The physicochemical properties, hardening, and corrosion resistance of austenitic chromium-nickel steels of type Kh18AN10 with different contents of impurities and different chromium concentrations (from the level of impurity to 0.220%) and of chromium-nickel-manganese steel Kh18N5AG9M2 are investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Austenitic steels of type Kh18AN10 are used widely as corrosion-resistant, heat-resistant, and cryogenic materials [1–8].
The complexity and the multifactor nature of the action of nitrogen are responsible for the absence of precise criteria for choosing its rational content and the structure to be obtained for steels intended for various purposes.
Nitrogen, just like carbon, provides solid-solution hardening and precipitation hardening of steels due to formation nitrides and carbonitrides. Since the diameter of a nitrogen atom is somewhat smaller than that of carbon, it provides stronger solid-solution hardening and strain hardening.
Nitrides in chromium-nickel steels are finer and more uniformly distributed than carbides, which makes it possible to obtain high characteristics of strength, ductility and toughness simultaneously.
Another effective means is grain-boundary hardening of nitrogen steels due to precipitation of fine nitrides and carbonitrides over boundaries and sub-boundaries and formation of finer grains under heat treatment.
Stable chromium-nickel and chromium-manganese austenitic steels alloyed with nitrogen are often hardened by cold plastic deformation and aging.
Increase in the content of N and N + C intensifies supersaturation of the solid solution and may enhance the effect of aging during the deformation and after it. In its turn, this may change the processes of structure formation and worsen the corrosion and other special properties. Specifically, the long-term strength may be lowered as a result of precipitation of nitrides and carbonitrides Cr23(CN)6, Cr2(CN). In this case it is necessary to correct the alloying in order to preserve the properties the steel, i.e., reduce the nitrogen content or introduce additionally other alloying elements.
Thermomechanical treatment is also effective for nitrogen-containing austenitic steels if applied in a rational regime. The heating temperatures in high-temperature thermomechanical treatment of such steels may be higher than those used for nitrogen-free steels [9, 10].
Alloying of a steel with nitrogen raises the resistance to local kinds of corrosion and to intercrystalline corrosion [4–8]. However, the corrosion strength is sensitive to the structural state of the steel, and therefore it is necessary to allow for the specific temperatures of precipitation and dissolution of nitrides.
The aim of the present work was to study the effect of nitrogen alloying on the hardening and corrosion resistance of austenitic chromium-nickel steel of type Kh18AN10 of different purity and of chromium-nickel-manganese steel Kh18N5AG9M2 under the conditions when nitrogen is located in the solid solution.
Methods of Study
We studied specimens of steels of type Kh18AN10 with 0.186 and 0.220% nitrogen, of chromium-nickel-manganese steel Kh18N5AG9M2 with 0.240% nitrogen and, for comparison, of steel 12Kh18N10T with chemical compositions presented in Table 1.
The steels were produced and treated preliminarily as follows. Steel 1 (12Kh18N10T) of commercial production was studied in hot-rolled condition. The nitrogen-alloyed steels were melted under laboratory conditions using blend components of different purity. Steel 2 (Kh18AN10 with 0.186% N) was melted in an induction furnace by remelting a commercial nitrogen-free steel of a close chemical composition with an addition of pure blend components to the required composition. Steel 3 (Kh18AN10 with 0.220% N) and steel 4 (Kh18N5AG9M20) were melted in a vacuum induction furnace from pure blend materials, i.e., electrolytic iron of grade 008ZhR (steel 3 ) or armco iron (steel 4 ), electrolytic nickel N1, electrolytic manganese Mn998, pure chromium Kh99 nitrided with ferrochrome FKhN8, and granulated aluminum.
The ingots were subjected to hot forging and subsequent hot rolling. The finishing treatment was the same for all the steels, i.e., quenching (treatment for solid solution) from the austenitic range from 1050°C in water.
The mechanical properties of all the steels were determined [11] by tensile tests of specimens in accordance with the GOST 1497–84 Standard.
The thermal conductivity was determined in a special device in the temperature range of 20 – 100°C with the help of a differential electrodeless thermocouple with plotting the temperature and time dependences. One end of the specimen was placed into boiling water with temperature T 2 (i.e., T 2 = const = 100°C). The rod itself was placed into foamed plastic to eliminate the heat exchange between the side surface and the ambient. The temperature of the second end of the rod was measured with a thermocouple by determining the time intervals t in which it attained a constant value. At the initial moment of time the rod had a temperature T 0 , then the temperature started to grow and tended asymptotically (at t = ∞) to value T 2 . The thermal diffusivity was determined by the formula
where T 3 is the temperature measured on the second end of the rod at moment t 3 ; L is the length of the rod.
The thermal expansion factor and the effects of the phase transformations were determined with the help of a DIL805 dilatometer in the following test mode: heating from room temperature to 1200°C at a rate of 5 K/sec, holding for 1 min, and cooling at a rate of 50 K/sec to 50°C.
We estimated the resistance of the steels to total, intercrystalline and pitting corrosion in various environments. The tests were performed with the use a Zive MP2 electrochemical station.
The susceptibility to total corrosion was determined in sea water (3% NaCl) and in an acid medium (0.5-M H2SO4), including blasting with H2S. We determined the potentials and the critical density of the passivation current. The test involved evaluation of the free corrosion potential (E fc) by the potentiostatic method (with 3-h hold), plotting of the polarization curve from negative values of the potential (from the cathode region) to the repassivation region (in an acid medium), or to the potential of formation of steady pits (in sea water). The critical passivation potential (E cr) was understood as a potential corresponding to the maximum current density in the zone of active dissolution; the total passivation potential (E t) was understood as a potential after which the scanning current density decreased by no more than 1 μA/cm2 (for 0.5-M H2SO4) or as a potential at which the current density was equal to 3% of the critical one (for 0.5-M H2SO4 + H2S); the critical passivation current density i cr was determined as the current density at the critical passivation potential, and the current density in the passivation range i p was determined as a minimum current density in the passivation range.
The repassivation potential was understood as a potential, the growth of which was not accompanied by decrease in the current density; for the steels studied the threshold value of the current density was about 5 μA/cm2 (for 0.5-MH2SO4) or 1 μA/cm2 (for 0.5-M H2SO4 + H2S).
The resistance to intercrystalline corrosion (ICC) was determined by the method of potentiodynamic scanning according to GOST 9.914–91 in a solution of 0.5-M H2SO4 + 0.01-M KSCN. The tests consisted in plotting polarization curves of active (from the cathode region to the anode one) and return (from the anode region to the cathode one) stroke for all the specimens. The ratio of the calculated total charges of return stroke (Q k ) to the charges of active stroke (Q a ) characterized the resistance of the specimen to intercrystalline corrosion; if it was less than 0.11 the specimen was assumed to be resistant to intercrystalline corrosion.
The resistance to pitting corrosion was determined according to the GOST 9.912 – 89 Standard in terms of the mean conventional rate of pitting corrosion. The tests consiste in holding preliminarily weighed specimens in a solution of iron chloride (III) for 24 h and second weighing. The loss in the mass was used to determine the mean conventional rate of pitting corrosion v m by the formula
where ∆m is the total loss in the mass of parallel (simultaneously tested) specimens (g), S is the total area of the surface of the parallel specimens (m2), and t is the duration of the test (h).
Results and Discussion
The strength characteristics determined earlier in [11] increased upon growth in the nitrogen content in steels 1 – 4 and with total alloying of the steel (4, Table 2) (from 230 to 545 MPa for σ0.2 and from 515 to 835 MPa for σr ); it can be seen that the resistance to low plastic deformation σ0.2 increased the most. After the tests, the specimens of steel 3 (Kh18AN10) were weakly magnetic and acquired some strain martensite (α-martensite). The other steels remained nonmagnetic even after tension until failure at room temperature.
All the steels had high ductility parameters under tension (Table 2). The purest steel 3 (the least alloyed of the steels studied and with a high nitrogen content) exhibited the highest ductility (δ = 63 % and ψ = 84%), a low strain resistance, and medium strength values.
The thermal diffusivity of the steels of type Kh18AN10 in the temperature range of 25 – 100°C had values typical for austenitic high-strength steels (Table 3). For example, the thermal diffusivity of steel 3 was 3.8 mm2/sec.
Analysis of the dilatograms of heating of the nitrogen steels shows that heating at 50 – 1200°C at a rate of about 5 K/sec may cause precipitation of excess nitrides and then their dissolution. Precipitation may occur at a temperature of 400 – 700°C, and dissolution may occur at a temperature exceeding 1000°C. This process should be controlled, because it may affect positively the strength and negatively the ductility, to say nothing of the corrosion resistance.
The thermal expansion factor αt for different temperature ranges has a usual order of magnitude; for example, for steel 3 in the range of 50 – 300°C (where precipitation of excess phases is known to be absent) it amounts to 20.5 × 10–6 K–1.
The mean values of thermal expansion factors for the ranges of 50 – 800°C and 50 – 1200°C are 25.3 × 10–6 K–1 and 31.7 × 10–6 K–1 respectively. The difference is connected with the change in the phase composition, may be used for rapid control of the processes of transformation, and should be taken into account when evaluating the effects of size changes. Articles from nitrogen steels after quenching should not be heated above 300°C to keep nitrogen in the solid solution, as it has been shown earlier in [12].
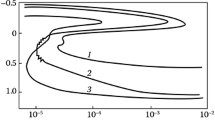
The polarization curves plotted in the tests in an acid environment (as an example, we present in Fig. 1 the polarization curve for steel 2 ) have a smooth outcome from the cathode zone into the anode one; the peak of active dissolution is well manifested in the range from –150 to +113 mV for steel 2, from –147 to +271 mV for steel 3, and from –100 to +112 mV for steel 4. The width of the passive domain for steels 2, 3, 4 amounts to 1145, 996 and 1580 mV respectively. The corresponding electrochemical results of the tests of the steels for resistance to total corrosion in an acid environment are presented in Table 4.
The surface of the specimens of steels 2 and 3 (Kh18AN10) after the tests is strongly etched, rough, and has well manifested boundaries of the working region (Fig. 2a and b ); the surface of the specimens of steel 4 (Kh18N5AG9M2) is the lowest, and a larger part of the surface preserves luster (Fig. 2c ).
Appearance of steel specimens after testing in a medium of 0.5-M H2SO4 (a – c), in a medium of 0.5-M H2SO4 with blasting H2S (d – f ), and in a 3% solution of NaCl (g – i ) (the external diameter of the specimens is 18 – 20 mm, the diameter of the functional zone is 11 mm, the thickness is 1.5 – 5.7 mm): a, d, g ) steel 2; b, e, h ) steel 3; c, f, i ) steel 4.
In an acid environment (0.5-M H2SO4, pH = 0.44) all the steels except for 3 are in a passive condition without application of an external potential, i.e., are more corrosion resistant in the acid medium. On the whole, the resistance of steel 4 (Kh18N5AG9M2) to corrosion in the acid environment is higher than that of the steels of type Kh18AN10 (2 and 3), because it has a wider zone of passivity (by 450 mV on the average) and a current density an order of magnitude lower (as compared to steel 2) in the zone of active dissolution and in the zone of passivity. It should also be noted that steel 4 does not loose the state of passivity upon the attainment of the potential of oxygen emission, whereas the steels of type Kh18AN10 are activated.
In the tests in a 0.5-M solution of H2SO4 with blasting H2S the polarization curves of the studied steels have a smooth outlet from the cathode zone into the anode one; the peak of active dissolution corresponds to the range from –147 to +510 mV for steel 2, from –182 to +623 mV for steel 3, and from –161 to +234 mV for steel 4. The passivation is abrupt and jumpwise, the width of the passive region is 701, 688, and 1473 mV for steels 2, 3, and 4 respectively. The results of the tests for resistance to total corrosion in an acid medium with blasting H2S are presented in Table 4. The erosion of the surface of the studied specimens is uniform, the depth of the erosion is low, but the surface becomes uniformly rough (Fig. 2d – f ); the black points observable on the surface of the specimens of steel 2 are commonly associated with selective dissolution of the components of the steel. In the range of active dissolution the surfaces of all the specimens acquire a black nonprotective (possibly due to porosity) film, which detaches upon the attainment f the critical passivation potential E cr . With allowance for the data of [13] this film should be nickel sulfide Ni2S. All the steels tested in a 0.5-M solution of H2SO4 without application of external potential have an active condition, i.e., their corrosion resistance in this environment is low. Blasting of the solution with H2S equally activates all the steels, i.e., the current density in the zones of active dissolution and passivity increases, the passivation potential shifts to the positive side, and the passivation range narrows. It is possibly that all the steels exhibit some change in the passive state due to formation of sulfide components in addition to the oxides; in the tests in the active range the specimens acquire a black coat not possessing protective properties, which is withdrawn completely before the passivation. Specifically, such behavior is typical for nickel in sulfur-containing media [14]. Experiments have shown formation of sulfides in the surface coat on nickel. Steel 4 is more resistant to corrosion in this environment than the other steels; the current density for it is an order of magnitude lower and the passivation range is wider by 800 mV on the average; the passive condition is preserved at the potential of oxygen emission.
The polarization curves of all the specimens tested for the resistance to total corrosion in a 3% solution of NaCl (sea water) do not have a zone of active dissolution, i.e., are in a passive condition during the whole of the test. The free corrosion potential (E fc) is much more negative than the pitting potential (E p). For this reason, we determined the potentials of free corrosion and pit formation (understood as the potential corresponding to the current density of 1 mA/cm2) for all the specimens. The electrochemical characteristics obtained in the tests in 3% solution of NaCl are presented in Table 5.
In this environment all the specimens acquired well manifested pits at inconsiderable total corrosion; the functional parts of all the specimens preserved high luster (Fig. 2g–i ). The pits were of the same kind on all the specimens and are shown in Fig. 3 (the pits on steel 2 are larger).
All the steels studied are susceptible to crevice corrosion in sea water; traces of crevice corrosion are observable on all the specimens over the edges of the functional zone (at the place of contact between the hydraulic insulation seal and the surface of the metal); they have the form of strong erosion shaped as rings or ring sectors. However, the susceptibility of the steels of type Kh19AN10 (2 and 3) to crevice corrosion is higher than that of steel Kh18N5AG9M2.
The results of the electrochemical tests show that steel 4 (Kh18N5AG9M2) has the highest corrosion resistance in chlorine-containing media of the steels studied (its pitting potential is 250 – 300 mV more positive on the average than those of the steels of type Kh18AN10). The pure steel 3 with 0.220% N is more resistant than the steel with 0.186% N (its E p is 120 mV more positive than that of steel 2).
All the steels have shown good resistance to intercrystalline corrosion (Table 6) in a standard environment of 0.5-M H2SO4 + 0.01-M KSCN; the ratio of the return stroke charge to the active stroke charge Q k /Q a for all the steels is less than 0.11. The erosion of the specimens during the tests is minimum and the luster of the surface is preserved. Note that the resistance to ICC is higher in the impurity-free steel 3 than in steel 2 of conventional purity and even than in the more alloyed steel 4.
The resistance of the steels studied to pitting corrosion in a solution of iron chloride evaluated in terms of the mean conventional rate of pitting corrosion is at a close level (Table 7), but the highest value is exhibited by steel 4. The pitting corrosion resistance of the nitrogen steel (2, Kh18AN10) and of the nitrogen-free steel (1, 12Kh18N10T) of usual purity is about the same and lower than that of the pure steel (3, Kh18AN10).
Thus, on the whole, the resistance of the studied nitrogen steels to different kinds of corrosion is quite close, but the values of the critical current, of the passivation potential, of the ratio of the charge of return stroke to the charge of active stroke in the evaluation of the resistance to ICC, and of the mean conventional rate of pitting corrosion allow us to rank the steels as follows (Table 8). Steel 4 (Kh18N5AG9M2) has the highest corrosion strength in all the environments studied. Then follows nitrogen steel 3 (Kh18AN10) clean with respect to impurities; it has higher resistance to ICC and resistance to total and pitting corrosion in chloride solutions as compared to conventional-purity steels.
Conclusions
-
1.
Alloying of austenitic chromium-nickel steel of type Kh18N10 with nitrogen in an amount of up to 0.220% in the absence of additional precipitation of nitrides is a promising measure for raising the corrosion resistance in little-aggressive chloride-containing environments (sea water).
-
2.
Alloying with nitrogen increases insubstantially the resistance of steels of type Kh18AN10 to corrosion in highly aggressive environments (H2SO4 and, especially, H2SO4 + H2S).
-
3.
Additional increase in the corrosion strength of steels of type Kh18AN10 is attained by lowering the content of impurities.
-
4.
The corrosion resistance of nitrogen-alloyed chromium-nickel-manganese steel Kh18N5AG9M2 in acid environment (0.5-M H2SO4) and in sea water (3% solution of NaCl) with respect to different parameters (ICC, general, pitting and crevice corrosion) is not lower or higher than that of steels of type Kh18AN10 and of the traditional nitrogen-free corrosion-resistant steel.
-
5.
The proportion of the parameters of corrosion resistance and strength in steel Kh18N5AG9M2 is higher than in the steels of type Kh18AN10 and 12Kh18N10T, in which the values of σy and σr are twice lower despite the fact that the content of nickel in Kh18N5AG9M2 is almost twice less.
The results have been obtained within the framework of State Assignment RFMEF157514X0071 of the Ministry of Education and Science of Russia.
References
O. Bannykh, “Progress in the research and application of nitrogen-alloyed steels,” in: Proc. 10th Int. Conf. on High Nitrogen Steels [in Russian], Moscow, MISiS (2009), pp. 24 – 27.
H. Berns, S. Riedner, and V. Gavriljuk, “High interstitial stainless austenitic steels. Part 1. Constitution, heat treatment, properties, applications,” in: Proc. 10th Int. Conf. on High Nitrogen Steels [in Russian], Moscow, MISiS (2009), pp. 129 – 139.
A. G. Svyazhin, J. Siwka, and L. M. Kaputkina, “High-nitrogen steels — the current state and development trends,” in: Proc. Int. Conf. Advanced Steels, Metallurgical Industry Press, Beijing, China (2010), pp. 352 – 356.
S. Yu. Mushnikova, Yu. L. Legostaev, A. A. Khar’kov, et al., “A study of the effect of nitrogen on the resistance of austenitic steels to pitting corrosion,” Vopr. Materialoved., No. 2(38), 126 – 135 (2004).
H. Baba, T. Kodama, and Y. Katada, “Role of nitrogen in the corrosion behavior of austenitic stainless steels,” Corros. Sci., 44, 2393 – 2407 (2002).
U. K. Mudali, S. Ningshen, and B. Raj, “Passive films and localised corrosion—role of nitrogen,” in: Proc. 10th Int. Conf. on High Nitrogen Steels [in Russian], Moscow, MISiS (2009), pp. 271 – 280.
I. V. Gorynin, V. A. Malyshevskii, G. Yu. Kalinin, et al., “Corrosion-resistant high-strength nitrogen steels,” Vopr. Materialoved., No. 3(59), 7 – 16 (2009).
M. V. Kostina, O. A. Bannykh, V. M. Blinov, et al., “Nitrogenalloyed chromium corrosion-resistant steels of new generation,” Materialovedenie, No. 2, 35 – 44 (2001).
A. G. Rakhshtadt, L. M. Kaputkina, and S. D. Prokoshkin (eds.), Metal Science and Heat Treatment of Steel and Cast Iron, Vol. 2 [in Russian], Intermet Engineering, Moscow (2005), 528 p.
V. M. Schastlivtsev, V. I. Zel’dovich, D. A. Mirzaev, et al., Advancement of the Ideas of Academician V. D. Sadovskii [in Russian], IFM UrO RAN, Ekaterinburg (2008), 409 p.
L. M. Kaputkina, M. G. Medvedev, V. G. Prokoshkina, et al., “Effect of nitrogen alloying on the hardening and stability of austenite in a steel of type Kh18N10,” Izv. Vysh. Uchebn. Zaved., Chern. Metall., 57(7), 43 – 50 (2014).
L. M. Kaputkina, V. G. Prokoshkina, G. E. Khadeev, et al., “Diagrams of hot and warm deformation and strain hardening of austenitic nitrogen-containing steels,” Metalloved. Term. Obrab. Met., No. 6, 38 – 43 (2013).
Y. Y. Andreev, T. V. Bobkov, A. V. Dub, et al., “Thermodynamics of chemisorption of sulfur from thiocyanate on nickel during electrochemical passivation,” Protect. Met. Phys. Chem. Surf., 4(49), 444 – 450 (2013).
V. P. Grigor’ev, V. M. Kravchenko, and I. M. Gershanova, “Visible activation energy of anode dissolution of nickel in sulfate solutions in the presence of Cl–and CNS–anions,” Zashch. Met., 40(3), 236 – 242 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 7, pp. 29 – 36, July, 2015.
Rights and permissions
About this article
Cite this article
Kaputkina, L.M., Smarygina, I.V., Kaputkin, D.E. et al. Effect of Nitrogen Addition on Physicochemical Properties and Corrosion Resistance of Corrosion-Resistant Steels. Met Sci Heat Treat 57, 395–401 (2015). https://doi.org/10.1007/s11041-015-9895-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-015-9895-1