The effect of adding aluminum on steel specific strength and corrosion resistance is studied. It is established by calculation that aluminum is the most effective element for reducing steel density. The effect of aluminum content on steel specimen corrosion resistance is studied by electrochemical methods. The possibility of improving steel corrosion resistance by alloying with aluminum in an amount of 8–12% is demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Development of the very abundant hydrocarbon raw material deposits within the Russian Federation is carried out under increasingly extreme natural and climatic conditions, including the Arctic shelf. Apart from equipment and other technology objects intended for recovery, storage, and shipping of gas and oil, there is also an increasing requirement for other structures built for various purposes in order to create a required infrastructure both within the Arctic and Antarctic. Features of natural and climatic conditions of these regions give rise to special requirements for metal products for these objects with the aim of providing prolonged operating life. These conditions involve not only extreme temperatures and related thermal and mechanical loads, but also active operation of corrosive media, i.e., sea water, inorganic bioactive media, etc.
In order to provide prolonged operating life under the conditions in question, steels are required that should exhibit apart from a set of good mechanical property indices good resistance to different forms of corrosion and corrosion-mechanical breakdown. It is also important to resolve the question of reducing equipment structural weight, which may be achieved by improving the specific strength of the steels used.
The main approach to resolving questions of improving steel specific strength [1–3] includes not only an increase in strength properties, but also a reduction in density, and this is achieved by alloying with such elements as aluminum and silicon.
An approximate empirical formula has been given in [4] for calculating steel density (see formula (1)). Steel density of different grades may be determined approximately by varying the density of pure iron (7880 kg/m3) by a value proportional to the amount (%) of alloying component within steel, in accordance with the values of corrections for one per cent of impurity provided in Table 1.
If these corrections are calculated as a percentage of iron density (7880 kg/cm3), then we obtain:
Thus, alloying with aluminum reduces steel density most strongly, i.e., by 1.97% for each per cent of aluminum in steel (Table 2).
According to data in [1], as a result of alloying steel with aluminum with a content of 14–28% Mn and 1% C steel density is reduced by 17% with addition of 12% aluminum, and this means a reduction in density by 1.4% for 1% of aluminum, but a change in manganese content gave rise to a small effect on steel density compared with changing aluminum content.
Another important advantage of alloying steel with aluminum is the possibility of improving steel corrosion resistance. As for chromium, aluminum may form protective films at a steel surface. According to data in [5], alloy Fe–8% Al exhibits the same oxidation resistance as 20%Cr–80%Ni alloy. At the same time, in [6] a low property level has been noted for these steels, i.e., strength and ductility, and these disadvantages are overcome by means of alloying with chromium.
Data for the effect of aluminum in combination with some other elements on steel and alloy corrosion resistance based on iron, including in sea water, are contained in more recent work [7–9]. It is noted that in spite of a marked reduction in overall steel corrosion rate within sea water an increase in chromium content of more than 3–5% leads to the occurrence of pitting corrosion, but this may be prevented by simultaneous alloying with other elements (molybdenum, nickel, copper). A marked increase in corrosion resistance in sea water and chloride solutions is observed on alloying steel with aluminum, especially together with chromium. The corrosion resistance of steel containing 2% Cr increases with an increase in aluminum content up to 0.4%. In the opinion of the authors in [10], steel alloying with aluminum is desirable up to 3–5%.
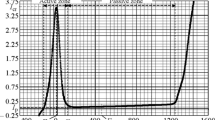
A study of alloys of the Fe–Al system has been the subject of many papers [11–18]. In particular, in [17, 18] corrosion resistance of alloys of Fe–Al–Cr with 10 wt.% Al w\s studied in oxidizing and sulfide-oxidizing atmospheres at 500°C. It may be seen from the curves provided in Fig. 1 that in order to provide corrosion resistance at elevated temperature in oxidizing and sulfide-oxidizing atmospheres alloy of this system Fe–10 wt.% Al should additionally be alloyed with chromium. Alloy Fe–10 wt.% Al–5 wt.% Cr showed almost no weight loss in both atmospheres for 500 h of testing.
Corrosion resistance of alloys of the Fe–10%Al–Cr system at 500°C [17].
Nonetheless, currently there are no systematized data for the effect of aluminum on steel corrosion resistance in sea water and generally in aqueous media with an increased chloride content. Recently, a series of electrochemical methods have been developed, making it possible to evaluate corrosion resistance of carbon and low-alloy steels in aqueous media containing chlorine ions [19].
The aim of this work is estimation of the effect of aluminum content in alloys based on iron on their corrosion resistance indices, determined according to the Bardin TsNIIchermet enterprise standard STO 00190242-001–2008.
The method involves derivation of a potentiodynamic curve (PDC) for direct and reverse path in aqueous media, containing chlorine ions, and determining values of parameters making possible to evaluate steel resistance to local corrosion.
Parameters determined with PDC derivation:
-
maximum current density i max, mA/cm2;
-
current density at potential –300 mV (i –300), mA/cm2 (saturated silver chloride electrode); and
-
current density maximum potential E imax, mV.
Depending on values of these parameters in accordance with the procedure steel is classified in one of three classes for local corrosion resistance: 1 is stable, 2 is satisfactorily stable, 3 is unstable. Ranges of values of parameters determined, recorded for each class, are provided in Table 3.
Melting of binary alloys of the Fe–Al system was performed in vacuum and open induction furnaces from pure material charges (iron grade ZhR008; lump aluminum). For vacuum melting of binary Fe–Al alloys, a Balzer vacuum furnace was used with a capacity of 20 kg, and pouring was carried out into two ingots weighing 10 kg each. Metallurgical finishing of ingots was carried out by a scheme: partial cleaning of ingots with abrasive wheels, forging at 1200°C to sheet bar with a size of 40 × 100 × L mm, shaving (or total abrasive cleaning) of sheet bar, hot rolling at 1200°C to strip 6 and 2.5 mm thick.
In order to study binary Fe–Al–alloys, they were also melted in an open induction furnace with a capacity of 10 kg. Melting was performed under cryolite slag, ingots were cast weighing 10 kg. Metallurgical finishing of ingots was carried out by a scheme: total cleaning of ingots with an abrasive wheel, forging at 1200°C into sheet bar with a size of 40 × 100 × L mm, shaving of sheet bar, hot rolling at 1200°C into strip 6 and 2.5 mm thick.
The chemical composition of alloys of the Fe–Al system is provided in Table 4.
It is seen that alloys of vacuum melting contain apart from iron and aluminum silicon in an amount of not more than 0.07%. Alloys melted in an open arc furnace had a higher silicon content, i.e., 0.18–0.60%. In addition, alloy with conditional number 3 had an increased manganese and phosphorus content compared with the rest of the alloys (see Table 4).
Specimens for the study were cut from hot-rolled strip. The specimens’ heat treatment was carried out in a vacuum furnace with a degree of rarefaction of not less 10–3 mmHg, temperature maintenance accuracy ±0.2°C. The specimens’ quenching was carried out with heating in an open tubular furnace at 1000°C, accuracy of temperature maintenance ±0.5°C.
Before performing corrosion tests, half of the specimens were heat treated, i.e., soaked at 850°C for 30 min followed by cooling in boiling water, which according to the phase diagram for the Fe–Al system (Fig. 2) should provide a more uniform ferrite structure without intermetallics and areas of layering.
Results of specimen testing before and after heat treatment (average of values for two specimens for each alloy and condition) are provided in Table 5 and in Fig. 3.
The main steel corrosion resistance index, not inclined towards passivation in the potential ranges considered, is the maximum value of current density. The dependence of this property on aluminum content is provided in Fig. 3.
It is seen that with an increase in aluminum content, particularly up to 8% or more, corrosion resistance of Fe–Al–alloys prepared in a vacuum furnace increases both for hot-forged and also for heat-treated specimens. Steel resistance with a similar aluminum content does not differ significantly for hot-forged and heat-treated specimens, which points to the specific contribution of chemical composition. Some steel structural inhomogeneity with a high aluminum content after hot rolling (in particular, the presence in the ferrite matrix of intermetallic particles) did not have an unfavorable effect on steel corrosion resistance.
Testing of alloy specimens prepared in an open arc furnace demonstrated markedly better corrosion resistance compared with specimens prepared in a vacuum furnace.
Resistance to local corrosion of all specimens independent of aluminum concentration, corresponded to the first class, and values of current density appeared to be less than 2 mA/cm2 for specimens after heat treatment and less than 1 mA/cm2 for specimens without heat treatment. Possibly, the appearance in Fe–Al alloys of silicon leads to protective film formation considerably more resistant than in alloys without silicon. This makes it possible to provide good corrosion resistance with low aluminum concentrations.
Conclusion. Thus, the results obtained point to a possibility of improving steel corrosion resistance by alloying with aluminum in an amount of 8–12%. It is expedient to continue research in the direction of optimizing the content of other elements in order to improve not only corrosion resistance, but also mechanical properties.
References
G. Frommeyer and U. Brux, “Microstructures and mechanical properties of high-strength Fe–Mn–Al–C light-weight TRIPLEX steels,” Steel Res. Int., 77, 627–633 (2006).
R. Spina and L.Tricarico, “Laser welding of aluminum-steel clad materials for naval applications,” in: Laser Welding, Italy (2010), pp. 77–107.
W. Szkliniarz, E. Hadasik, A. Koscielna, and I. Schindler, “Melting, casting and rolling problems in Fe–Al based alloy,” METAL, 1–8, (2004).
B. Cox, Corrosion, 18, 33 (1962).
G. G. Ulig and R. U. Revi, Corrosion and Combatting It: Introduction to Corrosion Science and Technology, Khimiya, Leningrad (1989), pp. 204–207.
K. Satoshi and W. Tuneyash, “Sea water corrosion of low alloy steels,” Boshoku Gijutsu, Corros. Eng., 25, No. 3, 173–190 (1976).
V. T. Abakov,V. P. Kharchevnikov, and D. A. Litvinenko, “Production properties and application of atmospheric corrosion resistant steels,” Stal, No. 11, 1042–146 (1978).
J. Peterson, “Das Verhalten von Ironbauteilen in Meerwasser,” Werkst. und Korros., 28, No. 11, 248–254 (1997).
H. Coriou, L. Grall, C. Mahieu, et al., “Comportment a la corrosion par J’eau de mer d’aciers faiblement allies ferchromealuminium,” Proc. 5th Int. Symp. Fresh Water from the Sea, Athens, (1976), pp. 365–372.
M. Jablonska, K. Rodak, and G. Niewielski, “Characterization of the structure of Fe–Al alloy after hot deformation,” J. Achiev. Mater. Manuf. Eng., 18, 107–110 (2006).
I. Bednarczyk and M. Jablonska, “Plasticity of low aluminum alloys from Fe–Al system,” Arch. Met. Mater., 57, No. 1, 271–276 (2012).
Z. Belamri, D. Hamana, I. S. Golovin, and I. B. Chudakov, “Study of ordering in Fe–25%Al–Cr Alloys by dilatometry, heat flow and mechanical spectroscopy,” Metallofiz. Nov. Tekhnol., 35, No. 2, 209–223 (2013).
H. Yasuda, K. Fukushima, K. Kouzai, and T. Edahiro, “Effect of Ni doping on strength and damping capacity of Fe–Al alloys,” ISIJ Int., 53, No. 4, 704–708 (2013).
I. Aaltio, K. Ullakko, and H. Hänninen, “Properties of Fe–Al–Si high-damping steel,” Smart Struct. Mater., 2720, 378–387 (1996).
V. S. Raja, “High temperature oxidation behaviour of carbon containing two phase iron aluminides and Fe–Al alloys,” Trans. Indian Inst. Met., 57, No. 5, 525–535 (2004).
J. R. Regina, J. N. DuPont, and A. R. Marder, “Gaseous corrosion resistance of Fe–Al-based alloys containing Cr additions. Part I: Kinetic results,” Mater. Sci. Eng., A 404, 71–78 (2005).
J. R. Regina, J. N. DuPont, and A. R. Marder, Weldability of Fe–Al–Cr Overlay Coatings for Corrosion Protection in Oxidizing/Sulfidizing Environments, Research under subcontract 19X-SU604V with UT Battelle Research Corp. Lehigh University Energy Research Center (2003).
I. G. Rodionova, A. I. Zaitsev, O. N. Baklanova, et al., Contemporary Approaches to Improving Corrosion Resistance and Operating Reliability of Steels for Oil Industry Pipelines, Metallurgizdat, Moscow (2012).
The work was carried out within the scope of subsidy agreement No. 14.624.21.0001 from 08.11.2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, No. 12, pp. 63–67, December, 2014.
Rights and permissions
About this article
Cite this article
Rodionova, I.G., Baklanova, O.N., Udod, K.A. et al. Evaluation of the Effect of Aluminum Content on Steel Corrosion Resistance Indices and Specific Strength. Metallurgist 58, 1098–1104 (2015). https://doi.org/10.1007/s11015-015-0046-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-015-0046-0