The nature of corrosion process development is studied by observing surface condition with potentiometric polarization. It is established that an increase in martensite content within steel structure increases the intensity of corrosion process development considerably. The high sensitivity of the corrosion test method developed to steel structural state (structural component content), and also prospects for using it instead of tests in accordance with GOST 9.911−89 are demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The corrosion resistance of automotive sheet steels is one of the key service properties governing operating reliability and endurance of the objects manufactured. In spite of extensive use in automobile building of components with protective coatings, it is impossible to ignore the corrosion of automotive sheet steels since in the case of coating damage in areas of defect occurrence corrosive processes may proceed at an extremely high rate. In view of this an important task is development of adequate procedures for diagnosing automotive sheet steel resistance to overall and local corrosion. This is especially important for new high-strength multiphase nanostructured automotive sheet steels, since they are intended for preparing the most critical automobile assemblies and other means of transport, building, and engineering structures.
Proceeding from operating features of components made from automotive sheet steels it may be concluded that the most adequate method for evaluating their corrosion resistance is testing according to GOST 9.911−89 “Steel Atmosphere Resistance.” The essence of the test includes exposure of test steel specimens in a sealed chamber in an atmosphere of sulfur dioxide for 72 h, after which specimens are subjected to 48 h of testing by alternating immersion: 10 min exposure in aqueous H2SO4 solution (pH 5) followed air drying in air for 50 min. After the end of a test for corrosion resistance it is possible to judge from a series of properties, including specimen weight loss (g), corrosion rate (mm/year), average pitting depth (μm), etc. The main disadvantage of corrosion tests in accordance with GOST 9.911−89 is their duration, more than five days. It is apparent that use of this method is limited, particularly under industrial production conditions.
The task of the present work was creation of a procedure for determining corrosion resistance of multiphase automotive sheet steel no worse with respect to quality than results obtained by GOST 9.911−89, and more rapid and less labor-consuming.
The majority of existing methods for corrosion tests give special attention to electrochemical methods distinguished by simplicity of implementation and a relatively short test time. In addition, these methods for studying corrosion resistance of steels and alloys have been used as the main methods over a prolonged period, and a considerable amount of data have been accumulated for using different measurement methods, the equipment required, and so forth [1, 2].
In the majority of electrochemical methods for corrosion tests the dependence of current strength (density J) on potential I = ƒ(E), or polarization curves, are determined. Construction and analysis of these dependences makes it possible to determine the nature of processes occurring at an electrode/electrolyte phase interface, and a number of important corrosion properties of a test steel or alloy such as current strength i cor (density j) and corrosion potential (E cor), potential of pitting formation φpi, repassivation φrp, and others.
In order to resolve the stated problem polarization curves were studied that were obtained in the course of potentiodynamic polarization of specimens in the range of potentials (E) from −1000 to 300 mV with a rate of potential change on 1 mv/sec. The electrolyte composition is similar to that used in tests according to GOST 9.911−89. i.e., an aqueous solution of 5·10−6 mol/dm3 H2SO4.
Generally steel corrosion resistance is determined by such factors as chemical composition, nonmetallic inclusion (NI) content, and structure type. The content of the main elements, impurities, NI, governing the intensity of corrosion process development depends primarily on steel melting parameters. The structural state of steel is determined by rolling and heat treatment regimes that are selected proceeding from specification of mechanical properties. It is well known that the strength of automotive sheet steels increases with an increase in the proportion of martensite within the structure, and therefore for steel of a single composition it is possible to obtain rolled product of different strength classes by controlling structure formation processes. With an identical composition the content of impurities and NI, rolled product of different strength classes differs exclusively with respect to steel structure. In accordance with this situation, the procedure developed for determining corrosion resistance of multiphase automotive sheet steels should be sensitive to structure type.
Research was performed on metal melted in a laboratory vacuum induction furnace with a capacity of 30 kg with steel pouring into two billets. Experimental melt composition was as follows (wt.%): C 0.09, Si 0.4, Mn 1.54, P 0.006, S 0.008, Cr 0.62, Ni 0.045, Мо 0.08, Cu 0.12, Al 0.020, Ti 0.002, V 0.021, Nb 0.001, N 0.005.
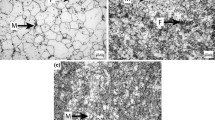
Billet rolling was carried in a duo-300 laboratory single-stand rolling mill by different regimes. Rolling of the first billet was performed at 1250°C (roughing rolling), after which sheet was heated in an intermediate furnace to 1050°C and the second rolling stage (finish rolling) was carried out with a temperature at the end of rolling T fr above 850°C. The rolled product obtained was cooled rapidly to 200°C, and strip winding into a coil was simulated by soaking in a furnace at 350°C for 60 min and subsequent furnace cooling. The structure of metal specimen No. 1 after rolling was ferritic-martensitic, with a ferrite volume fraction F = 80%, and martensite M = 20% (Fig. 1a ).
The rolling regime for the second billet was similar to that given above, with a difference that after finish rolling it was oil quenched from a temperature ≈900°C. The structure of specimen No. 2 rolled product and after rolling and quenching was almost entirely martensitic, i.e., its proportion exceeded 95% (Fig. 1b). Thus, from steel of one composition with an identical impurity and NI content specimens rolled product of two types were prepared (Nos. 1 and 2) with a different structure. In order to perform corrosion tests specimens were cut from them with a size of 25 × 40 mm. Specimens were polished to a mirror finish. A copper lead in insulation was soldered to a specimen for the possibility of connection to an electric circuit. After this a specimen was degreased and a working section was separated, i.e., a square with a side of 6–8 mm. The rest of a specimen surface was carefully insulated with liquid paraffin. Then a specimen with a separated working surface was placed in a Petri dish and connected by means of the copper lead to an IPC-Pro universal potentiostat/galvanostat. The end of a silver chloride reference electrode was placed at a distance of 2–3 mm from the specimen working surface. A graphite rod was used as the subsidiary electrode. The Petri dish with the installed electrodes was placed beneath the eyepiece of an inverted-stage microscope whose construction made it possible to observe the specimen surface through a layer of electrolyte during polarization. After assembly of the Petri dish with a specimen and electrodes it was filled with electrolyte (aqueous solution 5·10−6 mole/dm3 H2SO4) and a potential of E = −1000 mV was applied. With this potential a specimen was in the cathodic region, indicated by negative current values. The external appearance of a Nos. 1 and 2 specimen surface after 300 sec exposure with E = −1000 mV is given in Fig. 2. Polarization was started from a potential of E = −1000 mV in the anodic direction at a rate of 1 mV/sec, and ceased on reaching E = 300 mV. Specimen surface condition was observed during polarization. For greater clarity in the solution above the working surface several droplets of potassium (II) hexacyanoferrate (potassium ferrocyanide) were added as an indicator for presence of Fe3+ ions. The main task of observation was determination of the potential for the start of corrosion and its nature (see Table 1).
From data of Table 1 it follows that appearance of visible surface corrosion traces for both specimens are recorded with similar potential values, but the nature of corrosion is different. Corrosion of the surface of specimen No. 1 proceeds slowly, i.e., barely distinguishable areas of dissolving matrix are seen. At the surface of specimen No. 2 with a potential greater than E cor start clearly distinguished corrosion sites appear that rapidly occupy the whole working surface area (Fig. 3). Blue areas at a specimen surface (result of reaction of potassium (II) hexacyanoferrate and Fe3+ ions) (“Prussian blue”) are areas where there is transfer of iron ions into solution, i.e., the steel corrodes.
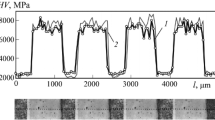
It is clearly seen (see Fig. 3) that the amount and size of blue areas at the surface of specimen No. 2 is much greater than at the surface of specimen No. 1. At the instant of the end of polarization with E = 300 mV) specimen No. 2 is in a condition of active dissolution with almost linear dependence of current density on potential (Fig. 4). The maximum current density for specimen No. 2 with a martensitic structure (J max = 1.61 mА/cm2) significantly exceeds the current density (J max = 0.6 mА/cm2) for specimen No. 1 with a significantly smaller proportion of martensite (20%) within the structure (see Fig. 4). Since specimens of rolled Nos. 1 and 2 were prepared from steel of the same melt, then it is apparent that the difference recorded in maximum current density is due to their different structure, mainly by a different proportion of martensite. This confirms the sensitivity of the procedure developed for corrosion tests of multiphase automotive sheet steels towards the structural state (content of different structural components) of steel.
For proof of the adequacy of the procedure developed the data obtained were compared with results of corrosion for specimens Nos. 1 and 2 according to GOST 9.911−89. In order to estimate the degree of local corrosion development a “maximum pitting depth” index was used. It was determined as the average arithmetic result of measuring the depth of the deepest pits. Measurement was performed in an optical microscope by double-focusing. No fewer than 40 pits were measured in each specimen. The maximum pitting depth for specimen No. 2 was 42 μm, and for specimen No. 1 it was 24 μm. Results were compared with electrochemical test data (Fig. 5).
Results obtained by the two different methods are in good agreement. This points to the reliability of the procedure developed and the possibility of its use instead of corrosion tests according to GOST 9.911−89. Time and labor consumption are reduced in performing corrosion tests with greater information content and comparable quality for results.
Conclusions. The nature of corrosion development has been studied for multiphase automotive sheet steels with a different structure using a method of direct observation for specimen surface condition with potentiodynamic polarization.
It has been shown that an increase in martensite content within a steel structure considerably increases the intensity of corrosion development.
A procedure has been developed for electrochemical determination of the corrosion resistance of multiphase automotive sheet steels having high sensitivity towards steel structural state (structural component content).
The promising nature and advantages of using the method developed instead of testing by GOST 9.911−89 have been demonstrated.
References
L. I. Freiman, V. A. Makarov, and I. E. Bryksin, Potentiostatic Methods in Corrosion Research, Khimiya, Moscow (1972).
Z. Galyus, Theoretical bases of Electrochemical Analysis [Russian translation], Mir, Moscow (1974).
This work was performed within the scope of an agreement for supply of a subsidy No. 14.625.21.0008 of 10.23.2014. Unique PNI identifier (project) REMEF162514X0008.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Metallurg, No. 12, pp. 54–57, December, 2015.
Rights and permissions
About this article
Cite this article
Kazankov, A.Y., Zaitsev, A.I., Rodionova, I.G. et al. Study of Multiphase Automotive Sheet Steel Local Corrosion Resistance by Electrochemical Methods. Metallurgist 59, 1204–1208 (2016). https://doi.org/10.1007/s11015-016-0238-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-016-0238-2