1:10.
Then, 200 ml of water was passed through the column in order to remove solvent particles not secured within the volume of the column. Then, 2 liters of vanadium solution with a concentration of 30 mg/dm3 were passed with a linear rate of 18 m/h. In the course of the process, samples 50 ml in volume were collected in order to monitor vanadium concentration in the final solution after passage through the column. After saturation of the whole volume of sorbent, desorption of vanadium compounds was carried out with ammonium chloride solution with concentration of 100 g/dm3. The fundamental layout for the adsorption unit is given in Fig. 1.
The kinetics of the adsorption process for vanadium compounds on a column are characterized by the relationship shown in Fig. 2.
The first linear section of the dependence of vanadium equilibrium concentration on volume of solution passed (see Fig. 2) was used for determining the static (equilibrium) volume capacity (SVC) of adsorbent “up to breakthrough”, i.e., up to development of the first appearance of vanadium ions in filtrate. The SVC of modified MM was 0.26 mmole/g.
The next inclined section of the curve for the dependence was used for calculating the dynamic volumetric capacity (DVC) equal to 0.38 mmole/g. The sum of the DVC and SVC reflects the overall volumetric capacity (OVC) of sorbent equal to 0.64 mmole/g, which may also be calculated from the results of sorbent regeneration.
The DVC specifies action of sorbent with constant adsorbent-regeneration cycles and points to its efficiency. This parameter depends directly in medium pH, regenerating agent concentration, column construction, and sorbent content time with solution. The OVC for each sorbent is a constant value representing the number of active adsorption centers (functional groups) related to dry sorbent weight.
The SVC is a process parameter specifying the ratio of solution volume and sorbent weight. This index depends on ion concentration and medium pH and is used in calculating actual process parameters.
It been demonstrated in [9] that reaction of modified MM and vanadium poly-anions proceeds according to the ionic associate type, and therefore during sorbent regeneration replacement of a vanadium poly-anion by a chloride-ion proceeds without any difficulty. A considerable advantage of the process is the possibility of increasing the vanadium concentration in process solution by more than a factor of three (100 mg/dm3) compared with the original (30 mg/dm3). The curve for desorption of vanadium compounds by ammonium chloride is shown in Fig. 3.
The sorbent total volumetric capacity (TVC) was determined using the data obtained (see Fig. 3). The value of TVC equals the area beneath the curve (0.64 mmole/g), which coincides with the value of TVC calculated as the sum of DVC and SVC. The volume of modified MM with the column version of vanadium adsorption is less than the volume by a factor of 2.5 obtained by the volumetric method. The main factor affecting divergence of results obtained by different procedures is diffusion of vanadium compounds to active sorbent centers. If the flow rate is gradually reduced, the amount of sorbent is increased by a ratio with respect to inert carrier these values with converge asymptotically.
After regeneration, the sorbent may be used again for extracting vanadium compounds. The solution after regeneration contains ammonium vanadate and ammonium chloride, i.e., vanadium may be separated from solution by hydrolytic precipitation [2]:
Previously solution was acidified with hydrochloric acid to pH 1.6 since in fact with this value of pH reaction of hydrolytic precipitation proceeds more completely (the process temperature was 80°C). Polyvanadium acids were separated from solution in the form of a reddish-brown deposit. With creation of optimum hydrolysis conditions, the residual content of vanadium in solution after precipitation is 0.03–0.05 g/dm3.
Polyvanadium acid on heating to 300°C breaks down into vanadium pentoxide and water. An IR-spectrum of residue obtained after washing sorbent, concentration, and drying at 60°C is given in Fig. 4.
The main part of the hydration water is molecular water with hydrogen bonds for which there are typically different energies (Fig. 4). Therefore, in the spectrum intense regions are present for absorption of 2800–3600 cm–1, typical valence oscillations and less intense regions of absorption 1610–1640 cm–1, typical for deformation oscillations [10].
For vanadium, there are typically intense adsorption bands in the short-wave region. Within the spectrum for vanadium poly-acids, absorption bands should be observed at 760 and 530 cm–1 [11], which corresponds to deformation oscillations (V–O···H) and deformation oscillations (V–O–V). The absorption bands are present in the spectrum in Fig. 4. However, the spectrum in the short-wave region is complicated to analyze since it is nit separated into individual peaks that most probably is connected with the combined deposition of a number of vanadium poly-acids: 3V2O5·2Н2O (H4V6O17), V2O5·Н2O (HVO3), V2O5·2Н2O (Н4V2O7), V2O5·3Н2O (H3VO4), V2O5·0.5Н2O (Н2V4O11), Н6V10O28. Within the structure of these compounds, there is a considerable amount of hydration water, which is removed at 300°C.
Sorbent MM after regeneration was transferred again into a highly dispersed conditions and was suitable for repeated use. After five cycles, the absorption properties did not deteriorate.
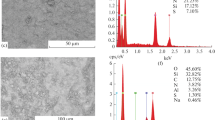
The high efficiency of vanadium compound absorption on modified MM is confirmed by results of studies in treating washing water of the Chusovskoi Metallurgical Plant (ChMZ). With an original vanadium concentration of 0.18 g/dm3, extraction of vanadium was 95.5%. The final product contained phases V2O5 (95.2%) and MnV2O6 (4.8%). The weight content of vanadium in terms of pentoxide was 99.01%.
Conclusions
-
1.
The possibility has been demonstrated of modifying sorbent based on montmorillonite (MM) for selective extraction of vanadium from solution.
-
2.
Good efficiency has been established for absorption of vanadium compounds on modified MM. Absorption is due to the formation in surface layers of compounds of vanadate ions with amines of the ionic associate type.
-
3.
During sorbent regeneration, vanadate ions are replaced by chloride ions by an ion exchange mechanism, and vanadium anions are removed from the sorbent surface by washing in a solution of ammonium chloride.
-
4.
During absorption of vanadium compounds on modified MM, the value of total volume capacity is 0.64 mmole/g with a linear solution passage rate of 18 m/h, which is 20% better than the capacity of ionite AK-22 (0.51 mmole/g with a linear solution passage rate of 5 m/h).
References
A. A. Fotiev, B. V. Slobodin, and M. Ya. Khodov, Vanadates. Composition, Synthesis, Structure, Properties, Nauka, Moscow (1988).
E. M. Rabinovich and V. G. Mizin, Comprehensive Treatment of Vanadium Raw Material: Metallurgy, UrO RAN, Ekaterinburg (2005).
V. N. Muzgin, L. B. Khamzina, V. L. Zolotavin, and I. Ya. Bezrukov, Vanadium Analytical Chemistry, Nauka, Moscow (1981).
L. Zhang, X. Liu, W. Xia, and W. Zhang, “Preparation and characterization of chitosan-zirconium (IV) composite for adsorption of vanadium (V),” Int. J. Biol. Macromol., No. 64, 155–161 (2014).
T. Wang et al., “The influence of vanadate in calcined Mg/Al hydrotalcite synthesis on adsorption of vanadium (V) from aqueous solution,” Chem. Eng. J., 181, 182–188 (2012).
S. U. Nve, A. V. Shilaev, and I. D. Troshkina, “Absorption extraction of vanadium from mineralized solutions with fiber ionite,” Usp. Khim. Tekhnol., 26, No. 6 (135), 126–129 (2012).
A. V. Sviridov, E. V. Ganebnykh, G. I. Mal’tsev, and K. L. Timofeeev, “Cleaning industrial effluent with aluminosilicate sorbents,” Tsvet. Met., No. 12, 42–47 (2015).
V. I. Vigdorovich, L. E. Tsygankova, V. V. Nikolenko, and A. I. Akulov, “Extraction of copper (II) ions and phenol in flowing solution of glauconite of the Bandar area of the Tambov region,” Sorb. Prom. Protsessy, 10, No. 6, 930–937 (2010).
D. P. Ordinartsev, A. V. Svirodov, S. S. Naboichenko, and V. V. Sviridov, “Absorption extraction of vanadium from acid solutions,” Butler. Soobsh., 46, No. 2, 22–28 (2016).
A. V. Sviridov, E. V. Ganebnykh, and V. A. Elizarov, “Aluminosilicate sorbents in water cleaning technology,” Ekol. Prom. Rossii, No. 11, 28–30 (2009).
V. L. Volkov, Interstitial Phases Based on Vanadium Oxides, UNTs AN SSSR, Sverdlovsk (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 10, pp. 79–82, October, 2017.
Rights and permissions
About this article
Cite this article
Ordinartsev, D.P., Sviridov, A.V., Naboichenko, S.S. et al. Sorption Extraction of Vanadium Compounds from Acid Solutions with Finely Divided Modified Aluminosilicate. Metallurgist 61, 912–916 (2018). https://doi.org/10.1007/s11015-018-0586-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-018-0586-1