Abstract
Neuroinflammation plays an essential role in the pathogenesis of Alzheimer’s disease. The preventive effect of physical exercise on attenuating neuroinflammation has not been completely defined. Levisticum officinale is known as a medicinal plant with antioxidant and anti-inflammatory properties. The current study was designed to investigate the neuroprotective impacts of treadmill running and Levisticum officinale on lipopolysaccharide (LPS)-induced learning and memory impairments and neuroinflammation in rats. Male Wistar rats ran on a treadmill and/or were pretreated with Levisticum officinale extract at a dose of 100 mg/kg for a week. Then, rats received intraperitoneal injection of LPS at a dose of 1 mg/kg. Treadmill running and/or treatment of extract lasted three more weeks. Behavioral, molecular, biochemical and immunohistochemical assessments were carried out after the end of the experiment. LPS administration resulted in spatial learning and memory impairments along with increased mRNA expression of interleukin-6 and malondialdehyde levels, as well as decreased superoxide dismutase activity and neurogenesis in the hippocampus. Moreover, treadmill running for four weeks, alone and in combination with Levisticum officinale extract attenuated spatial learning and memory deficits, decreased the mRNA expression of interleukin-6 and malondialdehyde levels, and enhanced superoxide dismutase activity and neurogenesis in the hippocampus. In conclusion, the advantageous effects of running exercise and Levisticum officinale extract on LPS-induced memory impairments are possibly due to the antioxidant and anti-inflammatory activity and enhancing neurogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is identified by degeneration of cholinergic neurons in the basal forebrain and clinical manifestations of memory impairment and dementia (Donev 2017). AD devotes up to 70% of dementia cases (WHO, 2021). Histopathological characteristics of AD are the accumulation of Aβ plaques outside neurons and neurofibrillary tangles within neurons that eventually causes synaptic dysfunction and neuronal loss (Murphy and LeVine 2010). Chronic inflammatory processes by activation of microglia and production of proinflammatory mediators and free radicals contribute to synaptic impairment and neuronal death in AD. Excessive amounts of proinflammatory mediators, including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) have been reported in the brain of patients with AD (Veerhuis 2011). Neuroinflammation also suppresses hippocampal neurogenesis and causes cognitive decline in AD (Ryan and Nolan 2016; Sung et al. 2020). Moreover, most studies have confirmed an increase in lipid peroxidation markers such as malondialdehyde in the brain of AD patients, particularly in the hippocampus and temporal cortex (Castellani et al. 2011).
Lipopolysaccharide (LPS) is a potent endotoxin derived from cell wall of Gram-negative bacteria. Evidence indicates that LPS administration induces learning and memory deficits in experimental animals and imitates AD in pathology and memory deficits (Zakaria et al. 2017). Systemic or central administration of LPS, results in neuroinflammation by activating nuclear factor-κB (NF-κB) and inducing the release of proinflammatory mediators such as IL-6, IL-1β and TNFα (Zhao et al. 2019; Bossu et al. 2012). Moreover, the elevation of these cytokines induces the expression of amyloid precursor protein (APP) and amyloidogenesis (Lee et al. 2008). Inflammatory responses induced by LPS also produce excessive amounts of free radicals and peroxides in the central nervous system (Ali et al. 2016). Systemic LPS also reduces neurogenesis and expression of BDNF in the hippocampus and deteriorates spatial memory (Valero et al. 2014; Amraie et al. 2020).
Exercise training is known to be beneficial for brain function. It was shown that regular physical activity postpones the occurrence of dementia and AD (Rovio et al. 2005; Zhu et al. 2022). Also, exercise promotes antioxidant defense system and protects against lipid peroxidation damage in aged individuals (Bouzid et al. 2018). Treadmill running prevents memory impairments by increasing neurotrophic factors and reducing oxidative stress in the hippocampus of aged rats (Vanzella et al. 2017). Exercise also enhances anti-inflammatory cytokines (Lovatel et al. 2013), long-term potentiation (LTP), neurogenesis and learning in healthy and aged animals (van Praag et al. 1999; Farmer et al. 2004; van Praag et al. 2005). Although physical exercise enhances memory function in healthy and aged rats, its mechanisms of action on learning and memory performance under brain inflammation conditions are not completely clarified.
Lovage (Levisticum officinale Koch) is a perennial aromatic plant from the Apiaceae family found in many European and East Asian countries (Downie et al. 2001). Levisticum officinale has been used as a medicinal plant due to its spasmolytic and diuretic properties. Previous studies have demonstrated that Levisticum officinale extract possesses anti-inflammatory, antioxidant (Złotek et al. 2019; Ghaedi et al. 2019), anti-apoptotic (Mollashahee-Kohkan et al. 2019) and anticholinesterase (Zarei et al. 2013) activities. Our previous study showed that the Levisticum officinale extract contains flavonoids (catechin, rutin, luteolin and quercetin) and phenolic acids (rosmarinic acid, chlorogenic acid and caffeic acid) (Amraie et al. 2020; Ghaedi et al. 2019).
The aim of the current study was to examine the neuroprotective effects of treadmill exercise, alone and in combination with Levisticum officinale extract on spatial learning and memory impairments and their mechanisms of action using LPS-induced brain inflammation in rats.
Materials and methods
Animals
Experiments were carried out on male Wistar rats, weighing 200–250 g (average age of 8 weeks). Animals were kept in a colony room under controlled environment (22 ± 2 °C), 12 h dark/light cycle with free access to a standard pellet diet and tap water. The maintenance and treatment of animals were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication, 8th edition, 2011). All experimental procedures were approved by Ethics Committee for Animal Experimentation at Isfahan University of Medical Sciences (IR.MUI.MED.REC.1396.1.067).
Levisticum officinale extract
The stems and leaves of Levisticum officinale were collected from Bardsir in Kerman province, and graciously identified at the MIR Herbarium of the Shahid Bahonar University of Kerman. The dried powdered plant was soaked in 80% methanol for 24 h. Then, the extract was filtered, evaporated by a rotary evaporator and oven-dried at 40 °C.
Treatments
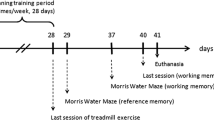
Rats were assigned to five groups (n = 10), including the control, LPS, LPS + exercise, LPS + extract and LPS + exercise + extract groups. LPS from E.coli (Sigma-Aldrich Co., USA) was administered intraperitoneally at a single dose of 1 mg/kg body weight (BW). Levisticum officinale extract was prepared in distilled water and administered orally to rats at a dose of 100 mg/kg BW. The control and LPS groups received distilled water by gavage at the same volume as the treated groups. Treadmill running or treatment of extract was carried out one week before the LPS injection for four weeks, according to previous protocols (Hou et al. 2014). Morris water maze (MWM) was used to assess spatial learning and memory on days 18–22 after LPS administration.
Tissue collection
After the end of behavioral experiments, rats were euthanized and the hippocampus was dissected out and kept at − 80 °C for biochemical and molecular assessments or placed in formaldehyde for histology.
Treadmill running
Exercise training was performed on rats using a five-lane motorized treadmill. Firstly, rats were trained on the treadmill at 5 min/day with 10 m/min speed for five days to reduce their stress. Thereafter, they ran on the treadmill at a speed of 20 m/min for 30 min once a day for four weeks. This protocol was performed one week before LPS injection for four weeks.
MWM task
MWM test was performed on post-injection days 18–22 to assess spatial learning and memory abilities. The maze was a black circular pool (150 cm diameter) filled with water (23 ± 1 °C) to a depth of 30 cm. A hidden platform (10 cm diameter) was located at a midpoint of the south-east quadrant. During acquisition training, rats were trained to reach the hidden platform within 60 s (4 trials per day × 4 days) with 60 s intersession interval. In each trial, the rat was released at one of the four starting points to find the platform. Computer software (NeuroVision, Tajhiz Gostar Co.) calculated the escape latency and traveled distance for each animal. On day 22, a probe test was carried out to assess memory consolidation. The platform was removed and rats had permission to swim in the maze for 60 s. The time spent in the target zone was recorded (Ahmadi et al. 2017).
Real-time PCR
Total RNAs from rat hippocampus were extracted using Total RNA Purification Kit (GeneAll; Korea) and reverse-transcripted into cDNA as explained previously (Amraie et al. 2020). Relative quantitative real-time PCR was performed using the SYBR kit. Relative mRNA expression of IL-6 gene was determined by 2−ΔΔCT method (Livak and Schmittgen 2001). GAPDH was used as a reference gene. Primers sequences were as:
(1) IL-6, forward: CTGGTCTTCTGGAGTTCCGT, reverse: TGGTCCTTAGCCACTCCTTCT; (2) GAPDH, forward: GTCTTCACCACCACGGAGAAGGC, reverse: ATGCCAGTGAGCTTCCCGTTCAGC.
Lipid peroxidation level
The malondialdehyde (MDA) level was measured in the serum and hippocampus, to estimate lipid peroxidation level. To measure serum MDA level, a mixture containing trichloroacetic acid, thiobarbituric acid, and HCL was added to samples and centrifuged at 10,000 g for 10 min. Then, the butylhydroxytoluene were added to the supernatant and the solution was placed in a boiling water bath for 15 min. Thereafter, it was centrifuged and the absorbance of the supernatant was read at 532 nm (Kurtel et al. 1992).
To measure hippocampal MDA level, the hippocampus was homogenized in sucrose and then centrifuged at 4 °C. Later, the sodium dodecyl sulphate and thiobarbituric acid were added to the supernatant and heated in a boiling water bath for 1 h. Trichloroacetic acid was added after cooling and then the mixture was centrifuged at 1000 g for 10 min. Finally, the absorbance was read at 532 nm against the blank. MDA level was measured using an extinction coefficient of 155 m/M − 1 cm − 1 (Ohkawa et al. 1979).
Catalase (CAT) and superoxide dismutase (SOD) enzymes activity
To measure antioxidant enzyme activity, the hippocampus was homogenized in phosphate buffer and centrifuged. The protein content of the supernatant was determined according to Bradford’s method using bovine serum albumin as the standard (Bradford 1976). CAT enzyme activity was measured as described before (Aebi 1984). One unit enzyme activity of CAT was defined to decompose 1 µmol of H2O2 per min at 240 nm.
For SOD activity, the supernatant was added to the reaction solution containing potassium phosphate buffer, Na–EDTA, methionine, riboflavin and nitroblue tetrazolium. After exposure to fluorescent light for 15 min, the absorbance was read at 560 nm. One unit SOD activity was defined to inhibit the nitroblue tetrazolium photoreduction to 50% (Giannopolitis and Ries 1977).
Histology
The histological method was performed as described before (Amraie et al. 2020). Briefly, the hippocampus (3 in each group) was placed in 10% formaldehyde and embedded with paraffin to make 5 μm sections using a microtome. Thereafter, some sections were deparaffinised, dehydrated and stained with hematoxylin and eosin (H&E). Sections were studied by a light microscope (Olympus BX41, Japan).
For Ki67 immunohistochemistry, some sections were processed by dewaxing, dehydration, and microwave antigen retrieval. Sections were placed in PBS for 5 min and H2O2 for 10 min. Then, sections were incubated with primary monoclonal mouse anti-human Ki67 antibody (DBS, USA) for 1 h. After washing with PBS, sections were incubated with a secondary antibody conjugated to HRP (Diagnostic BioSystems, USA) for 30 min. After coloring with diaminobenzidine (DAB) (DAKO, Denmark), sections were counterstained with hematoxylin. Ki67 + cells were counted within the dentate gyrus in 5 fields in each section of the animal using Image J.
Statistical analysis
Data were presented as the mean ± SEM and analyzed by one-way ANOVA and two-way repeated measures ANOVA followed by Tukey’s post hoc test. P < 0.05 was considered significant.
Results
Effects of exercise and extract on spatial learning and memory deficits
Analysis of data by repeated-measures ANOVA indicated that the escape latency (F(3,123) = 85.78, P < 0.001, Fig. 1 A) and traveled distance (F(3,126) = 56.85, P < 0.001, Fig. 1B) decreased over the four learning days in all groups, demonstrating acquisition of spatial learning (Fig. 1 A and B). Statistical analysis also revealed that LPS-injected rats spent more time (F(4,44) = 20.38, P < 0.001, Fig. 1 A) and distance (F(4,44) = 28.81, P < 0.001, Fig. 1B) to reach the hidden platform in comparison with the control group, indicating the impairment of spatial learning acquisition. In addition, treadmill running, treatment with the extract or both decreased the escape latency (P < 0.001, P < 0.001, P < 0.001) and traveled distance (P < 0.001, P < 0.001, P < 0.001) as compared to the LPS group rats (Fig. 1 A and B).
Effects of exercise training and/or extract on the performance of the spatial memory acquisition phase in Morris water maze, (A) escape latency, (B) traveled distance, (C) probe trial. Data are expressed as mean ± SEM for ten animals in each group. One-way and two-way repeated-measures ANOVA were performed. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group, +P < 0.05, ++P < 0.01, +++P < 0.01 vs. LPS group
On probe trial, the percentage of time spent in the south-east quadrant decreased in the LPS group in comparison with the control group (F(4,44) = 6.48, P < 0.01, Fig. 1 C). Moreover, treatment with extract (P < 0.05), exercise (P < 0.01) or both (P < 0.05) enhanced the percentage of time in comparison with LPS group (Fig. 1 C).
Effects of exercise and extract on hippocampal IL-6 gene expression
Analysis of data showed that the mRNA level of IL-6 was enhanced (Fvalue = 17.95, P < 0.001, Fig. 2) in the hippocampus of the LPS group in comparison with the control group. Moreover, extract treatment (P < 0.001), exercise (P < 0.01) or both (P < 0.01) significantly reduced the mRNA level of IL-6 in comparison with the LPS group (Fig. 2).
Effects of exercise and extract on serum and hippocampal MDA level
MDA level and antioxidant enzymes activity was measured in the hippocampus to evaluate the oxidative stress status. Results revealed that the levels of MDA were enhanced in the serum (Fvalue = 66.49, P < 0.001) and hippocampus (Fvalue = 32.95, P < 0.001, Fig. 3) in the LPS group in comparison with the control group. In addition, extract treatment (P < 0.001, P < 0.001), exercise (P < 0.001, P < 0.05) or both (P < 0.01, P < 0.05), significantly reduced the MDA level in the serum and hippocampus (Fig. 3). There was also a significant difference between the extract group with the exercise and extract + exercise groups in the serum (P < 0.01, P < 0.01) and hippocampal MDA levels (P < 0.05, P < 0.01).
Effects of exercise and extract on antioxidant enzyme activity
Data analysis showed that SOD enzyme activity (Fvalue = 32.86, P < 0.001) and CAT (Fvalue = 31.16, P < 0.001) significantly decreased in the hippocampus in the LPS group in comparison with the control group. Moreover, extract treatment (P < 0.01), exercise training (P < 0.05) or both (P < 0.05) enhanced the SOD activity in the hippocampus (Fig. 4). Also, treatment with extract increased CAT enzyme activity in the hippocampus (P < 0.05, Fig. 4).
Effects of exercise and extract on histopathology and cell proliferation
Histological observations demonstrated the presence of normal arrangement and density of the granule cells in the DG, and pyramidal neurons in the CA1 and CA3 areas of the hippocampus in the control group (Fig. 5 A). Moreover, we found the maximum changes including significant pyknosis (pyknotic neurons), cytoplasm vacuolization and dilated blood vessels in the DG, CA1, and CA3 areas of the hippocampus in the LPS group (Fig. 5B). In other groups, most of the neurons were normal, and minimal presence of pyknotic neurons and other changes were detected in the different areas of the hippocampus (Fig. 5 C, D and E).
Histological changes in the hippocampal regions of experimental groups. (A) Control group exhibits the normal thick layer and arrangement of the granular cells in the DG and the pyramidal neurons in CA3 and CA1 areas. (B) LPS group shows a significant reduction in the thickness of the granular cell layer in the DG, a significant increase in apoptotic neurons (arrow), and cytoplasm vacuolization (arrowhead) in the DG, CA3, and CA1 areas, accompanied by pyknotic nuclei and several dilated blood vessels (star) compared to other groups. (C, D, E) Extract, Exercise and Extract + exercise groups indicate the normal thickness of the DG, CA3, and CA1 regions with the preservation of pyramidal cells with rounded vesicular nuclei and prominent nucleoli (thick arrow). Magnification 4× (first left column), 20×, Scale bar = 100 μm
Ki67 immunohistochemistry revealed that the number of Ki67 + cells was decreased in the DG of the hippocampus in the LPS group in comparison with the control group (P < 0.05, Fig. 6 A, B). Moreover, treatment with Levisticum officinale extract (P < 0.01), exercise training (P < 0.05) or both (P < 0.001) enhanced the number of Ki67 + cells in comparison with the LPS group (Fig. 6 A, B). There was also a significant difference in the number of Ki67 + cells between the extract and exercise groups with the extract + exercise group (P < 0.001). These findings indicated the synergistic effects of the extract and exercise training on neuronal cell proliferation in the hippocampus.
Immunohistochemistry staining of the dentate gyrus of the hippocampus for the nuclear antigen Ki67 (A), and quantification analysis of Ki67 staining (B) in experimental groups. *P < 0.05 vs. the control group, +P < 0.05, ++P < 0.01, +++P < 0.001 vs. the LPS group. Magnification 20×, Scale bar = 100 μm
Discussion
The aim of the current study was to evaluate the neuroprotective potential of treadmill running and Levisticum officinale extract against LPS-induced memory deficits and find the potential mechanisms of action. Our findings demonstrated that LPS injection induced impairments in spatial learning and memory in the MWM via activation of neuroinflammation and oxidative stress, and decreased the cellular proliferation in the hippocampus. Our findings also demonstrated that treadmill running and supplementation of Levisticum officinale extract for four weeks improved memory deficits, decreased the expression of IL-6 mRNA and oxidative stress, and enhanced neurogenesis.
We used the LPS model since LPS injection induces memory deficits and pathology similar to AD (Zakaria et al. 2017). Evidence indicates that systemic LPS injection induces memory impairments by activating inflammatory responses and neurodegeneration (Chowdhury et al. 2018; Batista et al. 2019; Amooheydari et al. 2022). Consistent with previous studies, our findings showed that systemic LPS deteriorated learning and memory of rats in the MWM, since rats spent more time to find the hidden platform and less time on probe trial. Similarly, it was shown that acute systemic LPS deteriorates learning and memory of mice in MWM task (Ali et al. 2014), aversive memory (Lee et al. 2008), and performance in novel object recognition test (Ming et al. 2015).
Our results also indicated that treadmill exercise ameliorated spatial learning and memory impairments, as evident by a decrease in latency to find the hidden platform and an increase in time spent on the probe trial in exercised rats. The memory-enhancing effects of the exercise were previously shown in experimental animal models. For instance, it was reported that treadmill running improves spatial memory impairments in the MWM in transgenic AD mice (Liu et al. 2011) and cerebral ischemia model (Cechetti et al. 2012).
Our findings also showed that Levisticum officinale extract, alone and in combination with treadmill exercise ameliorated learning and memory impairments in the MWM. Conclusively, our results indicate that treadmill running and Levisticum officinale extract are effective in protecting against memory deficits elicited by LPS in rats.
Injection of LPS causes memory deficits by multiple mechanisms such as expression of proinflammatory mediators (Chowdhury et al. 2018), enhanced oxidative stress (Bai et al. 2016), impairment of neurogenesis (Ormerod et al. 2013), loss of cholinergic neurons (Houdek et al. 2014) and apoptosis.
Systemic LPS is known to produce inflammatory responses mainly via activating TLR4, subsequently NF-κB transcriptional activation of inflammatory genes, such as TNF-α and IL-6, and eventually loss of synapses, neurons and memory deficits (Brown 2019). The high production of IL-6 following LPS injection was shown to impair spatial and working memory in rodents (Chen et al. 2008; Dugan et al. 2009). It was also demonstrated that over-expression of IL-6 and TNF-α induces memory impairment in transgenic animal models (Zheng et al. 2016). In this study, we observed that LPS increased the mRNA expression of IL-6 in the hippocampus, which is consistent with prior studies (Chowdhury et al. 2018). Our findings also showed that treadmill running for four weeks decreased mRNA expression of IL-6 in the hippocampus, indicating the anti-inflammatory activity of exercise training against LPS-induced neuroinflammation in rats. The anti-inflammatory effect of treadmill exercise has been shown in several animal models such as cerebral ischemia (Zhang et al. 2017), Parkinson’s disease (Shahidani et al. 2022) and the transgenic AD mouse model (Leem et al. 2011). In line with this, it was shown that the expression of IL-6 and TNF-α was decreased in the brain of transgenic mice after treadmill training (Leem et al. 2011). While studies in the transgenic models of AD have reported the beneficial impact of exercise on cognitive function and pathology in AD, a few studies have evaluated the impact of exercise on LPS-induced neuroinflammation and memory deficits (Kelly 2018). In this context, several studies reported that treadmill running did not change LPS-induced expressions of IL-1β and TNF-α in the hippocampus (Wu et al. 2007), while other studies reported opposite results. For example, Mota and Kelly (2020) have shown that exercise exerts neuroprotective effects against LPS-induced impairment of spatial recognition memory along with down-regulation of TNF-α, IL-1β and IL-10 mRNA expression in the mice hippocampus (Mota and Kelly 2020). In line with this study, our study indicated that treadmill running reduced the mRNA expression of IL-6 in the hippocampus of LPS-administered rats.
Our results also demonstrated that treatment with Levisticum officinale extract, alone and in combination with treadmill exercise decreased mRNA expression of IL-6 in the hippocampus, indicating the anti-inflammatory activity of Levisticum officinale extract in LPS-induced neuroinflammation. The anti-inflammatory effect of Levisticum officinale was shown in previous studies (Złotek et al. 2019; Jakubczyk et al. 2020). Collectively, the beneficial impact of treadmill running and Levisticum officinale on memory dysfunction in LPS-injected rats could be partly attributed to anti-inflammatory activity of treadmill running and Levisticum officinale.
Evidence indicates a strong relation between oxidative stress and memory deficits in AD (Barnham et al. 2004). Production of high amounts of reactive oxygen species (ROS) during oxidative stress causes oxidative damage to lipids, proteins, DNA, and eventually neurodegeneration. MDA is considered as an index of lipid peroxidation. SOD and CAT scavenge free radicals or prevent their formation. SOD changes the superoxide anion to H2O2, which is removed by CAT (Ighodaro and Akinloye 2018). Administration of LPS causes the increased generation of ROS and development of oxidant/antioxidant imbalance (Bai et al. 2016, which leads to neuronal damage. Oxidative damage to synapses in the cerebral cortex and hippocampus contributes to memory deficits (Ahmadi et al. 2017). In this study, LPS induced memory deficits along with brain tissue oxidative stress since hippocampal MDA levels were increased, whereas the activities of SOD and CAT were decreased. Consistent with this, it was shown that LPS caused oxidative damage in brain tissues and memory deficits (Ali et al. 2016). According to our results, exercise training for four weeks reduced MDA levels in the serum and hippocampus and potentiated antioxidant defence of the brain by increasing SOD activity in the hippocampus. Therefore, the results demonstrate the protective action of exercise against LPS through its antioxidant activity. Consistent with our findings, previous reports have demonstrated the neuroprotective action of exercise by removing ROS and decreasing lipid peroxidation. For instance, studies in transgenic AD mice have shown that running wheel reduces lipid peroxidation and enhances glutathione level in the cerebral cortex (Garcı´a-Mesa et al. 2011). It was also revealed that swimming exercise for four months increases SOD activity in the hippocampus and cerebral cortex of normal rats (Devi and Kiran 2004). Moreover, an enhancement in the expression of the SOD enzyme in the hippocampus of transgenic mice following three weeks of treadmill running has been reported (Um et al. 2011).
According to the present results, treatment with Levisticum officinale extract, alone and in combination with treadmill exercise decreased MDA level and enhanced SOD activity in the hippocampus, indicating the antioxidant activity of Levisticum officinale in LPS-treated rats. Previous studies have also shown the antioxidant activity of Levisticum officinale. For example, it was demonstrated that aqueous extract of Levisticum officinale protects against the adverse impacts of paraquat on hepatocytes due to its antioxidant activity (Afarnegan et al. 2017). Taken together, the positive influence of treadmill exercise and Levisticum officinale on memory deficits could also be attributed to their antioxidant activity.
However, decreased neurogenesis is also involved in cognitive decline in AD, and targeting neurogenesis may ameliorate cognitive dysfunction in AD (Babcock et al. 2021). It was also revealed that neuroinflammation and oxidative stress negatively influences neurogenesis and cognition (Ryan and Nolan 2016; Suwannakot et al. 2022). In line with this, previous studies have shown the detrimental impacts of LPS and proinflammatory mediators on neurogenesis (Monje et al. 2003; Borsini et al. 2015). For example, it was revealed that systemic LPS injection reduces the proportion of doublecortin-positive new cells, demonstrating that LPS decreases neuronal differentiation (Monje et al. 2003). Similarly, in another study it was shown that the intracortical LPS injection reduced the production of new neurons (Ekdahl et al. 2003). It was also demonstrated that neurogenesis was reduced in the hippocampus in transgenic mice overexpressing IL-6 in astrocytes (Vallieres et al. 2002). Consistent with prior studies, present results showed that systemic LPS reduced neurogenesis, possibly due to the enhanced mRNA expression of IL-6 and oxidative stress in the hippocampus. Our results also showed that treadmill exercise enhanced neurogenesis, which is in agreement with previous studies. For instance, it was demonstrated that running wheel protects against reductions in hippocampal neurogenesis in the aged brain (Littlefield et al. 2015). Our results also demonstrated that Levisticum officinale extract alone and in combination with exercise enhanced neurogenesis in the hippocampus, which could be due to their anti-inflammatory and antioxidant activities. Collectively, according to our results, treadmill running and Levisticum officinale extract may improve spatial memory partly by synergistic effects on enhancing neurogenesis in LPS-treated rats.
It should be noted that in our previous studies, we reported the presence of flavonoids and phenolic acids in the Levisticum officinale extract by the HPLC method (Amraie et al. 2020; Ghaedi et al. 2019). Evidence indicates that phenolic acids and flavonoids possess anti-inflammatory and antioxidant properties (Amraie et al. 2020). Conclusively, the anti-inflammatory and antioxidant properties of Levisticum officinale extract could be due to the existence of flavonoids and phenolic acids in the extract.
It is noteworthy that other mechanisms might also be involved in the beneficial impact of exercise and plant extract on memory function in LPS injected rats. Previous studies have demonstrated that systemic LPS injection influences microglial phenotype, neuronal structure and function (Lonnemann et al. 2022), BDNF expression and Aβ formation (Lee et al. 2008; Beheshti et al. 2019). For instance, it was shown that neuroinflammation and activated microglia following LPS challenge reduces dendritic spine density in hippocampal neurons, and impairs LTP and cognitive abilities (Lonnemann et al. 2022; Hosseini et al. 2021). Evidence also indicates that treadmill exercise reduces the glial cell response (Mota and Kelly 2020; Zhang et al., 2022) and enhances structural and functional synaptic plasticity in the hippocampus and prefrontal cortex (Tsai et al. 2018; Mu et al. 2022). For instance, it was demonstrated that treadmill running for nine days improved memory performance in the object displacement task and reduced the microglia and astrocytes activation in the mouse brain following a single injection of LPS (Mota and Kelly 2020). In another studies, treadmill running enhanced CA1 neurons LTP, dendritic complexity (branch and length) and spine density of hippocampal neurons and upregulated the expression of BDNF in the mice hippocampus (Tsai et al. 2018; Lin et al. 2012). Therefore, above mechanisms may also involve in the positive influence of exercise and extract on memory deficits in LPS exposed rats due to their anti-inflammatory activity.
To sum up, treadmill running and Levisticum officinale extract, alone and combined together, ameliorated learning and memory impairments, decreased the mRNA expression of IL-6 and MDA level, and enhanced antioxidant enzymes activity and neurogenesis in the hippocampus. Comparing their effects revealed that Levisticum officinale extract possesses more antioxidant activity than exercise training, since the extract caused a more significant decrease in lipid peroxidation and increase in antioxidant enzymes activity in the hippocampus compared to exercise training. Furthermore, the synergistic effects of the plant extract and exercise training on neurogenesis in the hippocampus was found. The complementary mechanism of action of this combination on neurogenesis needs more studies; however, one possible mechanism could be related to antioxidant and anti-inflammatory activities of the extract and exercise.
Conclusion
The advantageous effects of running exercise and Levisticum officinale extract on memory impairments induced by LPS are possibly due to the antioxidant and anti-inflammatory activity and enhancing neurogenesis. Thus, treadmill exercise and Levisticum officinale could be used as a useful strategy for alleviating memory dysfunction in inflammatory disorders such as AD.
Data availability
On request from the corresponding author.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Afarnegan H, Shahraki A, Shahraki J (2017) The hepatoprotective effects of aquatic extract of Levisticum officinale against paraquat hepatocyte toxicity. Pak J Pharm Sci 30:2363–2368
Ahmadi M, Rajaei Z, Hadjzadeh MA, Nemati H, Hosseini M (2017) Crocin improves spatial learning and memory deficits in the Morris water maze via attenuating cortical oxidative damage in diabetic rats. Neurosci Lett 642:1–6
Ali MR, Abo-Youssef AM, Messiha BA, Khattab MM (2016) Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. NaunynSchmiedebergs Arch Pharmacol 389:637–656
Amooheydari Z, Rajaei Z, Alaei H, Esmaeil N (2022) Supplementation of carvacrol attenuates hippocampal tumor necrosis factor–alpha level, oxidative stress, and learning and memory dysfunction in lipopolysaccharide–exposed rats. Adv Biomed Res 11:33
Amraie I, Pouraboli I, Rajaei Z (2020) Neuroprotective effects of Levisticum officinale on LPS-induced spatial learning and memory impairments through neurotrophic, anti-inflammatory, and antioxidant properties. Food Funct 11:6608–6621
Babcock KR, Page JS, Fallon JR, Webb AE (2021) Adult hippocampal neurogenesis in aging and AD. Stem cell reports 16:681–693
Bai K, Xu W, Zhang J, Kou T, Niu Y, Wan X, Zhang L, Wang C, Wang T (2016) Assessment of free radical scavenging activity of dimethylglycine sodium salt and its role in providing protection against lipopolysaccharide-induced oxidative stress in mice. PLoS ONE 11:e0155393
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214
Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP (2019) Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci 20:2293
Beheshti F, Hashemzehi M, Sabeti N, Hashemi Sadr S, Hosseini M (2019) The effects of aminoguanidine on hippocampal cytokines, amyloid beta, brain-derived neurotrophic factor, memory and oxidative stress status in chronically lipopolysaccharide-treated rats. Cytokine 113:347–355
Borsini A, Zunszain PA, Thuret S, Pariante CM (2015) The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38:145–157
Bossù P, Cutuli D, Palladino I, Caporali P, Angelucci F, Laricchiuta D, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L (2012) A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J Neuroinflammation 9:101
Bouzid MA, Filaire E, Matran R, Robin S, Fabre C (2018) Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int J Sport Med 39:21–28
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown GC (2019) The endotoxin hypothesis of Neurodegeneration. J Neuroinflammation 16:180
Castellani RJ, Rolston RK, SmithMA (2011) Alzheimer disease. Dis Mon 56:484–546
Cechetti F, Worm PV, Elsner VR, Bertoldi K, Sanches E, Ben J, Siqueira IR, Netto CA (2012) Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem 97:90–96
Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW (2008) Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22:301–311
Chowdhury AA, Gawali NB, Shinde P, Munshi R, Juvekar AR (2018) Imperatorin ameliorates lipopolysaccharide induced memory deficit by mitigating proinflammatory cytokines, oxidative stress and modulating brain-derived neurotropic factor. Cytokine 110:78–86
Dementia WHO Newsletter; 2 September 2021
Devi SA, Kiran TR (2004) Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging 25:501–508
Donev R (2017) Advances in protein chemistry and structural biology, stress and inflammation in disorders. Academic Press
Downie S, Plunkett G, Watson M, Spalik K, Katz-Downie D, Valiejo-Roman C, Terentieva E, Troitsky A, Lee B, Lahham J, El-oqlah A (2001) Tribes and clades within Apiaceae subfamily Apioideae: the contribution of molecular data. Edinb J of Bot 58:301–330
Dugan LL, Ali SS, Shekhtman G, Roberts, Lucero J, Quick KL, Behrens MM (2009) IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS ONE 4:e5518
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100:13632–13637
Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague–dawley rats in vivo. Neuroscience 124:71–79
Garcı´a-Mesa Y, Lo´pez-Ramos JC, Gime´nez-Llort L, Revilla S, Guerra R, Gruart A, LaFerla FM, Cristo`fol R, Delgado-Garcı´a JM, Sanfeliu C (2011) Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimer Dis 24:421–454
Ghaedi N, Pouraboli I, Askari N (2019) Antidiabetic properties of hydroalcoholic leaf and stem extract of Levisticum officinale: an implication for α-amylase inhibitory activity of extract ingredients through molecular docking. Iran J Pharm Res 19:231–250
Giannopolitis CN, Ries S (1977) Superoxide dismutases. I. occurrence in higher plants. Plant Physiol 59:309–314
Hosseini M, Salmani H, Baghcheghi Y (2021) Losartan improved hippocampal long-term potentiation impairment induced by repeated LPS injection in rats. Physiol Rep 9:e14874
Hou Y, Xie G, Miao F, Ding L, Mou Y, Wang L, Su G, Chen G, Yang J, Wu C (2014) Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury inmice. Prog Neuropsychopharmacol Biol Psychiatr 54:92–102
Houdek HM, Larson J, Watt JA, Rosenberger TA (2014) Bacterial lipopolysaccharide induces a dose-dependent activation of neuroglia and loss of basal forebrain cholinergic cells in the rat brain. Inflamm Cell Signal 1:e47
Ighodaro O, Akinloye O (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 54:287–293
Jakubczyk A, Złotek U, Szymanowska U, Rybczyńska-Tkaczyk K, Jęderka K, Lewicki S (2020) In vitro antioxidant, anti-inflammatory, anti-metabolic syndrome, antimicrobial, and anticancer effect of phenolic acids isolated from fresh lovage leaves [Levisticum officinale Koch] elicited with jasmonicacid and yeast extract. Antioxid (Basel) 9:554
Kelly AM (2018) Exercise-induced modulation of neuroinflammation in models of Alzheimer’s disease. Brain Plast 4:81–94
Kurtel H, Granger DN, Tso P, Grisham MB (1992) Vulnerability of intestinal interstitial fluid to oxidant stress. Am J Physiol 263:G573–G578
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT (2008) Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 29:5–37
Leem YH, Lee YL, Son HJ, Lee SH (2011) Chronic exercise ameliorates the neuroinflammation in mice carrying NSE/htau23. Biochem Biophys Res Commun 406:359–365
Lin TW, Chen SJ, Huang TY, Chang CY, Chuang JI, Wu FS, Kuo YM, Jen CJ (2012) Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem 97:140–147
Littlefield AM, Setti SE, Priester C, Kohman RA (2015) Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J Neuroinflammation 12:138
Liu HL, Zhao G, Cai K, Zhao HH, Shi LD (2011) Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res 218:308–314
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25:402–408
Lonnemann N, Hosseini S, Ohm M, Geffers R, Hiller K, Dinarello CA, Korte M (2022) IL-37 expression reduces acute and chronic neuroinflammation and rescues cognitive impairment in an Alzheimer’s disease mouse model. eLife 11:e75889
Lovatel GA, Elsner VR, Bertoldi K, Vanzella A, Spindler C, Cechinel LR, Netto CA, Moutri AR, Siqueira IR (2013) Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol Learn Mem 101:94–102
Ming Z, Sawicki G, Bekar LK (2015) Acute systemic LPS mediated inflammation induces lasting changes in mouse cortical neuromodulation and behavior. Neurosci Lett 590:96–100
Mollashahee-Kohkan F, Saravani R, Khalili T, Galavi H, Sargazi S (2019) Levisticum Officinale extract triggers apoptosis and down-regulates ZNF703 gene expression in breast cancer cell lines. Rep Biochem Mol Biol 8:119–125
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765
Mota BC, Kelly ÁM (2020) Exercise alters LPS-induced glial activation in the mouse brain. Neuronal Signal 4:NS20200003
Mu L, Cai J, Gu B, Yu L, Li C, Liu QS, Zhao L (2022) Treadmill exercise prevents decline in spatial learning and memory in 3×Tg-AD mice through enhancement of structural synaptic plasticity of the hippocampus and prefrontal cortex. Cells 11:244
Murphy MP, LeVine H 3rd (2010) Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis 19:311–323
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ormerod BK, Hanft SJ, Asokan A, Haditsch U, Lee SW, Palmer TD (2013) PPARγ activation prevents impairments in spatial memory and neurogenesis following transient illness. Brain Behav Immun 29:28–38
Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, TuomilehtoJ, Soininen H, Nissinen A, Kivipelto M (2005) Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol 4:705–711
Ryan SM, Nolan YM (2016) Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neurosci Biobehav Rev 61:121–131
Shahidani S, Rajaei Z, Alaei H, Mohammadzadeh S (2022) The impact of sesamol and exercise on striatal TNF-α level, behavioral deficits and oxidative stress status in the rat model of Parkinson’s disease. Physiol Pharmacol 26:30–38
Sung PS, Lin PY, Liu CH, Su HC, Tsai KJ (2020) Neuroinflammation and neurogenesis in Alzheimer’s disease and potential therapeutic approaches. Int J Mol Sci 21:701
Suwannakot K, Sritawan N, Naewla S, Aranarochana A, Sirichoat A, Pannangrong W, Wigmore P, Welbat JU (2022) Melatonin attenuates methotrexate-induced reduction of antioxidant activity related to decreases of neurogenesis in adult rat hippocampus and prefrontal cortex. Oxid Med Cell Longev 2022:1596362
Tsai S-F, Ku N-W, Wang T-F, Yang Y-H, Shih Y-H, Wu S-Y, Lee C-W, Yu M, Yang T-T, Kuo Y-M (2018) Long-term moderate exercise rescues age-related decline in hippocampal neuronal complexity and memory. Gerontology 64:551–561
Um HS, Kang EB, Koo JH, Kim HT, Lee J, Kim EJ, Yang CH, An GY, Cho IH, Cho JY (2011) Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci Res 69:161–173
Valero J, Mastrella G, Neiva I, Sanchez S, Malva JO (2014) Long term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci 8:83
Vallieres L, Campbell IL, Gage FH, Sawchenko PE (2002) Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production ofinterleukin-6. J Neurosci 22:486–492
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431
van Praag H, Shubert T, Zhao C, Gage FH (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25:8680–8685
Vanzella C, Neves JD, Vizuete AF, Aristimunha D, Kolling J, Longoni A, Gonçalves CAS, Wyse ATS, Netto CA (2017) Treadmill running prevents age-related memory deficit and alters neurotrophic factors and oxidative damage in the hippocampus of Wistar rats. Behave Brain Res 334:78–85
Veerhuis R (2011) Histological and direct evidence for the role of complement in the neuroinflammation of AD. Curr Alzheimer Res 8:34–58
Wu CW, Chen YC, Yu L, Chen HI, Jen CJ, Huang AM, Tsai HJ, Chang YT, Kuo YM (2007) Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J Neurochem 103:2471–2481
Zakaria R, Wan Yaacob WM, Othman Z, Long I, Ahmad AH, Al-Rahbi B (2017) Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer’s disease. Physiol Res 66:553–565
Zarei S, Mohammadi P, Bakhtiari A, Moridi H, Janmohammadi E, Kaki A, Gholamhoseinian A, Sharifi-far F, Hatami M, Hosseini-Zijoud SM, Moradi MN (2013) Identification of anticholinesterase compounds from Berberis integerrima, Rheum ribes and Levisticum officinale. Ann Biol Res 4:138–142
Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, Zhu L (2019) Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9:5790
Zhang Q, Zhang J, Yan Y, Zhang P, Zhang W, Xia R (2017) Proinflammatory cytokines correlate with early exercise attenuating anxiety-like behaviour after cerebral ischemia. Brain Behav 7:e00854
Zhang L, Liu Y, Wang X, Wang D, Wu H, Chen H, Chen J, Liu Y (2022) Treadmill exercise improve recognition memory by TREM2 pathway to inhibit hippocampal microglial activation and neuroinflammation in Alzheimer’s disease model. Physiol Behav 251:113820
Zheng C, Zhou XW, Wang JZ (2016) The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener 5:7
Zhu J, Ge F, Zeng Y, Qu Y, Chen W, Yang H, Yang L, Fang F, Song H (2022) Physical and mental activity, disease susceptibility, and risk of dementia: a prospective cohort study based on UK Biobank. Neurology 99:e799–e813
Złotek U, Szymanowska U, Pecio U, Kozachok S, Jakubczyk A (2019) Antioxidative and potentially anti-inflammatory activity of phenolics from Lovage leaves Levisticum officinale Koch elicited with jasmonic acid and yeast extract. Molecules 24:1441
Funding
This study was supported by grants from the Council of Research, Isfahan University of Medical Sciences, and the Council of Research, Shahid Bahonar University of Kerman.
Author information
Authors and Affiliations
Contributions
ZR, IP designed the study; EA acquired data; ZR, IP, HS, EA analyzed and interpreted the data; ZR prepared the draft of the paper; ZR, IP, HS critically revised the article. All authors approved final version of the article.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The maintenance and treatment of animals were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication, 8th edition, 2011). The Ethic Committee for Animal Experiments at Isfahan University of Medical Sciences approved the study (Ethical code: IR.MUI.REC.1396.1.067).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amraie, E., Pouraboli, I., Salehi, H. et al. Treadmill running and Levisticum Officinale extract protect against LPS-induced memory deficits by modulating neurogenesis, neuroinflammation and oxidative stress. Metab Brain Dis 38, 999–1011 (2023). https://doi.org/10.1007/s11011-022-01140-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01140-z