Abstract
The aim of this study was to evaluate the effects of swimming in the brain and behavior of young and aged mice. Forty-eight male C57BL/6 J mice were randomly distributed into 4 groups (n = 12 per group, 3 and 18 months old). The subdivision of the groups was: 3 months-SED, 18 months-SED, 3 months-EXE, and 18 months-EXE. SED mice did not swim, while EXE mice performed the physical exercise protocol. Training was initiated 48 h after the adaptation week. Swimming sessions consisted of 30 min, with no overload, 5 days per week, for 4 weeks. After the exercise protocol, it was revealed working and spatial memory were impaired in the 18 months-SED group. Pre- and post-synaptic proteins were enhanced in the groups that swam when compared to the 3- and 8 months-SED groups. Lipid peroxidation was greater in the aged mice that did not perform the physical exercise protocol and might have contributed to the cognitive impairment in this group. In conclusion, an aerobic physical exercise protocol, performed through regular swimming sessions, inhibited cognitive impairment, memory loss and lipid peroxidation in the aged mice, while pre- and post-synaptic proteins were enhanced in the hippocampus of young and aged mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When faced with bioenergetic obstacles brought on by continual activity in neural circuits and ambient energetic stressors like physical exercise, brain cells typically adapt (Camandola & Mattson, 2017). These adaptive reactions take place at the cellular level by involving the creation of new synapses, the "strengthening" of preexisting ones, and the development of new neurons (Kandel, 2012). Bioenergetic challenges seen in Alzheimer's disease and aging activate transcription factors at the molecular level, resulting in various metabolic, oxidative, excitotoxic, and proteotoxic stresses that are involved in the pathogenesis of several brain disorders and different conditions (Camandola & Mattson, 2017). On the contrary, the physical exercise, also an intermittent bioenergetic challenge, can contribute to delay aging-related factors (De Sousa et al., 2021a, 2021b).
One of the main causes of diminished autonomy in the aging population is brain and cognitive deficits that come with natural aging process and sedentary lifestyle (Albinet et al., 2016). Nevertheless, it has been reported that aerobic exercise, which improves cardiorespiratory fitness, may be useful in mitigating or even reversing the rate of these age-related cognitive and cerebral deficits (De la Rosa et al., 2019; Sousa et al., 2021a, 2021b). Animal models subject to different type of physical exercises, such as resistance or aerobic training, have shown to be able to reverse or inhibit cognitive decline and memory loss in different conditions and diseases (De Sousa et al., 2020a, 2020b, 2020c). Besides the experimental studies using animal models have been successfully reversing dementia-related conditions and diseases through physical exercise protocols (Li et al., 2019) the same has not been seen in humans.
There are different type of memories, such as working and spatial memories, that can be stimulated through different mechanisms when using aerobic or resistance training (Cassilhas et al., 2012). Aerobic training is a type of physical exercise that involves mainly cardiovascular conditioning and, therefore, is also called “cardio” program. Swimming and running are well-known types of aerobic training. Aerobic training activates more oxidative fibers, while resistance training activates more glycolytic fibers (Pearen et al., 2012). Despite the brain size when compared to the rest of the body, the brain is responsible for consuming approximately 20% of the oxygen consumed by the body. Therefore, aerobic exercise protocols, such as swimming, can bring greater physiological benefits to it (Nonato et al., 2016).
In order to process information, healthy synaptic terminals send signals between cells (Leshchyns’Ka et al., 2015). As we age, there are fewer synapses and fewer impulses are transmitted by them what might favor the development of several conditions and neurodegenerative diseases, especially the ones related to cognitive function, memory, and behavior (Bečanović et al., 2021). Physical exercise has been used as a non-pharmacological tool that can delay or avoid the negative age-related physiological outcomes (Graham et al., 2019). We hypothesized that a physical exercise intervention would impact pre- and post-synaptic proteins and there would be consequences on the cognitive function and memory in an animal model of aging. Here, we evaluated the effects of an aerobic physical exercise protocol in the brain and behavior of young and aged mice.
Methods
Animals
Experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication, 1996), and authorized by the local Animal Use Ethics Committee at the Federal University of Jequitinhonha and Mucuri Valleys. Mice were acquired from our university mouse colony and housed in plastic cages with no more than 4 mice per cage at 22 °C on a 12:12 h light–dark cycle, with free access to standard rodent chow and water. We chose to work with 3- and 18- months old mice for multiple reasons. At 3 months old, mice are young adults and, therefore, even the animals that do not exercise should not present cognitive impairment and memory loss (Freitas et al., 2019). Thus, 3 months old mice that perform exercise should not present differences in cognitive and memory tasks to the 3 months old that does not exercise because they are too young either to have memory deficits or to enhance cognitive function and memory just because of the physical exercise. However, at 18 months of age, mice are old, and one of the consequences of this aging condition is the possibility of developing cognitive decline and memory loss, which could be inhibited by the regular practice of physical exercise (De la Rosa et al., 2019; Sousa et al., 2021a, 2021b). By using 3 months and 18 months old mice we have not only paired controls but also some expected responses like cognitive decline in the aged mice that do not exercise that will help to understand better how the pre- and post-synaptic mechanisms are affected by the physical exercise.
It is also important to discuss and mention that most scientists take sample size for granted and, as a rule, employ 10 (ten) mice per group. However, scientists must think about using a scientific justification to choose the sample size for every study (Charan & Kantharia, 2013). Just saying that the number of mice chosen was based on previous studies is not enough either. Usually, sample size can be determined by using dedicated software. It should be noted that an experiment is obviously powerful if it successfully rejects the null hypothesis, but a false-positive result is also possible. Conversely, a study may not have had enough power or there may not have been a significant enough treatment effect to warrant detecting it if it is unable to reject the null hypothesis (Festing, 2018; Serdar et al., 2021). The sole goal of a power analysis, which is the probability of finding an effect, is to plan experiments that will enable the detection of effect size, which is the expected mean difference between the groups, is big enough to be of scientific relevance (Festing, 2018). For example, for most experimental studies if you have 12 animals per group you should have an excellent sample size, power analysis and effect size. However, it is important to reaffirm that sample size will depend on the research question and study design, and the calculations when planning and before starting a study are necessary (Charan & Kantharia, 2013; Dell et al., 2002; Festing, 2018; Serdar et al., 2021). Therefore, the correct or incorrect choice of the sample size affects the hypothesis and the study design, and that’s why, according to our hypothesis, we used 12 mice per group in our study to guarantee the best possible results.
Physical Exercise Protocol
Male C57BL/6J mice were randomly distributed into 4 groups (n = 12 per group). The subdivision of the groups is presented as follows: 3 months-SED, 3 months-EXE, 18 months-SED, 18 months-EXE. All groups were exposed to the room environment where the swimming protocol occurred for the same amount of time. The exercised groups swam for 30 min each. While the 3 months-EXE group was swimming for 30 min, the 3 months-SED group was kept in the same room, but the animals did not perform the physical exercise protocol. The same routine was done with the other 2 groups: while the 18 months-EXE group was swimming for 30 min, the 18 months-SED group was kept in the same room, but the animals did not perform the physical exercise protocol.
Animals were familiarized with the swimming exercise for 1 (one) week. Animals went through an adaptation week, and they swam 10 min on the first day, 15 min on the second day, 20 min on the third day, 25 min on the fourth day, and 30 min on the 5th day, followed by 48 h rest before starting the swimming protocol. The training protocol consisted of swimming sessions with a duration of 30 min, with no overload, 5 days per week (Monday to Friday), for 4 weeks. Animals swam in groups of six animals in a plastic pool (60 cm depth × 150 cm diameter). The water temperature was always kept at approximately 24 °C. The same experienced researcher always conducted the experiments. The physical exercise protocol was adapted from De Sousa et al., 2020a, 2020b, 2020c (De Sousa et al., 2020a, 2020b, 2020c).
Behavioral Tasks
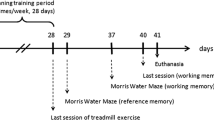
Animals performed a battery of behavioral tasks 24 h after the last swimming session. We evaluated locomotor activity, anxious profile, working and spatial memories. All tasks and their respective sessions were performed 24hs apart from each other. We used the Noldus Etho Vision XT V.16 video tracking system (Leesburg, VA, USA) to record test data. After the behavioral tasks their brain was harvested, and the hippocampus was collected for western blot analysis, lipid peroxidation and redox state parameters (Fig. 1).
Experimental design timeline. The first familiarization session lasted for 10 min, and sessions duration were increased by 5 min/day until reached a total of 30 min on the 5th day (last day of the adaptation week). Training protocol was initiated 48 h after the last day of the familiarization week. The exercise protocol consisted of 30 min of swimming, 5 days per week for 4 weeks. Thereafter, mice went under behavioral tasks. At the end of the behavioral tasks the animals were euthanized, and their brain harvested. Hippocampus was collected for western blot assays, lipid peroxidation and redox state analysis. Min minutes, OFT open field task, NOR novel object recognition task, DOR displaced object recognition task, WB western blot, MDA malondialdehyde, FRAP ferric-reducing antioxidant power
Open Field Task (OFT)
OFT is used to assess both rodent locomotor activity and anxiety-like behavior. The apparatus to perform this task is an arena that is 50 cm (length) × 50 cm (width) × 38 cm (height), made from white high density and non-porous plastic. The mouse was placed in the middle of the arena while tracking its movement for a ten-minute period. We measured total distance traveled along with other parameters such as time spent in central area, which evaluate locomotor activity and anxious profile, respectively, as previously described (De Sousa et al., 2020a, 2020b, 2020c).
Novel Object Recognition (NOR)
The task was carried out in the same arena used for the open field task. This task aims to evaluate the working memory. Initially, animals are allowed to freely explore the arena for 5 min (habituation phase). One hour later, the training phase consists of a 5 min session. Animals were placed at the center of the arena in the presence of two identical objects, with one being on the left and the other on the right side of the arena, with equal distances between them and the arena’s walls. Then, the amount of time spent exploring each object was recorded. Sniffing and touching the object were considered as exploratory behavior. The arena was cleaned with 70% ethanol between all the trials. One hour after the training, the animals were replaced in the arena for the test session, which also lasts 5 min. During the test session, one of the objects was replaced by a new one, and the amount of time spent exploring old and new objects was measured. If the mice did not spend a significant amount of time exploring the new object is an indicator of cognitive decline and memory loss. All the animals needed to explore both objects for a total period of no less than 2 s, otherwise they would be excluded from further analysis. The results were expressed as a percentage of time exploring the novel object (De Sousa et al., 2020a, 2020b, 2020c).
Displacement Object Recognition (DOR)
The major difference between the NOR task and the DOR recognition task occurs on the day of testing, when for the DOR one object is displaced to a novel location. The main measure is the time spent in exploration of the two objects at test. Both tasks were based on a rodent's innate preference for novelty. However, here the object is moved in the space. Thus, this task evaluates primarily the spatial memory. All the animals needed to explore both objects for a total period of no less than 2 s, otherwise they would be excluded from further analysis. Animals that remember the original training experience will preferentially explore the displaced object relative to the non-displaced object.
Western Blot
Levels of proteins were determined from the hippocampus of the mice by Western blot analysis. Hippocampal tissue was homogenized and lysed using 1 × RIPA Buffer with protease and phosphatase inhibitors. Protein concentration was determined by a bicinchoninic acid (BCA) protein quantification kit (Bio-Rad). Total protein lysates (30 μg) were separated in equal volume on 4–12% prepared gels. Proteins were subsequently transferred to an Immuno-Blot polyvinylidene fluoride membrane (PVDF cat. # 1620177) in transfer buffer 20% methanol. Membranes were blocked for 1 h in the tris buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) and 5% bovine serum albumin (BSA). Primary and secondary antibodies were prepared in TBS-T 5% BSA. Primary antibody incubations were done overnight at 4 °C, and secondary antibody incubations were done at room temperature for 1 h. Membranes were developed using super signal west Femto chemiluminescent detection kit (Thermo Scientific). The following primary antibodies were used: AKT (1:1000, Cell Signaling Technology, cat. #4060), phospho-AKT (Thr308) (1:1000, Cell Signaling Technology, cat. #13038), PKA (1:1000, Cell Signaling Technology, cat. #5842), phospho-PKA (1:1000, Cell Signaling Technology, cat. #4781), PSD95 (1:1000, Cell Signaling Technology, cat. # 2507), Synaptophysin (1:1000, Cell Signaling Technology, cat. #5461), β-Actin (1:1000, Cell Signaling Technology, cat. #3700). We used secondary antibodies goat anti-rabbit and goat anti-mouse IgG conjugated with Horseradish Peroxidase (1:5.000 and 1:10.000 dilution, Jackson Immuno Research Laboratories, Inc, US). To quantify the bands, we used the software Image J.
Lipid Peroxidation and Redox State
Quantification of thiobarbituric acid reactive substances (TBARS) was used to evaluate lipid peroxidation. The formation of TBARS during an acid-heating reaction was determined at 532 nm based on a standard curve with known concentrations of malondialdehyde (MDA) (1,1,3,3-tetramethoxypropane) (Sigma, MO, USA). The amount of MDA produced was interpreted as the TBARS levels. Results are expressed as nmol of MDA per mg protein (nmol.MDA/mg protein). TAC was estimated by the ferric-reducing antioxidant power (FRAP) assay. Sample capacity to reduce the ferric-tripyridyltriazine [Fe(III)-TPTZ] complex to ferrous tripyridyltriazine [Fe(II)-TPTZ] was measured at 550 nm. A standard curve of known concentrations of FeSO4 was used to estimate FRAP (nM FeSO4/mg protein). All measurements were performed in triplicate, as previously described (Freitas et al., 2019; Melo et al., 2019).
Statistical Analysis
We used the Noldus Etho Vision XT V.16 video tracking system (Leesburg, VA, USA) to record behavioral test data. Data were analyzed to behavioral, molecular and biochemical assays using GraphPad Prism (version 9.00 for Mac OS X, GraphPad Software, San Diego, CA, USA), and presented as mean ± standard error of the mean (s.e.m). One-way analysis of variance (ANOVA) corrected for multiple comparisons using Tukey post-hoc was employed to compare data among the groups. We adopted a significant result as *p < 0.05; **p < 0.01, ***p < 0.001, and ****p < 0.001.
Results
Working and Spatial Memory Were Impaired Due to Aging
The behavioral tasks (Fig. 2a) were the first analysis done after the physical exercise protocol was finished. Aged mice that did not exercise showed cognitive decline and memory loss in the working and spatial memory tasks when compared to all the groups (Fig. 2e, f). Locomotor activity was reduced in the aged mice that did not exercise, but there was not significant difference among the groups (Fig. 2b). The animals did not show any differences in the anxious profile either (Fig. 2c). As expected, the training session did not show differences between the objects and groups revealing that there were not any preferences for any of the objects (Fig. 2d). Together, these data indicate that aged mice that did not exercise presented memory impairment. These data corroborate our group previous finding showing that healthy young animals won’t show cognitive impairment or improvement after physical exercise (Freitas et al., 2019), while memory loss will be inhibited in aged mice that exercise (Yao et al., 2024).
Spatial and working memory are impaired in aged mice that do not exercise. a Representative scheme of behavioral tasks. b Total distance traveled (m) in the OFT. c Time spent in center in the OFT. d Time spent in the left object (%) during the training session of NOR. e Time spent in the novel object (%) during the test session of NOR. f Time spent in the displaced object (%) during the test session of DOR. Mice groups that exercised are presented in blue bars and single blue points represent the result for each mouse, and groups that did not exercised are shown in black bars with single black points representing the result for each mouse. Values are shown as mean ± s.e.m (n = 12/group), *p < 0.05, **p < 0.01
Next, we decided to evaluate memory-related proteins due to the behavioral deficits seen in the working and spatial memories, NOR and DOR tasks, respectively. We chose to evaluate synaptophysin, PSD95, PKA and pPKA, and AKT and pAKT because it has been shown that these are main biomarkers related to changes in cognitive function and memory (De Sousa et al., 2020a, 2020b, 2020c; Gylys et al., 2004; Kandel, 2012).
Pre- and Post-synaptic Proteins are Enhanced in the Groups that Performed the Physical Exercise Protocol Compared to the Aged Mice that Did Not Exercise
Pre-synaptic biomarker, synaptophysin, was enhanced in both exercised groups, 3 months-EXE and 18 months-EXE, when compared to the young and aged mice that did not exercise (Fig. 3a, b). Similarly, post-synaptic biomarker, PSD95, was also enhanced in both exercised groups when compared to the non-exercised groups (Fig. 3a, c). No significant differences were seen when comparing young and aged mice that did not exercise, despite the levels of the pre- and post-synaptic biomarkers being slightly lower in the 18 months-SED group. These results together indicate that pre- and post-synaptic proteins are enhanced in the groups that performed the physical exercise protocol, independently of their age, when compared to the young and aged mice that did not exercise. In a recent published manuscript, the authors revealed that treadmill moderate-intensity exercise routine improved spatial memory and synaptic function in the hippocampus of SAMP8 mice, an animal model of aging (Guo et al., 2023). Then, the authors concluded that enhancing synaptic function could be a strong mechanism through which aerobic exercise enhances memory retention, what is a very similar result to our current findings.
Physical exercise improves pre- and post-synaptic proteins in the hippocampus of young and aged mice. a Representative bands for synaptophysin, PSD95 and β-Actin, which is our control protein used for normalization. b Hippocampal protein levels for synaptophysin, and c PSD95. Mice groups that exercised are presented in red bars and single red points represent the result for each mouse, and groups that did not exercised are shown in black bars with single black points representing the result for each mouse. Values are shown as mean ± s.e.m (n = 6–8/group), *p < 0.05, **p < 0.01, ***p < 0.001
Phospho-PKA and Phospho-AKT Are Not Significantly Altered
The pathway that involves phosphorylation of PKA (pPKA) is one of the regulators of memory (Kandel, 2012). However, we did not find any statistical differences among the groups, besides a clear enhancement of it in the aged mice group that exercised (Fig. 4a, b). When comparing 18 months-EXE with 18 months-SED, and 3 months-EXE with 3 months-SED we can see that there is an increase in pPKA, even if it is not significant. Insulin resistance is known as one of the possible causes for cognitive decline and memory loss, whereas the phosphorylation of AKT (pAKT) plays an important role (De Sousa, 2018). Nevertheless, no differences were found by comparing all the group’s results (Fig. 4a, c). Similarly to what was seen in pPKA, when comparing the levels of pAKT of 18 months-EXE with 18 months-SED, and 3 months-EXE with 3 months-SED we can see that there is an increase in pAKT, even if it is not significant. Together, these results indicate that the improvement in memory tasks happened mainly due to the enhancement of the levels of pre- and post-synaptic proteins.
No significant changes were seen in pPKA and pAKT in the hippocampus of young and aged mice. a Representative bands for pPKA, PKA, pAKT, AKT, and β-Actin. Phosphorylated proteins were normalized by the total level of the same protein, and β-Actin is also shown. b Hippocampal protein levels for pPKA/PKA, and c pAKT/AKT. Mice groups that exercised are presented in pink bars and single pink points represent the result for each mouse, and groups that did not exercised are shown in black bars with single black points representing the result for each mouse. Values are shown as mean ± s.e.m (n = 6–8/group), *p < 0.05, **p < 0.01
It has been recently shown that a long-term running exercise, which lasted for 5 months could improve the spatial memory and enhancing the AKT levels in developing mice (Wan et al., 2024). We suggest that despite not being statistically significant, the mentioned differences in pAKT when comparing 18 months-SED, which presented reduction on its levels, with 18 months-EXE and 3 months-EXE may have contributed to avoid cognitive decline and memory loss. In addition, our protocol lasted just for 4 weeks, and if the mice are exposed to more time of swimming probably the pAKT levels will increase. It has also been reported that mice subjected to treadmill running for just 21 days and that had restraint stress applied for 9 days within the period of the exercise regimen inhibited the enhancement of the ratio pPKA/PKA in basal lateral amygdala what contributed to avoid the development of an anxious profile (Leem et al., 2019). Conversely, another recent study pointed out that pPKA level was enhanced after a swimming protocol of 60 min/day, 6 days/week for 6 weeks in the hippocampus of aged rats, what contributed to a better spatial memory (Jin et al., 2024). It seems that pPKA will be enhanced or diminished accordingly to the brain area, and with the exercise intervention protocol (i.e., intensity, duration, frequency), and the previous or induced condition or disease of the animal model. This is a topic that still needs to be better studied and elucidated in the future.
Lipid Peroxidation is Greater in Aged Mice that Does Not Exercise
It is known that the development of lipid peroxidation, oxidative stress and inflammation might be changed by physical exercise and can contribute to cell death and memory changes (De la Rosa et al., 2019; Yang & Stockwell, 2016). Lipid peroxidation was greater in the aged mice that did not perform the physical exercise protocol (Fig. 5a). In our study, the antioxidant mechanisms were present in the young-exercised group (Fig. 5b) when compared to the 18 months-SED group. Besides not being statistically significant, we can also see higher levels of the antioxidant mechanisms in the aged mice who exercised when compared to the aged mice that did not exercise (Fig. 5b). Here, we have shown that there was an enhancement of the activity of the antioxidant defense in the 3 months-EXE group, and that there was lipid peroxidation in the 18 months-SED group. This greater lipid peroxidation might have contributed to the memory impairment. It has been shown that aerobic exercise training reduced magnetic resonance imaging (MRI) detected abnormalities, insulin resistance and markers of oxidative stress and inflammation in old ApoE−/− mice (Chirico et al., 2016). In this study, the authors used young and old ApoE−/− mice, a similar approach to our study, and revealed the protective effect of exercise on neurovascular damage in these old mice, what corroborates our current findings.
Lipid peroxidation is greater in the hippocampus of aged mice that do not exercise. a MDA (nmol MDA/mg protein) level from whole brain lysates, and b FRAP (nm FeSO4/mg protein). Mice groups that exercised are presented in orange bars and single orange points represent the result for each mouse, and groups that did not exercised are shown in black bars with single black points representing the result for each mouse. Values are shown as mean ± s.e.m (n = 8/group), *p < 0.05, **p < 0.01
Discussion
In the present study, we revealed that young and aged mice after 4 weeks of swimming develop several neuroprotective mechanisms to inhibit the impairment of the cognitive function, memory loss, pre- and post-synaptic deficits, and lipid peroxidation. The results also showed an improvement of the redox state in young mice that exercised when compared to aged mice that did not. However, no significant changes were seen in locomotor activity, and anxiety. These results suggested that exercise rescues age-related memory impairment.
Learning tasks were the first behavioral systems to be analyzed aiming to elucidate the neural circuits that mediate the change on these behaviors and the critical synaptic sites where they may occur (Kandel, 2012). NOR and DOR tasks are two well-known behavioral tasks that are used to measure performance and relative well-being with the hippocampus, a brain area, playing a crucial role in memory and cognition. Both tasks take advantage of mice’s innate preference for novelty to look for objects that they have not encountered before or that were moved from their original positions (Denninger et al., 2018). However, the main difference between the two memory tasks is that the DOR focuses primarily on spatial learning, which relies mostly on hippocampus activity. The NOR relies on several brain regions when it comes to working memory, with one of its main brain areas involved being the hippocampus. Here, we showed that physical exercise had a neuroprotective effect in young and aged C57BL/6 mice.
Recent studies from our group showed that a high-intensity resistance training (De Sousa et al., 2020a, 2020b, 2020c) or high-intensity interval training (HIIT) (Freitas et al., 2019). Can induce changes in cognitive function and positive physiological effects that contributed to preserving cognition. Different types of physical exercise and protocols, which may vary in intensity, frequency and duration, have been successfully used to reverse and inhibit several negative outcomes of a vast number of conditions and diseases, such as in type 2 diabetes (Heled et al., 2005), obesity (de Lima et al., 2021), Alzheimer’s disease (Alkadhi & Dao, 2018), infections (De Sousa et al., 2020a, 2020b, 2020c), among many others (de Araujo et al., 2012; Fiorelli et al., 2019). Keeping physically active can be determinant whereas you will or not develop neurodegenerative diseases when getting older (Pedersen & Saltin, 2015). Investing in a healthy lifestyle is the cheapest and best approach to try to minimize the risks of aging with a bad health condition.
Loss of synaptic proteins and synaptic damage are vastly correlated with cognitive decline and memory loss (George & Hemachandra Reddy, 2019). There are several pathways that can potently reduce synaptic plasticity and inhibit hippocampal long-term potentiation (Gong et al., 2003), a master regulator of cognitive function and memory, such as oxidative stress and inflammatory mechanisms (Wang et al., 2019). It is well-known that physical exercise can prevent the loss of synaptic proteins and increase the levels of memory-related proteins, and also increase neurogenesis, mainly in hippocampus (Park et al., 2018). In the present study, the physical exercise protocol adopted was capable of significantly enhancing the level of pre- and post-synaptic proteins in the hippocampus of young and aged mice that exercised when compared to the ones that did not exercise. To our knowledge, this is the first study to use swimming as the physical exercise type and to compare changes in behavior and in memory-related proteins in young and aged mice.
There are hundreds of genes that are acutely regulated by synaptic activity, which may involve different mechanisms such as the cAMP/PKA-dependent activation (Hasel et al., 2017). PKA consists of two regulatory subunits that inhibit two catalytic subunits, which are the active phophorylating portions of the enzyme, and when the level of cAMP rises in the organism, the cAMP binds to the PKA regulatory subunits what leads it to undergo a conformational change that activates the catalytic subunits, and allows it to phosphorylate its substrates (Kandel, 2012). The cAMP-dependent PKA signaling cascade is a ubiquitous pathway targeting downstream a vast number of neuromodulators (Plattner et al., 2015).
It also has been reported that insulin resistance in the brain could be triggered by significant reduction in pAKT levels (De Sousa, 2018). The phosphatidylinositol 3 kinase (PI3K)-protein kinase B (AKT) pathway is involved in several roles like cell growth and proliferation, survival, and intracellular trafficking (Qiao et al., 2018). It is known that AKT phosphorylation can trigger mitochondrial biogenesis, enhance ATP production, and inhibit apoptosis mediated neuronal death. AKT is also known for its interactivity with cAMP/PKA pathway activation being both pathways, PI3K/AKT and cAMP/PKA, associated with cognitive and memory improvement (Ahmed et al., 2021).
In the present study, besides not having significantly higher levels of pPKA and pAKT in the hippocampus of the aged-exercised group, there is a clear enhancement of the pAKT levels in the 3 months-EXE and 18 months-EXE groups when compared to the 18 months-SED group. We suggest that this enhancement was enough to contribute to preserve the cognitive function and contributed to the improvement of pre- and post-synaptic proteins levels and avoid memory loss in the aged mice. Nevertheless, there are another components that have been indicated to be present in conditions that might lead to cognitive decline such as oxidative stress, and inflammation (de Oliveira et al., 2022).
Finally, lipid peroxidation is related to a vast number of pathologies and conditions, such as the development of ferroptosis, a new form of cell death (Latunde-Dada, 2017). The lipid peroxidation process occurs when free radicals or oxidants attack lipids, but especially polyunsaturated fatty acids. Phospholipids are abundant in brain tissues, and these phospholipids can be oxidized to lipid peroxidation (Garcia et al., 2005). It is known that physical exercise can inhibit lipid peroxidation and oxidative stress mechanisms (Cifuentes et al., 2010; Freitas et al., 2019). In our study, we showed that 18 months-SED mice presented significant higher lipid peroxidation levels when compared to the other groups, what certainly might have contributed to the cognitive decline and memory impairment. However, when evaluating the antioxidant mechanisms, we also observed that just the 3 months-EXE group had a significant improvement when compared to the 18 months-SED group.
Although the study presents interesting and promisor findings, there are some limitations that need to be addressed. Since the study was performed in male mice the current results of this study should not be applied to female mice. We used the whole hippocampus for our molecular and biochemical analyses and the expressed results can’t be limited to a specific hippocampal area, such as the dentate gyrus, CA1, CA2, or CA3. Physical exercise protocols are more complex than physical activity interventions. This occurs because physical exercise may include different modalities (i.e., swimming, running, cycling, etc.) performed at different intensities (i.e., low, moderate, high) with multiple different combinations of frequency, duration, and volume. Therefore, the results can’t be compared to high-intensity exercise interventions, for example. Finally, animal models may be a useful tool to study several types of interventions and their consequences in many conditions and diseases to find out possible hints that lead to the discoveries of a possible cure or attenuation of the symptoms of a specific condition or pathology. However, animal models, such as mice, are not humans and do not present the same physiology and, therefore, the results of this or any study with animal models should not be translated directly to humans. Clinical trials need always to be performed to confirm or not findings made by using animal models.
Conclusion
Aerobic physical exercise inhibits cognitive impairment, and memory loss in aged mice, and enhances pre- and post-synaptic biomarkers in the hippocampus of young and aged. Not incorporating physical exercise into the routine can lead to lipid peroxidation in aged mice. These results suggest a strong potential for physical exercise therapeutics, what could save a lot of money from the public safes that are spent every year to treat diseases in the population that could be avoided just by adopting a healthy and active lifestyle.
Data Availability
All data generated or analyzed during this study are included in this published article and further datasets are available from the corresponding author on reasonable request.
References
Ahmed, S., Kwatra, M., Gawali, B., Panda, S. R., & Naidu, V. G. M. (2021). Potential role of TrkB agonist in neuronal survival by promoting CREB/BDNF and PI3K/Akt signaling in vitro and in vivo model of 3-nitropropionic acid (3-NP)-induced neuronal death. Apoptosis, 26(1–2), 52–70. https://doi.org/10.1007/s10495-020-01645-x
Albinet, C. T., Abou-Dest, A., André, N., & Audiffren, M. (2016). Executive functions improvement following a 5-month aquaerobics program in older adults: Role of cardiac vagal control in inhibition performance. Biological Psychology, 115, 69–77. https://doi.org/10.1016/j.biopsycho.2016.01.010
Alkadhi, K. A., & Dao, A. T. (2018). Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Molecular and Cellular Neuroscience, 86, 25–29. https://doi.org/10.1016/j.mcn.2017.11.008
Bečanović, K., Muhammad, A., Gadawska, I., Sachdeva, S., Walker, D., Lazarowski, E. R., et al. (2021). Age-related mitochondrial alterations in brain and skeletal muscle of the YAC128 model of Huntington disease. Aging and Mechanisms of Disease, 7(1), 1–14. https://doi.org/10.1038/s41514-021-00079-2
Camandola, S., & Mattson, M. (2017). Brain metabolism in health, aging, and neurodegeneration. The EMBO Journal. https://doi.org/10.15252/embj.201695810
Cassilhas, R. C., Lee, K. S., Fernandes, J., Oliveira, M. G. M., Tufik, S., Meeusen, R., & De Mello, M. T. (2012). Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience, 202, 309–317. https://doi.org/10.1016/j.neuroscience.2011.11.029
Charan, J., & Kantharia, N. D. (2013). How to calculate sample size in animal studies? Journal of Pharmacology and Pharmacotherapeutics, 4(4), 303–306. https://doi.org/10.4103/0976-500X.119726
Chirico, E. N., Di Cataldo, V., Chauveau, F., Geloën, A., Patsouris, D., Thézé, B., et al. (2016). Magnetic resonance imaging biomarkers of exercise-induced improvement of oxidative stress and inflammation in the brain of old high-fat-fed ApoE −/− mice. The Journal of Physiology, 594(23), 6969–6985. https://doi.org/10.1113/JP271903
Cifuentes, D. J., Rocha, L. G., Silva, L. A., Brito, A. C., Rueff-barroso, C. R., Porto, L. C., & Pinho, R. A. (2010). Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthritis and Cartilage, 18(8), 1088–1095. https://doi.org/10.1016/j.joca.2010.04.004
de Araujo, C. C., Silva, J. D., Samary, C. S., Guimaraes, I. H., Marques, P. S., Oliveira, G. P., et al. (2012). Regular and moderate exercise before experimental sepsis reduces the risk of lung and distal organ injury. Journal of Applied Physiology, 112(7), 1206–1214. https://doi.org/10.1152/japplphysiol.01061.2011
De la Rosa, A., Solana, E., Corpas, R., Bartrés-Faz, D., Pallàs, M., Vina, J., et al. (2019). Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Scientific Reports, 9(1), 1–11. https://doi.org/10.1038/s41598-019-40040-8
de Lima, N. S., De Sousa, R. A. L., Amorim, F. T., Gripp, F., Dinizemagalhães, C. O., Henrique Pinto, S., et al. (2021). Moderate-intensity continuous training and high-intensity interval training improve cognition, and BDNF levels of middle-aged overweight men. Metabolic Brain Disease. https://doi.org/10.1007/s11011-021-00859-5
de Oliveira, L. R. S., Machado, F. S. M., Rocha-Dias, I., Magalhães, C. O. D. E., De Sousa, R. A. L., & Cassilhas, R. C. (2022). An overview of the molecular and physiological antidepressant mechanisms of physical exercise in animal models of depression. Molecular Biology Reports. https://doi.org/10.1007/s11033-022-07156-z
De Sousa, R. A. L. (2018). Brief report of the effects of the aerobic, resistance, and high-intensity interval training in type 2 diabetes mellitus individuals Diabetes mellitus. International Journal of Diabetes in Developing Countries, 38(2), 138–145. https://doi.org/10.1007/s13410-017-0582-1
De Sousa, R. A. L., Caria, A. C. I., De Jesus Silva, F. M., Dinizemagalhães, C. O., Freitas, D. A., Lacerda, A. C. R., et al. (2020a). High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiology & Behavior, 223(6), 1–7. https://doi.org/10.1016/j.physbeh.2020.112998
De Sousa, R. A. L., Harmer, A. R., Freitas, D. A., Mendonça, V. A., Lacerda, A. C. R., & Leite, H. R. (2020b). An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Molecular Biology Reports, 47, 6347–6356. https://doi.org/10.1007/s11033-020-05693-z
De Sousa, R. A., Peixoto, M. F., Leite, H. R., Oliveira, L. R., Freitas, D. A., Silva-Júnior, F. A., Oliveira, H. S., Rocha-Vieira, E., Cassilhas, R. C., & Oliveira, D. B. (2020c). Neurological consequences of exercise during prenatal Zika virus exposure to mice pups. International Journal of Neuroscience, 21, 1–11. https://doi.org/10.1080/00207454.2020.1860970
De Sousa, R. A. L., Rocha-Dias, I., de Oliveira, L. R. S., Improta-Caria, A. C., Monteiro-Junior, R. S., & Cassilhas, R. C. (2021a). Molecular mechanisms of physical exercise on depression in the elderly: a systematic review. Molecular Biology Reports. https://doi.org/10.1007/s11033-021-06330-z
Dell, R. B., Holleran, S., & Ramakrishnan, R. (2002). Sample Size Determination. ILAR Journal, 43(4), 207–213. https://doi.org/10.1093/ilar.43.4.207
Denninger, J. K., Smith, B. M., & Kirby, E. D. (2018). Novel object recognition and object location behavioral testing in mice on a budget. Journal of Visualized Experiments, 2018(141), 1–10. https://doi.org/10.3791/58593
Festing, M. F. (2018). On determining sample size in experiments involving laboratory animals. Laboratory Animals, 52(4), 341–350. https://doi.org/10.1177/0023677217738268
Fiorelli, C. M., Ciolac, E. G., Simieli, L., Silva, F. A., Fernandes, B., Christofoletti, G., & Barbieri, F. A. (2019). Differential acute effect of high-intensity interval or continuous moderate exercise on cognition in individuals with Parkinson’s Disease. Journal of Physical Activity and Health. https://doi.org/10.1123/jpah.2018-0189
Freitas, D. A., Rocha-Vieira, E., De Sousa, R. A. L., Soares, B. A., Rocha-Gomes, A., Chaves Garcia, B. C., et al. (2019). High-intensity interval training improves cerebellar antioxidant capacity without affecting cognitive functions in rats. Behavioural Brain Research, 376, 112181. https://doi.org/10.1016/j.bbr.2019.112181
Garcia, Y. J., Rodríguez-Malaver, A. J., & Peñaloza, N. (2005). Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. Journal of Neuroscience Methods, 144(1), 127–135. https://doi.org/10.1016/j.jneumeth.2004.10.018
George, E. K., & Hemachandra Reddy, P. (2019). Can healthy diets, regular exercise, and better lifestyle delay the progression of dementia in elderly individuals? Journal of Alzheimer’s Disease, 72(s1), S37–S58. https://doi.org/10.3233/JAD-190232
Gong, Y., Chang, L., Viola, K. L., Lacor, P. N., Lambert, M. P., Finch, C. E., et al. (2003). Alzheimer’s disease-affected brain: Presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proceedings of the National Academy of Sciences of the United States of America, 100(18), 10417–10422. https://doi.org/10.1073/pnas.1834302100
Graham, L. C., Grabowska, W. A., Chun, Y., Risacher, S. L., Philip, V. M., Saykin, A. J., et al. (2019). Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiology of Aging, 80, 154–172. https://doi.org/10.1016/j.neurobiolaging.2019.03.018
Guo, L., Li, S., Zhang, Y., Yang, X., Zhang, Y., Cui, H., & Li, Y. (2023). Effects of exercise intensity on spatial memory performance and hippocampal synaptic function in SAMP8 mice. Neurobiology of Learning and Memory, 203, 107791. https://doi.org/10.1016/j.nlm.2023.107791
Gylys, K. H., Fein, J. A., Yang, F., Wiley, D. J., Miller, C. A., & Cole, G. M. (2004). Synaptic changes in Alzheimer ’ s disease accompanied by decreased PSD-95 fluorescence. Neurobiology, 165(5), 1809–1817.
Hasel, P., Dando, O., Jiwaji, Z., Baxter, P., Todd, A. C., Heron, S., et al. (2017). Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nature Communications, 8(May), 15132. https://doi.org/10.1038/ncomms15132
Heled, Y., Dror, Y., Moran, D. S., Rosenzweig, T., Sampson, S. R., Epstein, Y., & Meyerovitch, J. (2005). Physical exercise increases the expression of TNFalpha and GLUT 1 in muscle tissue of diabetes prone Psammomys obesus. Life Sciences, 77(23), 2977–2985. https://doi.org/10.1016/j.lfs.2005.05.033
Jin, Y., Li, X., Wei, C., & Yuan, Q. (2024). Effects of exercise-targeted hippocampal PDE-4 methylation on synaptic plasticity and spatial learning/memory impairments in D-galactose-induced aging rats. Experimental Brain Research, 242(2), 309–320. https://doi.org/10.1007/s00221-023-06749-9
Kandel, E. R. (2012). The molecular biology of memory: CAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Molecular Brain, 5(14), 1–12.
Latunde-Dada, G. O. (2017). Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochimica Et Biophysica Acta - General Subjects, 1861(8), 1893–1900. https://doi.org/10.1016/j.bbagen.2017.05.019
Leem, Y.-H., Jang, J.-H., Park, J.-S., & Kim, H.-S. (2019). Exercise exerts an anxiolytic effect against repeated restraint stress through 5-HT2A-mediated suppression of the adenosine A2A receptor in the basolateral amygdala. Psychoneuroendocrinology, 108, 182–189. https://doi.org/10.1016/j.psyneuen.2019.06.005
Leshchynska, I., Liew, H. T., Shepherd, C., Halliday, G. M., Stevens, C. H., Ke, Y. D., et al. (2015). Aβ-dependent reduction of NCAM2-mediated synaptic adhesion contributes to synapse loss in Alzheimer’s disease. Nature Communications. https://doi.org/10.1038/ncomms9836
Li, B., Liang, F., Ding, X., Yan, Q., Zhao, Y., Zhang, X., et al. (2019). Interval and continuous exercise overcome memory deficits related to β-Amyloid accumulation through modulating mitochondrial dynamics. Behavioural Brain Research, 376(8), 112171. https://doi.org/10.1016/j.bbr.2019.112171
Melo, C. S., Rocha-Vieira, E., Freitas, D. A., Soares, B. A., Rocha-Gomes, A., Riul, T. R., et al. (2019). A single session of high-intensity interval exercise increases antioxidants defenses in the hippocampus of Wistar rats. Physiology and Behavior, 211(3), 112675. https://doi.org/10.1016/j.physbeh.2019.112675
Nonato, L. F., Rocha-Vieira, E., Tossige-Gomes, R., Soares, A. A., Soares, B. A., Freitas, D. A., et al. (2016). Swimming training attenuates oxidative damage and increases enzymatic but not non-enzymatic antioxidant defenses in the rat brain. Brazilian Journal of Medical and Biological Research, 49(10), 6–10. https://doi.org/10.1590/1414-431X20165310
Park, H. S., Cho, H. S., & Kim, T. W. (2018). Physical exercise promotes memory capability by enhancing hippocampal mitochondrial functions and inhibiting apoptosis in obesity-induced insulin resistance by high fat diet. Metabolic Brain Disease, 33(1), 283–292. https://doi.org/10.1007/s11011-017-0160-8
Pearen, M. A, Eriksson, N. A, Fitzsimmons, R. L., Goode, J. M., Martel, N., Andrikopoulos, S., & Muscat, G. E. O. (2012). The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Molecular endocrinology (Baltimore, Md.), 26(3), 372–84. https://doi.org/10.1210/me.2011-1274
Pedersen, B. K., & Saltin, B. (2015). Exercise as medicine - Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scandinavian Journal of Medicine and Science in Sports, 25, 1–72. https://doi.org/10.1111/sms.12581
Plattner, F., Hayashi, K., Hernández, A., Benavides, D. R., Tassin, T. C., Tan, C., et al. (2015). The role of ventral striatal cAMP signaling in stress-induced behaviors. Nature Neuroscience, 18(8), 1094–1100. https://doi.org/10.1038/nn.4066
Qiao, X., Gai, H., Su, R., Deji, C., Cui, J., Lai, J., & Zhu, Y. (2018). PI3K-AKT-GSK3β-CREB signaling pathway regulates anxiety-like behavior in rats following alcohol withdrawal. Journal of Affective Disorders, 235, 96–104. https://doi.org/10.1016/j.jad.2018.04.039
Serdar, C. C., Cihan, M., Yücel, D., & Serdar, M. A. (2021). Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochemia Medica, 31(1), 27–53. https://doi.org/10.11613/BM.2021.010502
Sousa, R. A. L. D., Improta-Caria, A. C., de Souza, B. S., & F. (2021b). Exercise–linked irisin: Consequences on mental and cardiovascular health in type 2 diabetes. International Journal of Molecular Sciences, 22(4), 1–15. https://doi.org/10.3390/ijms22042199
Wan, C., Shi, L., Lai, Y., Wu, Z., Zou, M., Liu, Z., et al. (2024). Long-term voluntary running improves cognitive ability in developing mice by modulating the cholinergic system, antioxidant ability, and BDNF/PI3K/Akt/CREB pathway. Neuroscience Letters, 836, 137872. https://doi.org/10.1016/j.neulet.2024.137872
Wang, K., Song, F., Xu, K., Liu, Z., Han, S., Li, F., & Sun, Y. (2019). Irisin attenuates neuroinflammation and prevents the memory and cognitive deterioration in streptozotocin-induced diabetic mice. Mediators of Inflammation. https://doi.org/10.1155/2019/1567179
Yang, W. S., & Stockwell, B. R. (2016). Ferroptosis: Death by lipid peroxidation. Trends in Cell Biology, 26(3), 165–176. https://doi.org/10.1016/j.tcb.2015.10.014
Yao, R., Yamada, K., Izawa, S., Kito, T., Sawada, H., Chihara, T., et al. (2024). FNDC5/irisin mediates the protective effects of Innovative theta-shaking exercise on mouse memory. Heliyon, 10(8), e29090. https://doi.org/10.1016/j.heliyon.2024.e29090
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—(001).
Author information
Authors and Affiliations
Contributions
RALS: Conceptualized, executed the study, carried out the data analysis, prepared results, and prepared the manuscript. CODM executed the study, carried out the data analysis, and prepared results. PPC: Executed the study and reviewed the manuscript. GHBO: Executed the study and reviewed the manuscript. JTACP: Executed the study and reviewed the manuscript. CMAF: Executed the study and reviewed the manuscript. RRS: reviewed and corrected the manuscript. RCC: Conceptualized, funding acquisition, supervision, reviewed, and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Sousa, R.A.L., Diniz-Magalhaes, C.O., Cruz, P.P. et al. Physical Exercise Inhibits Cognitive Impairment and Memory Loss in Aged Mice, and Enhances Pre- and Post-Synaptic Proteins in the Hippocampus of Young and Aged Mice. Neuromol Med 26, 31 (2024). https://doi.org/10.1007/s12017-024-08798-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12017-024-08798-x