Abstract
Doxorubicin (DOX) is an effective anticancer drug, however, side effects such as cognitive impairment and cardiotoxicity have limited its clinical use. Juglanin (JUG) is a flavonoid with excellent antioxidant, anti-inflammatory, neuroprotective and anticancer properties. This study investigated the protective effects of JUG against DOX-induced cognitive decline, oxidative stress and inflammatory response in rats. The rats were orally administrated with JUG or JUG in combination with DOX. After treatment, the animals were subjected to series of behavioral test including Morris water maze, Y-maze and forced swimming tests. After the study, the rats were sacrificed and the level of acetylcholinesterase (AchE), superoxide dismutase (SOD), glutathione (GSH), catalase (CAT), malondialdehyde (MDA), interleukin 6 (IL-6), interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), caspase 3 and Nuclear factor kappa B (NF-кB) were assayed in the brain. Histopathological analysis was also performed on the brain of the rats. JUG significantly protected against DOX-induced cognitive impairment and depressive behaviors. In addition, JUG attenuated altered brain histopathological architecture, reduced oxido-inflammatory responses, acetylcholinesterase and caspase 3 activity in the brain of the treated rats. Collectively, the results suggested that JUG offered neuroprotection against DOX induced Chemobrain via ameliorating oxidative stress and inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DOX) is an anthracycline and antibiotic antitumor agent that is widely used for treating a wide range of solid tumors and cancers, including soft tissue sarcomas, lymphomas, breast, bladder, ovarian, lungs and blood cancers (Ibrahim et al. 2021; Songbo et al. 2019). Mechanistically, DOX antitumor effects is mediated by its ability to interacts with DNA, inhibition of macromolecular biosynthesis and topoisomerase II, leading to a halt in DNA replication (Sergazy et al. 2020) Besides, DOX also exerts its cytotoxicity by increasing free radical production and oxidative stress (Shafei et al. 2017). Although DOX has shown promising efficacy in the management of cancers and solid tumors, its clinical applications has been significantly curtailed due to severe toxicities including cardiotoxicity, myelosuppression, alopecia and neurotoxicity (El-Agamy et al. 2018; Granados-Principal et al. 2010). In fact, due to these side effects DOX has been nick named “red devil” or “red death. Although the penetration of DOX into the blood brain barrier is relatively poor, several reports have indicated that DOX can accumulate in the brain and cause significant toxicity through a secondary mechanism via elevated peripheral TNF-α which penetrates the blood brain barrier and impede antioxidant defense Du et al. 2021; Liao et al. 2018; Eide and Feng 2020). DOX induced neurotoxicity (Chemobrain) is typically characterized by impaired cognitive function and neuropsychiatric disorders (Merzoug et al. 2014; Liao et al. 2018).
Multiple studies have implicated ROS generation, oxidative stress and neuroinflammation in the pathogenesis of DOX-induced chemo brain. As such any therapy that significant modulates these factors may serve as a preventive agent against DOX-induced Chemobrain (Liao et al. 2018; Shaker et al. 2021; El-Agamy et al. 2018).
Juglanin (JUG; Fig. 1), a flavonoid glycoside isolated from Polygonum aviculare has received attention in recent years due to its excellent anti-inflammatory, antioxidant and anti-cancer effects (Wang et al. 2021a, b; Liu et al. 2020; Zhang et al. 2021). Ample pharmacological studies have brought into limelight the neuroprotective efficacy of JUG. For instance, JUG was reported to show inhibitory effect against LPS-induced neuroinflammation through its anti-inflammatory properties (Zhang et al. 2018). Moreover, JUG showed preventive effects against neuronal injury induced by cerebral ischemia (Liu et al. 2020). Inspired by these findings, we envisaged that JUG could also exert protective effects against DOX-induced Chemo brain through its ability to inhibit oxidative stress and inflammation. As such this study evaluated the protective effects of JUG against DOX-induced cognitive dysfunction in rats.
Materials and methods
Chemicals and reagents
JUG was graciously provided by Prof. Jian Tang (Bozhou University. Anhui, China). DOX was procured from Fresenius Kabi Oncology Ltd., India. Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6 and IL-1β were purchased from Abcam (Cambridge, UK). Biochemical kits for SOD, GSH, CAT and MDA analysis were procured from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China). All the other chemicals were of analytical grade purity and used as purchased.
Animals and treatments
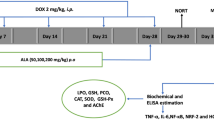
Twenty four (6 weeks old male Sprague Dawley rats; 180 ± 40 g) were used in this experiment. The animals were housed at an experimental animal house facility with standard conditions of temperature 22 ± 2 °C and light-controlled environment (12 h light and dark cycles). The animals were acclimatized for 1 week and fed ad libitum with standard rat diet and water. After acclimatization, the rats were randomly assigned to four groups (n = 6) as follows: normal control, JUG, DOX and JUG + DOX groups. The rats in DOX group were intraperitoneally administered with 5 mg/kg DOX once a week for 3 weeks (total dose of 15 mg/kg), while the rats in JUG + DOX group were orally pretreated with JUG (30 mg/kg/day) for a week before receiving a weekly injection of DOX as stated above together with daily doses of JUG for 3 weeks. The normal control, DOX group were treated with 5% DMSO, while the JUG groups were treated with JUG (30 mg/kg) for 4 weeks. The doses of JUG and DOX used in this study was adopted from previous studies (Zhang and Xu 2018; Rizk et al. 2017; Kwatra et al. 2016). Upon completion of the treatment, all the rats were subjected to series of neurobehavioral studies. The schematic treatment protocol for the study is illustrated in Fig. 2. All animals were handled according to the National Institute of Health (NIH) guidelines on the ethical use of animals and the experiment received approval from the ethics Committee of Wuhu Second Peoples Hospital (Approval NO. AHWHSEY2021-08-18).
Neurobehavioral studies
Morris water maze test
The MWM test was utilized to access the memory and learning ability of the DOX treated rats (Huang et al. 2021). Briefly, the MWM apparatus consisted of a circular water container filled with water at 25 °C. A hidden platform was submerged 1 cm below the surface of the water in one of the quadrant of the apparatus and the water surface was covered with powdered milk. The animals were given four days of training, during this period rats were placed in the water tank and allowed to locate the position of the hidden platform within 60 s and rats that were unable to achieve this task within 60 s were guided to the position of the platform. On the fifth day, the platform was removed and the time spent by the rats in the target quadrant, the swimming speed and the number of target quadrant crossings were recorded.
Y-maze test
The Y maze test was used for evaluating spatial memory and learning using spontaneous alternation test (Fan et al. 2021). In brief, the maze was made up of three identical arms positioned at 120 °C to each other in a Y shaped structure. The rats were individually placed in the middle of the maze and allowed to freely explore the maze for 5 min. The percentage alternation of each rats was evaluated based successive entries into alternate arm (Fan et al. 2021).
Forced swimming test
The FST was used to evaluate depressive-like behavior in DOX treated animals (Liao et al. 2018). For the pretest, the rats were placed in a transparent cylindrical shaped glass (40 cm high and 30 cm diameter) containing 30 cm of water at 25 °C and allowed to swim for 15 min. After 24 h, the rats were subjected to the same experimental conditions stated above for 5 min. The swimming, climbing and immobility time was recorded.
Animal sacrifice
At the end of the neurobehavioral experiment, all the rats were sacrificed and the brain was immediately excised, cleaned with phosphate buffer saline and weighed.
Histopathological observation
The brain of the rats were fixed in 10% buffered formalin solution, embedded in paraffin wax and cut into 4 μm thickness sections. The sections were further deparaffinized, stained with hematoxylin and eosin and visualized under a light microscope.
Biochemical analysis
The whole brain was homogenized in 0.1 M of phosphate buffer, pH 7.4 (1:9 w/v) and centrifuged at 6,000 rpm for 30 min at 4 °C. The supernatant obtained after decanting were stored at -80 °C until used for further analysis.
Inflammatory mediators measurement
The brain concentration inflammatory mediators including TNF-α, IL-1β, IL-6 and NF-κB were measured in the brain homogenate with the help of ELISA kits following the instructions of the manufacturer (Abcam, Cambridge, UK).
Oxidative stress bio-markers measurement
The brain oxidative stress bio-markers including malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT) were measured in the brain homogenate using kits provided by Nanjing Jiancheng Bioengineering Institute.
Acetylcholinesterase (AChE) measurement
The brain activities of AchE were assayed using the method of Ellman et al., (1961). Briefly, a mixture containing phosphate buffer, 10 mM DTNB, 50 mM of acetylthiocholine iodide and an aliquot of brain tissue homogenate. The reaction mixture was incubated at 37 ◦C for 10 min and the absorbance of the yellow color formed was measured at 412 nm.
Caspase 3 activity measurement
Caspase 3 activity in the brain homogenate was measured using ELISA kits from Cusabio following the manufacturer’s instructions.
Statistical analysis
All data analyses were performed using GraphPad Prism (version 5.0) software and data were expressed as the mean ± SD. Statistical analysis was performed using one way ANOVA followed by Tukey post hoc test for multiple comparison. p<0.05 was considered statistically significant.
Results
Effect of JUG on DOX-induced body weight loss
As shown in Fig. 3A, the DOX-treated rats showed marked reduction in their body weight relative to the normal control and JUG rats. Moreover, the JUG treated rats demonstrated significant increase in their body weight compared to DOX group (Fig. 3A).
Effects of juglanin on memory and cognitive dysfunctions in doxorubicin injected rats. (A) Body weight (B) Percentage of spontaneous alternation in the Y-maze test (C) Total number of arm entries in the Y-maze test (D) Latency of escape on during training days in MWM test (E) Time spent in the target quadrant in the MWM test (F) Number of platform crossing. Data were presented as mean ± SD. **p<0.05 compared to the normal control and JUG groups; ##p<0.05 compared to DOX group
Effect of JUG on DOX-induced behavioral deficits
The results shown in Fig. 3B indicated that DOX treated rats showed significantly lower percentage spontaneous alternation compared to the normal control and JUG rats in the Y maze test (Fig. 3B). Whereas, in the JUG+DOX treated rats, the percentage spontaneous alternation was observed to be significantly increased compared to DOX alone treated rats (Fig. 3B). Albeit there were no significant differences in the total arm entries of all the groups (Fig. 3C). In the MWM test, the escape latency of the DOX treated rats was significantly prolonged compared to the normal control and JUG group during the four days of the position navigation training (Fig. 3D). In contrast, treatment with JUG significantly improved learning and memory as portrayed by reduced latency of escape compared to the DOX group. Furthermore, in the space exploration test (day 5), the time spent and number of crossings in the target quadrant where the hidden platform was originally located were significantly increased in the JUG+DOC treated rats compared to the DOX alone group (Fig. 3E-F).
Effect of JUG on DOX-induced anxiety and depression-like behaviors
There were obvious differences in the immobility time of the DOX treated rats when compared to the normal control and JUG rats. The rats in the DOX treated group showed significantly increased immobility time, while the swimming and climbing time were concurrently reduced compared with the normal control and JUG groups (Fig. 4A-C). Interestingly, these changes were markedly reversed in JUG treated rats. The immobility time was significantly reduced, while the swimming and climbing time were increased accordingly compared to the DOX alone treated group (Fig. 4A-C).
Effect of JUG on DOX-induced neuroinflammation
It is well known that increased oxidative stress and pro-inflammatory cytokines plays a critical role in DOX-induced Chemobrain, leading to neuroinflammation. The concentrations of pro-inflammatory cytokines including TNF-α (Fig. 5A), IL-1β (Fig. 5B) and IL-6 (Fig. 5C) in the brain of the DOX alone treated rats were significantly augmented compared to the normal control and JUG groups. Additionally, NF-kB level (Fig. 5D) was also observed to be marked increased in the brain of DOX alone treated rats. Whereas, TNF-α, IL-1β, IL-6 and NF-kB were notably decreased in JUG+DOX treated rats compared with the DOX group (Fig. 5A-D).
Effect of JUG on DOX-induced oxidative stress in the brain of rats
As shown in Fig. 6A, MDA level in the brain was increased significantly in the DOX treated rats compared to the normal control and JUG groups, while treatment with JUG significantly decreased brain MDA level (Fig. 6A). Consistently, the brain antioxidation capacity of the DOX alone treated rats was compromised as indicated by significant decreases in SOD (Fig. 6B), CAT (Fig. 6C) and GSH (Fig. 6D) compared with normal control and JUG groups. Howbeit compared to the DOX alone treated group, JUG significantly increased the activities of these brain antioxidant enzymes in addition to reducing MDA level (Fig. 6A-D).
JUG attenuated brain histopathological changes in DOX treated rats
The therapeutic effect of JUG in DOX treated rats was further confirmed by histopathological evaluation. As indicated in Fig. 7A-B, the normal control and JUG only-treated rats showed normal brain histological structures of the cerebral cortex, compared to the DOX alone treated group that showed gross histopathological alterations including pyknosis, congested blood vessels, degenerated and swollen neurons (Fig. 7C). Interestingly, in the JUG+DOX group, most of the pathological alteration were significantly alleviated compared to the DOX alone treated group (Fig. 7A-D).
Effects of Juglanin on DOX-induced histopathological alterations of rat brain. Photomicrographs of hematoxylin and eosin stained sections from (A) Normal control group (B) JUG group (C) DOX group (D) JUG + DOX group. Magnification power X400. Black arrow: pyknosis and swollen neutrons. Red arrow: congested blood vessels
Effect of JUG on brain AChE activity in DOX treated rats
As shown in Fig. 8A, the level of AChE in the brain of the DOX alone treated rats were markedly increased compared to the normal control and JUG groups, while JUG treatment notably reduced the activities of AchE in comparison to the DOX alone rats.
Effect of JUG on brain caspase 3 activity in DOX treated rats
As shown in Fig. 8B, the administration of DOX to rats significantly increased caspase 3 in the brain of the rats as compared with the normal and JUG rats. Whereas, administration of JUG with DOX significantly lowered caspase 3 activity as compared with the DOX alone treated rats (Fig. 8B).
Discussion
The global fight against cancer has resulted in the discovery and development of several anticancer drugs including platinum based anticancer drugs (cisplatin, carboplatin and oxaliplatin), anthracyclines (doxorubicin, idarubicin and mitoxantrone) and alkylating antineoplastic agents (cyclophosphamide and ifosfamide). However, majority of these chemotherapeutic drugs have varying degrees of side effects, some of which could be fatal and life threatening. Amongst them DOX is one of the prominently used anticancer drug. The anticancer effect of DOX is associated with inhibition of topoisomerase II and formation of complexes that disrupts DNA or RNA synthesis (Cavalcanti et al., 2021). However, DOX presents several side effects including cardiotoxicity and neurotoxicity that has curtailed its clinical application (Ikewuchi et al. 2021)). DOX-induced neurotoxicity has been proposed to be related to ROS generation, oxidative stress and proinflammatory cytokine upsurge leading to neuronal death and brain oxidative damage (Cappetta et al. 2017; Bredlau et al. 2018; Ibrahim et al. 2021). Since DOX-induced oxidative stress and inflammation has been implicated in the onset and progression of Chemobrain, it is widely believed that antioxidant agents may be beneficial in preventing or modulating these side effects (Songbo et al. 2019). In this sense, this study investigated the protective effects of juglanin against DOX-induced Chemobrain. This present investigation found that treatment with JUG decreased memory and learning impairment, anxiety like behaviors, brain proinflammatory cytokines and oxidative stress in the DOX treated rats, suggesting that JUG possesses neuroprotective activities which may be associated with its ability to modulate oxidative stress and inflammation.
Neurobehavioral analyses including MWM and Y maze tests has been extensively used for examining learning and memory capabilities as well as the willingness of the animals to explore new environments. Moreover, previous studies have revealed that DOX-induced Chemobrain is associated with impairment in spatial memory and learning functions in the MWM and Y maze test as evidenced by increase in platform escape latency and reduced percentage alterations (Mounier et al. 2021; El-Agamy et al. 2018). Furthermore, this study observed significant depressive like behaviors in the DOX alone treated rats as portrayed by enhanced immobility duration in the forced swimming test, which was consistent with previous studies (Kwatra et al. 2016; Wu et al. 2016) Contrarily, treatment with JUG significantly prevented cognitive deficits and depressive like behaviors delineating the memory enhancing effect of JUG.
The results from this study indicated that DOX administration enhanced the activity of AchE in the brain of the rats. Numerous studies have illustrated the vital role that acetylcholine plays in cognition. Increase in AchE activity has been widely linked to rapid decline in cognitive function especially in dementias like Alzheimer’s disease (Khadrawy et al., 2021; El-Agamy et al. 2018). JUG treatment reduced brain AchE activity in the treated rats, thus enhancing their cognitive abilities.
Oxidative stress resulting from excessive accrual of ROS have been conspicuously identified as a major player mediating DOX-induced toxicity including DOX-induced cognitive deficits. Several studies have suggested that the production of highly reactive and toxic free radicals are involved in the oxidation reaction involving DOX binding to cell membranes (Cappetta et al. 2017; Ibrahim et al. 2021). In addition, quinone containing molecules like DOX encourages redox recycling that leads to the generation of large amounts of superoxide anion radicals, hydrogen peroxide, hydroxyl radical and peroxynitrite leading to massive depletion in endogenous antioxidant defense (Zhu et al. 2016). DOX induced oxidative stress was evidenced in this study as portrayed by gross reduction in the activities of brain catalase, glutathione and superoxide dismutase as well as apparent increase in lipid peroxidation levels in the brain. These observations were obviously in agreement with previous studies (Mounier et al. 2021; Kwatra et al. 2016; Wang et al. 2021a, b). The antioxidant effect of JUG was evident in reversing the altered oxidative stress parameters in the brain of the DOX treated rats. JUG treatment decreased the elevated MDA levels and markedly restored the altered antioxidant defense enzyme activities i.e. GSH, SOD and CAT in the brain. The antioxidant potential of JUG may be attributed to its free radicals scavenging capabilities which agreed with previous reported studies (Ge et al. 2020; Kong and Xu 2020).
Moreover, DOX prompted ROS and oxidative stress have been reported to activate several redox sensitive inflammatory transcriptional factor such as NF-κB which subsequently enhances the production and release of inflammatory cytokines including TNF-α, IL-1β, and IL-6 (Wei et al. 2020; Liao et al. 2018). These proinflammatory cytokines such as TNF-α have the ability to bypass the blood brain barrier to exert neuroinflammation resulting in brain damage (Ibrahim et al. 2021; Tangpong et al. 2006, 2007). In addition, mounting evidences have shown that neuroinflammation is critically involved in the pathophysiology of neurodegenerative and brain related disorders via the TNF-α and NF-κB pathways (Frank-Cannon et al. 2009; Chtourou et al. 2015; El-Naga et al. 2014). We observed that treatment with DOX provoked significantly high concentration of inflammatory mediators including TNF-α, NF-κB, IL-1β and IL-6, suggesting neuroinflammation, which was supported by previous studies (Ramalingayya et al. 2017; Ali et al. 2020; Mounier et al. 2021). Whereas, treatment with JUG markedly suppressed DOX-induced increase in TNF-α, IL-1β, IL-6 and NF-κB in the brain of treated rats.
Caspase 3, an important marker of cellular apoptosis has been widely reported to be influenced by oxidative stress and inflammation in several diseases including DOX induced toxicity (Abu Gazia and El-Magd 2018). The findings from this study indicated elevated level of caspase 3 in the brain tissues of DOX treated rats. Several studies have shown an increase in caspase 3 activity following exposure to DOX (El-Agamy et al. 2016, 2018; Rizk et al. 2017). In contrast to DOX treated rats, JUG administration significantly reduced the level of caspase 3 in the brain tissues of the treated rats.
Conclusions
Conclusively, the results obtained in this study suggested that JUG protected against DOX induced cognitive dysfunction by improving memory and learning capabilities, decreasing depressive like behaviors and ameliorate oxidative stress and inflammation in the brain tissues of DOX treated rats. The results stated above demonstrated that JUG may offer protection in chemotherapy induced toxicity.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abu Gazia M, El-Magd MA (2018) Ameliorative effect of Cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cells Tissues Organs 206:62–72
Ali MA, Menze ET, Tadros MG, Tolba MF (2020) Caffeic acid phenethyl ester counteracts doxorubicin-induced chemobrain in Sprague-Dawley rats: Emphasis on the modulation of oxidative stress and neuroinflammation. Neuropharmacology 181:108334
Bredlau AL, Motamarry A, Chen C, McCrackin MA, Helke K, Armeson KE, Bynum K, Broome AM, Haemmerich D (2018) Localized delivery of therapeutic doxorubicin dose across the canine blood-brain barrier with hyperthermia and temperature sensitive liposomes. Drug Deliv 25:973–984
Cappetta D, De Angelis A, Sapio L, Prezioso L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S, Urbanek K (2017) Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev 2017:1521020
Chtourou Y, Aouey B, Kebieche M, Fetoui H (2015) Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chemico-Biol Inter 239:76–86
Du J, Zhang A, Li J, Liu X, Wu S, Wang B, Wang Y, Jia H (2021) Doxorubicin-induced cognitive impairment: The mechanistic insights. Front Oncol 11:673340
Eide S, Feng ZP (2020) Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur J Pharmacol 881:173078
El-Agamy SE, Abdel-Aziz AK, Wahdan S, Esmat A, Azab SS (2018) Astaxanthin ameliorates doxorubicin-induced cognitive impairment (Chemobrain) in experimental rat model: Impact on oxidative, inflammatory, and apoptotic machineries. Mol Neurobiol 55:5727–5740
El-Agamy DS, Abo-Haded HM, Elkablawy MA (2016) Cardioprotective effects of sitagliptin against doxorubicin-induced cardiotoxicity in rats. Exp Biol Med 241:1577–1587
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
El-Naga RN, Ahmed HI, Abd Al Haleem EN (2014) Effects of indole-3-carbinol on clonidine-induced neurotoxicity in rats: impact on oxidative stress, inflammation, apoptosis and monoamine levels. Neurotoxicology 44:48–57
Fan SR, Ren TT, Yun MY, Lan R, Qin XY (2021) Edaravone attenuates cadmium-induced toxicity by inhibiting oxidative stress and inflammation in ICR mice. Neurotoxicology 86:1–9
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47
Ge C, Tan J, Zhong S, Lai L, Chen G, Zhao J, Yi C, Wang L, Zhou L, Tang T, Yang Q, Lou D, Li Q, Wu Y, Hu L, Kuang G, Liu X, Wang B, Xu M (2020) Nrf2 mitigates prolonged PM2.5 exposure-triggered liver inflammation by positively regulating SIKE activity: Protection by juglanin. Redox Biol 36:101645
Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, Sanchez-Rovira P, Ramirez-Tortosa MC (2010) New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food Chem Toxicol 48:1425–1438
Huang Y, Liu C, Song X, An M, Liu M, Yao L, Famurewa AC, Olatunji OJ (2021) Antioxidant and anti-inflammatory properties mediate the neuroprotective effects of hydro-ethanolic extract of Tiliacora triandra against cisplatin-induced neurotoxicity. J Inflamm Res 14:6735–6748
Ibrahim SS, Abo Elseoud OG, Mohamedy MH, Amer MM, Mohamed YY, Elmansy SA, Kadry MM, Attia AA, Fanous RA, Kamel MS, Solyman YA, Shehata MS, George MY (2021) Nose-to-brain delivery of chrysin transfersomal and composite vesicles in doxorubicin-induced cognitive impairment in rats: Insights on formulation, oxidative stress and TLR4/NF-kB/NLRP3 pathways. Neuropharmacology 197:108738
Ikewuchi JC, Ikewuchi CC, Ifeanacho MO, Jaja VS, Okezue EC, Jamabo CN, Adeku KA (2021) Attenuation of doxorubicin-induced cardiotoxicity in Wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. J Ethnopharmacol 274:114004
Kwatra M, Jangra A, Mishra M, Sharma Y, Ahmed S, Ghosh P, Kumar V, Vohora D, Khanam R (2016) Naringin and sertraline ameliorate doxorubicin-induced behavioral deficits through modulation of serotonin level and mitochondrial complexes protection pathway in rat hippocampus. Neurochem Res 41:2352–2366
Kong YH, Xu SP (2020) Juglanin administration protects skin against UVB–induced injury by reducing Nrf2–dependent ROS generation. Int J Mol Med 46:67–82
Khadrawy YA, Hosny EN, El-Gizawy MM, Sawie HG, Aboul Ezz HS (2021) The effect of curcumin nanoparticles on cisplatin-induced cardiotoxicity in male Wistar albino rats. Cardiovasc Toxicol 21: 433-443.
Liao D, Xiang D, Dang R, Xu P, Wang J, Han W, Fu Y, Yao D, Cao L, Jiang P (2018) Neuroprotective effects of dl-3-n-butylphthalide against doxorubicin-induced neuroinflammation, oxidative stress, endoplasmic reticulum stress, and behavioral changes. Oxid Med Cell Longev 2018:9125601
Lima Cavalcanti ID, de Santos Soares JC, de Britto Lira Nogueira MC (2021) Fátima Ramos dos Santos Medeiros SM, Ferro Cavalcanti IM, Can antioxidant vitamins avoid the cardiotoxicity of doxorubicin in treating breast cancer? PharmaNutrition 16:100259
Liu J, Chen L, Zhang X, Pan L, Jiang L (2020) The Protective effects of juglanin in cerebral ischemia reduce blood-brain barrier permeability via inhibition of VEGF/VEGFR2 signaling. Drug Des Devel Ther 14:3165–3175
Merzoug S, Toumi ML, Tahraoui A (2014) Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn-Schmiedeberg’s Arch Pharmacol 387:921–933
Mounier NM, Wahdan SA, Gad AM, Azab SS (2021) Role of inflammatory, oxidative, and ER stress signaling in the neuroprotective effect of atorvastatin against doxorubicin-induced cognitive impairment in rats. Naunyn-Schmiedeberg’s Arch Pharmacol 394:1537–1551
Ramalingayya GV, Cheruku SP, Nayak PG, Kishore A, Shenoy R, Rao CM, Krishnadas N (2017) Rutin protects against neuronal damage in vitro and ameliorates doxorubicin-induced memory deficits in vivo in Wistar rats. Drug Des Devel Ther 11:1011–1026
Rizk HA, Masoud MA, Maher OW (2017) Prophylactic effects of ellagic acid and rosmarinic acid on doxorubicin-induced neurotoxicity in rats. J Biochem Mol Toxicol 31:e21977
Sergazy S, Shulgau Z, Fedotovskikh G, Chulenbayeva L, Nurgozhina A, Nurgaziyev M, Krivyh E, Kamyshanskiy Y, Kushugulova A, Gulyayev A, Aljofan M (2020) Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci Rep 10:14720
Shafei A, El-Bakly W, Sobhy A, Wagdy O, Reda A, Aboelenin O, Marzouk A, El Habak K, Mostafa R, Ali MA, Ellithy M (2017) A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacother 95:1209–1218
Shaker FH, El-Derany MO, Wahdan SA, El-Demerdash E, El-Mesallamy HO (2021) Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci 269:119078
Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S (2019) Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett 307:41–48
Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA (2006) Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis 23:127–139
Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA (2007) Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem 100:191–201
Wang T, Wang J, Sun T, Li Y (2021a) Amelioration of juglanin against LPS-induced activation of NLRP3 inflammasome in chondrocytes mediated by SIRT1. Inflammation 44:1119–1129
Wang C, Zhao Y, Wang L, Pan S, Liu Y, Li S, Wang D (2021b) C-phycocyanin mitigates cognitive impairment in doxorubicin-induced Chemobrain: Impact on neuroinflammation, oxidative stress, and brain mitochondrial and synaptic alterations. Neurochem Res 46:149–158
Wei S, Ma W, Li X, Jiang C, Sun T, Li Y, Zhang B, Li W (2020) Involvement of ROS/NLRP3 inflammasome signaling pathway in doxorubicin-induced cardiotoxicity. Cardiovasc Toxicol 20:507–519
Wu YQ, Dang RL, Tang MM, Cai HL, Li HD, Liao DH, He X, Cao LJ, Xue Y, Jiang P (2016) Long chain omega-3 polyunsaturated fatty acid supplementation alleviates doxorubicin-induced depressive-like behaviors and neurotoxicity in rats: Involvement of oxidative stress and neuroinflammation. Nutrients 8:243
Zhang F, Huang X, Qi Y, Qian Z, Ni S, Zhong Z, Zhang X, Li D, Yu B (2021) Juglanin inhibits osteoclastogenesis in ovariectomized mice via the suppression of NF-κB signaling pathways. Front Pharmacol 11:596230
Zhang FX, Xu RS (2018) Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson’s disease and cell culture via inactivating TLR4/NF-kappaB pathway. Biomed Pharmacother 97:1011–1019
Zhu H, Sarkar S, Scott L, Danelisen I, Trush MA, Jia Z, Li YR (2016) Doxorubicin redox biology: Redox cycling, topoisomerase inhibition, and oxidative stress. React Oxyg Species (Apex) 1:189–198
Author information
Authors and Affiliations
Contributions
OJO designed the experiment. TW, LW, TJA and OJO performed the performed the experiment. JT provided the biological sample. OJO wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared by all authors.
Ethical approval
All animals were handled according to the National Institute of Health (NIH) guidelines on the ethical use of animals and the experiment received approval from the ethics Committee of Wuhu Second Peoples Hospital (Approval NO. AHWHSEY2021-08-18).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, T., Wang, L., Tang, J. et al. Protective effect of Juglanin against doxorubicin-induced cognitive impairment in rats: Effect on oxidative, inflammatory and apoptotic machineries. Metab Brain Dis 37, 1185–1195 (2022). https://doi.org/10.1007/s11011-022-00923-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-00923-8