Abstract
Non-coding RNAs have recently attracted much attention with the potential in the treatment of cerebral ischemia/reperfusion (I/R) injuries. In this study, we investigated the role of miR-32-5p in cerebral I/R injuries by using oxygen–glucose deprivation/reperfusion (OGD/R) PC12 cells and middle cerebral artery occlusion/reperfusion (MCAO/R) rats. The expression of genes and proteins were detected by RT-qPCR and Western blot, respectively. The function of OGD/R PC12 cells was detected using MTT assay and flow cytometry analysis. The influences of MCAO/R on rats was evaluated by measuring the infarct volume and brain water content. Bioinformatics analysis and luciferase gene reporter assay were used to identify the relationship between miR-32-5p and PTEN. The results showed that miR-32-5p had neuroprotective effects on OGD/R induced PC12 cells and MCAO/R injured rats’ brain. The level of miR-32-5p was significantly reduced after OGD/R. Overexpression of miR-32-5p significantly reduced MCAO/R-induced brain damages in rats. Moreover, PTEN was found to be a target of miR-32-5p, and overexpression of PTEN attenuated the effects of miR-32-5p overexpression on cerebral I/R injuries. In addition, miR-32-5p was able to activate PI3K/AKT signaling by inhibiting PTEN. In conclusion, miR-32-5p prevents brain I/R injuries through modulating PTEN/PI3K/AKT signaling pathway.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the most common clinical cerebrovascular disease, stroke seriously affects the quality of life of patients, and brings a heavy burden to the family and society (Trialists’ Collaboration 2013). Its etiology involves a series of complex pathophysiological changes such as thrombosis, bleeding and embolism (Party 2012). Although timely restoration of blood supply to the brain tissues is the best way to save the life of patients with brain ischemia, restoration of blood flow perfusion brings new damages to patients’ hearts and brain tissues, which is known as ischemia–reperfusion (I/R) injuries (Wang et al. 2017). The mechanism of cerebral I/R injury is complex. Despite of increasing efforts, dealing with cerebral I/R injuries still remains a challenge in current treatments (Arslan et al. 2019; He et al. 2019b). Therefore, well understanding of specific molecular mechanisms involved in brain I/R injuries will help to develop new and effective therapeutic targets for ischemic stroke.
MicroRNAs (miRNAs) are a class of highly-conserved non-coding small RNAs of 20 to 24 nucleotides (Li et al. 2017). MiRNAs can bind to the 3′-untranslated region (3′-UTR) of the target gene mRNA, inducing transcriptional degradation or translational inhibition (Ma et al. 2017). MiRNAs have been implicated in many cell procedures including neuro-degenerative diseases (Liang et al. 2018). After ischemic stroke, miRNAs dysregulation has a profound impact on the ischemic processes. Changes in miRNAs expression are closely related to inflammation and cell death. MiR-32-5p is an important miRNA and has been reported to be related with several human cancers, such as pancreatic cancer and renal cell carcinoma (Gao et al. 2017; Wang et al. 2018). We have previously performed a miRNAs transcriptome analysis in a rat ischemic stroke model and found that miR-32-5p was differentially expressed in peripheral venous blood between ischemic and healthy rats (Li et al. 2015).
Phosphatase and tensin homologue (PTEN) located on autosomal 10q23.3 has activities of both lipid and protein phosphatases (Sha et al. 2019). Previous studies have found that miR-32-5p is a negative regulator of pancreatic cancer progression and functions by binding to PTEN (Gao et al. 2017) which can activate its downstream phosphatidylinositol 3-hydroxy kinase (PI3K)/protein kinase B (Akt) signaling pathway to regulate stress induced by cerebral ischemia–reperfusion (Zheng et al. 2019). However, currently, very few studies have explored the effects of miR-32-5p on ischemic stroke and PTEN/PI3K/Akt signaling pathway, which is known to directly or indirectly regulate the activity of apoptosis-related proteins, thereby disturbing cell proliferation and arresting cell cycle and ultimately leading to neuronal apoptosis (Wang et al. 2020a). Therefore, in this study, we investigated the association of miR-32-5p with PTEN and PI3K/AKT in the regulation of cerebral ischemia–reperfusion injuries. Our study may provide potential new therapeutic targets and diagnostic markers for ischemic stroke injuries.
Materials and methods
Cell culture, oxygen–glucose deprivation/reperfusion (OGD/R) PC12 cell establishment and transfections
PC12 cells (ATCC, VA, USA) were cultured in DMEM (HyClone, Logan, USA) with 10% FBS. OGD/R PC12 cell model was established as previously described with minor modifications. Briefly, cells were washed twice with glucose-free DMEM (Gibco, USA) and transferred to a hypoxic incubator chamber (Thermo, USA) containing N2/CO2 (95:5%) (v/v) at 37 °C for 10 min to reduce oxygen content to less than 1% (v/v). Thereafter, glucose-free DMEM was added, and these cells were cultured at 37 °C for 2 h (OGD period). Then glucose-free DMEM was completely replaced with normal DMEM/F12 medium containing 10% (v/v) FBS and cells were incubated under normoxic conditions with 5% (v/v) CO2 for the indicated times (reoxygenation period). Cells in the control group were treated identically without OGD/R. MiR-32-5p mimic, miR-32-5p inhibitor, negative control, and PTEN overexpression plasmids were purchased from Genepharma (Shanghai, China). For cell transfection, briefly, a mixture of transfection agent Lipofectamine 2000 (Invitrogen, Carlsbad, USA) and miR-32-5p mimic or PTEN-overexpressing plasmid were prepared and added into cells cultured in a 24-well plate to make a final concentration of 100 μM. After 6 h exposure to the mixture, cells were cultured in standard media for another 48 h. At the end, cells were collected for mRNA, protein and other analyses.
Cell viability assay
Cell viability was indirectly evaluated using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay based on its positive correlation with metabolic stress as previously described (Guo et al. 2014). Briefly, PC12 cells were evenly seeded at an approximate density of 1 × 104 cells per well in 96-well culture plates. At the end of each treatment, 50 μl of MTT (5 mg/mL in PBS) was added to each well and the plates were incubated for another 4 h at 37 °C. After removal of the supernatant, 150 μl DMSO was added to each well to dissolve the insoluble purple formazan product for quantification. The absorbance (A) value at 570 nm was measured using a microplate reader (Epoch, BioTek, USA). Cell viability was expressed as percentage of the control group and calculated as (A570, sample—A570, blank)/(A570, control—A570, blank) × 100%.
RT-qPCR
Total RNA of each sample was extracted using Trizol (TakaRa Biotechnology Co., Ltd., Dalian, China) according to the manufacturer’s description. The purity and concentration of extracted RNA were determined spectrophotometrically. A total of 100 ng RNA from each sample was used for reverse transcription using a commercial transcription Kit (DRR037A, TaKaRa). miR-32-5p and PTEN expression levels were quantitatively determined by real-time PCR using SYBR Premix Ex Taq (TaKaRa) on an ABI 7300 Real-Time PCR System and primer sets 5′-CGGCCATGCCTTGAGTGTA-3′ and 5′-GCAGGGTCCGAGGTATTC-3′ for miR-32-5p, 5′-CTCGCTTCGGCAGCAC-3′ and 5′-ACGCTTC ACGAATTTGC-3′ for U6, 5′-ACCAGTGGCACTGTTGTTTCAC-3′ and 5′-TTCCTCTGGTCCTGGT ATGAAG-3′ for PTEN, and 5′-ACAACTTTGGTATCGTGGAAGG-3′ and 5′-GCCATCACGCCACAG TTTC-3′ for GAPDH. Data analysis was performed with the comparative Ct method by using the ABI software. MiR-32-5p expression was normalized to U6 and PTEN expression was normalized to GAPDH.
Western blot
Total protein from each sample was extracted using a commercial kit (Beyotime, Shanghai, China). Protein concentration was determined using BCA Protein Assay kit (Beyotime). A total of 40 μg protein from each sample was mixed with loading buffer, denaturized at 99℃ for 5 min, separated on 10% sodium dodecyl sulphate–polyacrylamide gels (SDS-PAGE) and transferred onto polyvinylidene fluoride membranes. The membranes were then incubated with anti-PTEN (1:1,000, ab32199, Abcam), anti-AKT (1:500, ab8805, Abcam), anti-p-AKT (1:1,000, ab38449, Abcam), anti-GSK3β (1:5,000, ab32391, Abcam), anti-p-GSK3β (1:1,000, ab75745, Abcam), anti-pc-Raf (1:1,000, 9421, CST), anti-p-BAD (1:1,000, 5248, CST) and anti-GAPDH (1:10,000, 9851, CST) antibodies. After wash, the membranes were then incubated with corresponding IgG-HRP secondary antibody (1:2,000, sc-2004, Santa Cruz) for 1 h. The signals were visualized and quantified using the GelDoc-2000 Imagine system and protein expression levels were normalized to GAPDH.
Flow cytometry
Apoptosis of PC12 cells was determined by Annexin V-FITC (Beyotime, China). In brief, 1 × 105 PC12 cells were re-suspended in 200 µl binding buffer and stained by Annexin V-EGFP and propidium iodide. The differently treated cells were separated by flow cytometry and incubated with 10 μM DCFH-DA for 30 min. The formation of reactive oxygen species (ROS) after OGD/R was measured based on cell fluorescence intensity.
Dual luciferase gene reporter assay
The PTEN 3′-UTR fragments containing the miR-32-5p binding site or its corresponding mutant were inserted into pGL3 reporter vector (Promega, USA) downstream of the firefly luciferase gene. The recombinant constructs were co-transfected with miR-32-5p mimics or NC miRNA into PC12 cells. Two days after transfections, luciferase activity was evaluated on a microplate reader (BioTek) through dual-luciferase reporting system (Promega, USA).
Rat model
A total of 120 male adult Sprague–Dawley (SD) rats (10–12 weeks, weighing 260–320 g) were from Sparford, China and kept in a pathogen-free room (23 ± 2 ºC, 55% ± 5% humidity) with a 12-h light/12-h dark cycle. These rats were randomly allocated to four groups (n = 6 per group) and intraventricularly injected miR-32-5p mimic/inhibitors or controls. In detail, miR-32-5p mimic or inhibitor was injected into the right lateral ventricle (2.0 mm posterior, 1.5 mm dorsoventral and 1.8 mm lateral to bregma) at a rate of 0.2 μl/min using micro-syringes (Hamilton, Nevada, USA). Two days later, rats were anesthetized by intraperitoneal injection of 100 mg/kg pentobarbital sodium and the middle cerebral artery occlusion (MCAO) was established by operation within 2 h after anesthesia. Then the right internal carotid, internal carotid arteries (ICA) and common carotid artery (CCA) were separated. The right external carotid artery (ECA) and CCA were ligated to the distal and proximal ends and a piece of nylon filament was inserted from the ECA into ICA. After 2 h of ischemia, the filament was removed and blood reperfusion was restored for 24 h. During the whole surgical course, rats were maintained at approximately 37 ± 0.5 °C and rat exhibited no signs of peritonitis or ileus following the administration pentobarbital sodium. Sham surgery was performed without nylon filaments. At the end of reperfusion, neurological deficit score was assessed. Then rats were sacrificed by cervical spine dislocation under deep anesthesia by intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg body weight, Sigma, USA). The brain tissues were harvested for measurement of infarct volume and expression of mRNA or protein. To exclude the interference of operative failures, rats subjected to MCAO with no detectable neurological deficits were eliminated from the subsequent experiments and analyses. The drop-out, mortality rate was ∼18% due to individual resistance to the surgery and vascular variation. The outcomes of MCAO/R were evaluated by measuring the infarct volume and brain water content. The experiments were approved by the Animal Use Committee.
Evaluation of infarct volumes, neurological deficits, and brain water contents
After 24 h of reperfusion, the rats were anesthetized, the brain was dissected and sectioned. The brains were sectioned into 5 coronal sections of 1.5 mm thickness, immersed in 1% 2,3,5-triphenyltetrazole chloride (TTC) for 30 min and fixed by 4% paraformaldehyde. The lesion areas that were not stained red with TTC were quantitatively analyzed. The neurological deficits of brain I/R rats were evaluated blindly based on the following scoring system: 0 point for rats behaved normally, 1 point for rats unable to fully extend their left forelegs, 2 points for rats circled when their tails were pulled and rested in a normal posture, 3 points for rats fell to the left, and 4 points for rats showed no spontaneous activity and had a sluggish level of consciousness. The cerebral water content was measured 24 h after reperfusion. The infarcted cerebral hemispheres were quantified using an electronic scale (wet weight), dried overnight in a drying box at 105 °C, and weighed (dry weight). The total brain water was calculated as (wet weight-dry weight)/wet weight × 100%.
Statistical analysis
SPSS software (version 18.0, Chicago, IL, USA) was used for statistical analysis. All experiments were independently performed for six times and data are presented as mean ± standard deviation (SD). Data sets were first analyzed for assumptions of normality using Shapiro–Wilk test and equal variance using modified Levene test prior to the use of parametric statistical analysis. Differences between means were evaluated using Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s multi-range post hoc test if data conformed to normality and homogeneity of variance or using a non-parametric method (Kruskal–Wallis test) followed by the Mann–Whitney U-test using Bonferroni correction if not. P < 0.05 was considered as the significant threshold.
Results
MiR-32-5p reduces brain I/R damage both in vivo and in vitro
RT-qPCR illustrated that tmiR-32-5p expression was reduced in the MCAO/R-treated rats compared with the sham operation rats (Fig. 1a, t test, t(10) = 14.202, P = 0.01) and increased by its mimic (Fig. 1b, sham groups, ANOVA, F(3,20) = 300.907, P = 0.000; Tukey test, t = -19.719, P < 0.01; I/R groups, ANOVA, F(3,20) = 300.907, P = 0.000; Tukey test, t = -3.880, P < 0.05). In addition, rats’ neurological score was greatly increased by brain I/R (Fig. 1c, K-W test, H = 21.027, P < 0.01) but attenuated by miR-32-5p mimic (K-W test, H = 21.027, P < 0.01). Moreover, infarct volume was dramatically enhanced in the I/R group compared with the sham operation group (Fig. 1d, ANOVA, F(3,20) = 221.264, P = 0.000; Tukey test, t = -21.670, P < 0.01), but was reduced by miR-32-5p mimic (Fig. 1d, ANOVA, F(3,20) = 221.264, P = 0.000; Tukey test, t = 12.267, P < 0.01). Besides infarction, brain water content was markedly increased by brain I/R (Fig. 1e, ANOVA, F(3,20) = 99.667, P = 0.000; Tukey test, t = -13.770, P < 0.01), but significantly reduced by miR-32-5p mimic (ANOVA, F(3,20) = 99.667, P = 0.000; Tukey test, t = 10.313, P < 0.01). These results indicated that miR-32-5p could reduce brain I/R damage in vivo.
Neuroprotective effects of miR-32-5p on brain I/R injuries in vivo. (a) The expression of miR-32-5p in MCAO/R rats was measured by RT-qPCR. (b) Expression of miR-32-5p was determined by RT-PCR in SD rats by miR-32-5p mimic. (c) Nervous system defects evaluated in MCAO/R rats administered with miR-32-5p mimic. (d) Infarct volumes in MCAO/R rat by miR-32-5p mimic. (e) Brain water content of MCAO/R rat injected with miR-32-5p mimic. n = 6. *p < 0.05, ** p < 0.01
The effects of miR-32-5p were also assessed in vitro using OGD/R PC12 cells. RT-PCR results revealed that miR-32-5p was down-regulated (t test, t(10) = 16.948, P < 0.01) in OGD/R PC12 cells compared with the control group (Fig. 2a) and miR-32-5p expression was augmented by miR-32-5p mimic in vitro (Fig. 2b, control groups, ANOVA, F(3,20) = 364.231, P = 0.000; Tukey test, t = -23.571, P < 0.01; OGD/R groups, ANOVA, F(3,20) = 364.231, P = 0.000; Tukey test, t = -3.723, P < 0.01). Overexpression of miR-32-5p significantly improved the viability of PC12 cells (Fig. 2c, ANOVA, F(3,20) = 189.426, P = 0.000; Tukey test, t = -13.887, P < 0.01). Moreover, apoptosis analysis showed that enhanced apoptotic rate of PC12 cells in the OGD/R group (ANOVA, F(3,20) = 190.471, P = 0.000; Tukey test, t = -18.633, P < 0.01), but decreased in the miR-32-5p mimic group compared with the negative control group (ANOVA, F(3,20) = 190.471, P = 0.000; Tukey test, t = 14.855, P < 0.01) (Fig. 2d). Furthermore, ROS production was increased in OGD/R group compared with the control group (ANOVA, F(3,20) = 73.438, P = 0.000; Tukey test, t = -12.903, P < 0.01), while but decreased after miR-32-5p mimic expression (Fig. 2e, ANOVA, F(3,20) = 73.438, P = 0.000; Tukey test, t = 3.937, P < 0.01 or P < 0.05), indicating that miR-32-5p could relieve OGD/R-induced PC12 cell injuries. Together, these results suggested that miR-32-5p could protect brain from I/R induced injuries both in vivo and in vitro.
Neuroprotective effects of miR-32-5p on brain I/R injuries in vitro. (a) The expression of miR-32-5p in OGD/R-induced PC12 was measured by qRT-PCR. (b) Expression of miR-32-5p in PC12 cell transfected with miR-32-5p mimic. (c) Survivals of OGD/R-treated PC12 cells transfected with miR-32-5p expression plasmids detected by MTT assay. (d) Apoptosis of OGD/R PC12 cells transfected with miR-32-5p mimic measured by flow cytometry. The upper left, lower left, upper right and lower right quadrants show the nonviable necrotic cells (annexin V−/PI+), viable cells (annexin V−/PI−), late apoptotic cells (annexin V+/PI+) and early apoptotic cells (annexin V+/PI−), respectively. (e) ROS productions were measured by flow cytometry in OGD/R PC12 cell with a plasmid expressing miR-32-5p. n = 6. * p < 0.05, ** p < 0.01
PTEN is a direct target of miR-32-5p
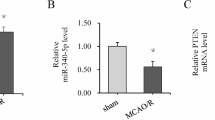
TargetScan indicated that PTEN is a possible target for miR-32-5p (Fig. 3a). As shown in Fig. 3b, miR-32-5p over-expression reduced the luciferase activity of WT-PTEN, while miR-32-5p silencing increased it (ANOVA, F(7,40) = 88.077, P = 0.000; Tukey test, t = 11.320, P < 0.01). It was noted that miR-32-5p mimic suppressed OGD/R-induced PTEN expression in PC12 cells (ANOVA, F(7,40) = 88.077, P = 0.000; Tukey test, t = -12.928, P < 0.01), while miR-32-5p inhibitor increased PTEN expression at both mRNA and protein levels (Fig. 3c, ANOVA, F(3,20) = 242.291, P = 0.000; Tukey test, t = -10.836, P < 0.01; Fig. 3d, ANOVA, F(3,20) = 201.860, P = 0.000; Tukey test, t = -11.587, P < 0.01) (Fig. 3c, d). Similarly, in vivo experiments showed that miR-32-5p mimic reduced PTEN expression in the I/R brain (1/1#, ANOVA, F(5,30) = 297.197, P = 0.000; Tukey test, t = 22.878, P < 0.01; 2/2#, ANOVA, F(3,20) = 201.860, P = 0.000; Tukey test, t = 20.470, P < 0.01; 3/3#, ANOVA, F(3,20) = 201.860, P = 0.000; Tukey test, t = 21.674, P < 0.01) (Fig. 3e). Taken together, these results indicated that miR-32-5p reduces PTEN expression by directly targeting PTEN.
PTEN is directly targeted by miR-32-5p. (a) Binding site between miR-32-5p and PTEN. (b) PTEN 3′-UTR-wild type or mutant luciferase activities determined in a PC12 cell expressing miR-32-5p inhibitor. (c) The mRNA level of PTEN measured by RT-PCR in OGD/R-simulated PC12 cells with miR-32-5p expression/inhibition plasmids. (d) The protein expression of PTEN measured by Western blot in OGD/R- simulated PC12 cells expressing miR-32-5p/inhibitory plasmids. (e) Expression of PTEN measured by Western blot in MCAO/R rat brains after injected with miR-32-5p expression plasmid. 1/1#, repeat once, 1, I/R + NC, 1#, I/R + miR-32-5p mimic, n = 6; 2/2#, repeat twice, 2, I/R + NC, 2#, I/R + miR-32-5p mimic, n = 6; 3/3#, repeat third, 3, I/R + NC, 3#, I/R + miR-32-5p mimic, n = 6. **p < 0.01
PTEN attenuates neuroprotective effect of miR-32-5p on the brain I/R injuries
MTT analysis illustrated that miR-32-5p mimic significantly promoted OGD/R-induced PC12 cell survival (ANOVA, F(3,20) = 134.284, P = 0.000; Tukey test, t = -12.392, P < 0.01), while PTEN overexpression reduced the effect of miR-32-5p mimic on cell viability (ANOVA, F(3,20) = 134.284, P = 0.000; Tukey test, t = 4.499, P < 0.05) (Fig. 4a). As shown in Fig. 4b, miR-32-5p mimic attenuated the apoptosis rate of PC12 cells induced by OGD/R (ANOVA, F(3,20) = 168.050, P = 0.000; Tukey test, t = 13.505, P < 0.01), while over-expression of PTEN reversed miR-32-5p mimic’s effect on apoptosis (ANOVA, F(3,20) = 134.284, P = 0.000; Tukey test, t = -11.838, P < 0.01). In addition, compared with the OGD/R group, miR-32-5p mimic inhibited ROS production (ANOVA, F(3,20) = 82.274, P = 0.000; Tukey test, t = 9.443, P < 0.01), while the PTEN vector reversed this effect (ANOVA, F(3,20) = 134.284, P = 0.000; Tukey test, t = -10.140, P < 0.01) (Fig. 4c). Moreover, similar results were also obtained in vivo. It was noticed that miR-32-5p mimic reduced infarct volume (ANOVA, F(3,20) = 213.563, P = 0.000; Tukey test, t = 12.908, P < 0.01) and cerebral water content (ANOVA, F(3,20) = 61.124, P = 0.000; Tukey test, t = 9.546, P < 0.01), while PTEN overexpression overturned these effects (Fig. 4d, ANOVA, F(3,20) = 213.563, P = 0.000; Tukey test, t = -10.295, P < 0.01; Fig. 4e, ANOVA, F(3,20) = 61.124, P = 0.000; Tukey test, t = -4.533, P < 0.01) (Fig. 4d, e). In summary, our data indicated that PTEN reverses the neuroprotection effects of miR-32-5p on the brain I/R injuries.
PTEN attenuates the neuroprotective effect of miR-32-5p in the brain I/R injuries. (a) Survival of PC12 cells with a plasmid expressing miR-32-5p or OGD/R in combination with a vector expressing PTEN, as detected by MTT assay. (b) Effect of miR-32-5p mimic or combination with PTEN on the apoptosis of OGD/mimic PC12 cells was detected and quantified by flow cytometry. (c) ROS productions by flow cytometry in PC12 cell with miR-32-5p or OGD/R in combination with PTEN. (d) Infarct volumes of MCAO/R rats by miR-32-5p mimic or miR-32-5p mimic + PTEN through TTC staining. The upper left quadrant shows the nonviable necrotic cells (annexin V− /PI+). The lowerleft quadrant shows viable cells, which exclude PI and are negative for FITC-Annexin V binding (annexin V− /PI−). The upper right quadrant contains the late apoptotic cells positive for FITC-Annexin V binding and for PI uptake (annexin V+ /PI+). The lower right quadrant represents the early apoptotic cells. (e) Brain water content of MCAO/R rats by miR-32-5p mimic or miR-32-5p mimic + PTEN. n = 6. * p < 0.05, ** p < 0.01
MiR-32-5p modulates PTEN/PI3K/AKT axis
As shown in Fig. 5a-e, the levels of p-AKT (ANOVA, F(3,20) = 135.231, P = 0.000; Tukey test, t = 12.856, P < 0.01), p-GSK3β (ANOVA, F(3,20) = 77.933, P = 0.000; Tukey test, t = 9.323, P < 0.01), p–c-Raf (ANOVA, F(3,20) = 156.708, P = 0.000; Tukey test, t = 12.620, P < 0.01), and p-BAD (ANOVA, F(3,20) = 110.999, P = 0.000; Tukey test, t = 11.423, P < 0.01) were greatly reduced in OGD/R induced PC12 cells than in the control group. Compared with the OGD/R group, up-regulation of miR-32-5p resulted in a significant increase in p-AKT level (ANOVA, F(3,20) = 135.231, P = 0.000; Tukey test, t = -11.099, P < 0.01), while overexpression of PTEN showed the opposite effect (ANOVA, F(3,20) = 135.231, P = 0.000; Tukey test, t = 4.179, P < 0.05) (Fig. 5b). Moreover, compared with the OGD/R group, overexpression of PTEN significantly restored the decreased levels of p-GSK3β (ANOVA, F(3,20) = 77.933, P = 0.000; Tukey test, t = 3.198, P < 0.05), pc-Raf (ANOVA, F(3,20) = 156.708, P = 0.000; Tukey test, t = 4.281, P < 0.05), and p-BAD (ANOVA, F(3,20) = 110.999, P = 0.000; Tukey test, t = 4.521, P < 0.05) induced by miR-32-5p mimic (Fig. 5c-e). No difference was observed in the expression levels of AKT and GSK3β between various incubations. It was obvious that miR-32-5p promoted PI3K/AKT, which is suppressed by PTEN in the brain I/R injuries, suggesting that miR-32-5p activated the PI3K/AKT signaling by inhibiting PTEN.
MiR-32-5p modulates PTEN/PI3K/AKT axis. (a) Western blotting for protein expression of AKT, p-AKT, GSK3β, p-GSK3β, pc -Raf and p-BAD. (b) Using miR-32-5p or PTEN to quantify the relative density of p-AKT/AKT in PCG cells by OGD/R. (c) The relative density of p-GSK3β/GSK3β in PC12 cells by OGD/R was quantified using a plasmid expressing miR-32-5p or a vector expressing PTEN. (d) Quantification of p–c-Raf levels in PC12 cells by OGD/R using a plasmid expressing miR-32-5p or a vector expressing PTEN. (e) Quantification of p-BAD levels in PC12 cells by OGD/R using a plasmid expressing miR-32-5p or a vector expressing PTEN. n = 6. * p < 0.05, ** p < 0.01
Discussion
Stroke caused by cerebrovascular occlusion or rupture is harmful to people's health, with high morbidity, high mortality and high disability (Xiao et al. 2017). Although timely restoration of blood supply to the brain tissues is the best way to save the life of patients with brain ischemia, it could also lead to cerebral ischemia–reperfusion injury, further damaging the brain tissues (Yang et al. 2018). To avoid these adverse events, three key directions have been identified for advancing stroke treatment: (1) developing systems of care for endovascular therapy (ET) in large vessel occlusion stroke, (2) developing therapeutic approaches adjunctive to ET, and (3) exploring clinical benefit of ET in insufficiently studied patient populations. Moreover, methodological issues such as optimal trial design and outcome measures have also been addressed (Jovin et al. 2016; Savitz et al. 2019). In this study, we demonstrated for the first time that the expression of miR-32-5p was suppressed in MCAO/R-treated rats. MiR-32-5p overexpression significantly reduced neurological score, infarct volume and brain water content of MCAO/R-treated rats.
MiRNAs play important roles in almost every aspect of brain functions, including neurogenesis, neurodevelopment, and cellular responses that lead to changes in synaptic plasticity (Zheng et al. 2019). They are also involved in neuro-degenerative diseases and ischemic conditions (Dehaini et al. 2019). It was shown previously that many miRNAs could exert neuroprotection effects against cerebral ischemia–reperfusion injuries (Yang et al. 2018). For example, one previous study reported the neuroprotective effects of microRNA-99a against focal cerebral ischemia–reperfusion injury in mice (Tao et al. 2015). Besides, miRNA treatment before or after MCAO both have beneficial effects on I/R injuries (Yang et al. 2021; Zhang et al. 2021). In this study, both MCAO/R-treated rats and OGD/R PC12 cells were used to examine the effects of miR-32-5p. We found that miR-32-5p expression was suppressed in MCAO/R-treated rats. MiR-32-5p treatment before MCAO could significantly reduce the increased neurological score, infarct volume and brain water content in MCAO/R, suggesting that miR-32-5p not only plays an important role against cerebral ischemia–reperfusion injuries, but also provided evidence that intervention with miR-32-5p before stroke can yield promising results. As far as we know, this is the first report on the expression pattern of miR-32-5p in MCAO/R-treated rats. Besides, miR-32-5p was down-regulated in OGD/R PC12 cells, and miR-32-5p overexpression promoted the survival of PC12 and decreased the production of ROS. Taken together, our study indicated that miR-32-5p has neuroprotection effects against cerebral ischemia–reperfusion injuries both in vivo and in vitro. These suggest that miR-32-5p may play its protection role in cerebral ischemia–reperfusion injuries by regulating the neuron survival and ROS production.
It has been reported that miR-32-5p can bind with PTEN and regulate metastasis of pancreatic cancer (Gao et al. 2017). In this study, using TargetScan and luciferase gene reporter assay, our results indicated that PTEN is a direct target of miR-32-5p, which is consistent with previous studies (Gao et al. 2017). Moreover, we found that miR-32-5p overexpression promoted OGD/R-induced PC12 cells survival, while PTEN significantly reduced this effect of miR-32-5p on OGD/R-induced PC12 cells survival. MiR-32-5p overexpression attenuated the apoptosis rate of PC12 cells induced by OGD/R, while PTEN reversed miR-32-5p mimic’s effect on the apoptosis rate of PC12 cells. This trend is also true for ROS production, neurological outcomes, infarct volume, and cerebral water content. Our results established the fact that PTEN could reverse the neuro-protective effects of miR-32-5p on brain I/R injuries both in vitro and in vivo. These results also suggest that PTEN mediates the neuroprotection role of miR-32-5p in cerebral ischemia–reperfusion injuries.
AKT is a threonine protein kinase widely expressed in various tissues in the body (Wang et al. 2019). Studies have shown that AKT can be stimulated by growth factors, such as insulin-like growth factor-1 and neurotrophic factors via PI3K, which is activated by phosphorylation (He et al. 2019a). Activation of PI3K/AKT pathway protects brain, and inhibits apoptosis (Tian et al. 2019). Moreover, the expression of pc-Raf, and p-BAD were also reported to be involved in the brain I/R injuries (Wang et al. 2020b). In our study, we found that miR-32-5p increased the expression of p-AKT, while overexpression of PTEN inhibited the effect of miR-32-5p on the expression of p-AKT. Moreover, PTEN also could significantly restore the decreased levels of p-GSK3β, pc-Raf, and p-BAD induced by miR-32-5p mimic. These results suggest that miR-32-5p promotes the expression of PI3K/AKT, which is mediated by PTEN in brain I/R injuries, thereby protect organs from reperfusion-induced damages. In addition, since our current study only focused on short-term outcomes of miR-32-5p in cerebral ischemia–reperfusion injury, we will conduct long-term stroke recovery studies in future studies to evaluate long-term outcomes through a variety of behavioral tests.
In conclusion, this study demonstrated that miR-32-5p prevents brain I/R injuries through modulation of PTEN/PI3K/AKT both in vivo and in vitro. Our results may provide novel treatment directions for ischemia–reperfusion injuries.
Data availability
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
References
Arslan E, Gel MS et al (2019) The Effects of Rifampicin on Experimental Cerebral Ischemia/Reperfusion Injury in Rats. Iran Red Crescent Med J (In Press)
Dehaini H, Awada H et al (2019) MicroRNAs as Potential Pharmaco-targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells 8(2):152
Gao ZQ, Wang JF et al (2017) Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci 7(1):66
Guo QQ, Wu ZM et al (2014) Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH. Soft Matter 10(6):911–920
He H, Zeng Q et al (2019a) Bone marrow mesenchymal stem cell transplantation exerts neuroprotective effects following cerebral ischemia/reperfusion injury by inhibiting autophagy via the PI3K/Akt pathway. Brain Res 1707:124–132
He Q, Li Z et al (2019b) Parkin-Dependent Mitophagy Is Required for the Inhibition of ATF4 on NLRP3 Inflammasome Activation in Cerebral Ischemia-Reperfusion Injury in Rats. Cells 8(8):897
Jovin TG, Albers GW et al (2016) Stroke Treatment Academic Industry Roundtable: The Next Generation of Endovascular Trials. Stroke 47(10):2656–2665
Li P, Shen M et al (2017) An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol 54(4):2901–2921
Li PF, Teng FM et al (2015) Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol 35(3):433–447
Liang Y, Xu J et al (2018) Inhibition of miRNA-125b decreases cerebral ischemia/reperfusion injury by targeting CK2α/NADPH oxidase signaling. Cell Physiol Biochem 45(5):1818–1826
Ma J, Shui S et al (2017) microRNA-200a silencing protects neural stem cells against cerebral ischemia/reperfusion injury. PLoS ONE 12(2):e0172178
Party, ISW (2012) National clinical guideline for stroke, Citeseer
Savitz SI, Baron JC et al (2019) Stroke Treatment Academic Industry Roundtable X Brain Cytoprotection Therapies in the Reperfusion Era. Stroke 50(4):1026–1031
Sha R, Han X et al (2019) The Effects of Electroacupuncture in a Rat Model of Cerebral Ischemia-Reperfusion Injury Following Middle Cerebral Artery Occlusion Involves MicroRNA-223 and the PTEN Signaling Pathway. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 25:10077
Tao Z, Zhao H et al (2015) Neuroprotective effect of microRNA-99a against focal cerebral ischemia–reperfusion injury in mice. J Neurol Sci 355(1–2):113–119
Tian X, An R et al (2019) Tamibarotene Improves Hippocampus Injury Induced by Focal Cerebral Ischemia-Reperfusion via Modulating PI3K/Akt Pathway in Rats. J Stroke Cerebrovasc Dis
Trialists’ Collaboration, SU (2013) Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 9(9)
Wang J, Cao B et al (2017) Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis 8(1):71
Wang J, Wang A et al (2019) Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed Pharmacother 112:108692
Wang M, Sun Y et al (2018) Preclinical studies using miR-32-5p to suppress clear cell renal cell carcinoma metastasis via altering the miR-32-5p/TR4/HGF/Met signaling. Int J Cancer 143(1):100–112
Wang X, Shi C et al (2020a) MicroRNA-22 exerts its neuroprotective and angiogenic functions via regulating PI3K/Akt signaling pathway in cerebral ischemia–reperfusion rats. J Neural Transm 127:35–44
Wang ZW, Han YF et al (2020b) Lupeol Alleviates Cerebral lschemia-Reperfusion Injury in Correlation with Modulation of PI3K/Akt Pathway. Neuropsychiatr Dis Treat 16:1381–1390
Xiao Q, Ye QF et al (2017) Mild hypothermia pretreatment protects hepatocytes against ischemia reperfusion injury via down-regulating miR-122 and IGF-1R/AKT pathway. Cryobiology 75:100–105
Yang CC, Wei XP et al (2021) Down-regulating microRNA-20a regulates CDH1 to protect against cerebral ischemia/reperfusion injury in rats. Cell Cycle 20(1):54–64
Yang X, Ji H et al (2018) Downregulation of circ_008018 protects against cerebral ischemia–reperfusion injury by targeting miR-99a. Biochem Biophys Res Commun 499(4):758–764
Zhang YM, Liu JY et al (2021) Exosomal microRNA-22–3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/BMF axis. Stem Cell Res Ther 12(1)
Zheng T, Shi Y et al (2019) MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed Pharmacother 117:109117
Zheng Y, Zhao P et al (2019) MiR-340–5p alleviates oxygen-glucose deprivation/ reoxygenation-induced neuronal injury via PI3K/Akt activation by targeting PDCD4. Neurochem Int 104650
Acknowledgments
The authors would like to express our gratitude for those who have critically reviewed this manuscript and those who give us help during this experiment.
Funding
The research was supported by Shenzhen “Sanming Project” (grant no.: SZSM201610039), Science Technology Innovation and Industrial Development of Shenzhen Dapeng New District (Grand No. YL202001-14), and Scientific Research Project of Shenzhen Dapeng New District Medical and Health Group(Grand No. 2020JTLCYJ01).
Author information
Authors and Affiliations
Contributions
Yao Wang and Shangjie Chen were the guarantor of integrity of the entire study. Yulong Wang provided the study concepts. Yao Wang and Shangjie Chen designed the experiments. Weiyi Pan defined the intellectual content.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Affiliated Baoan Hospital of Shenzhen, Southern Medical University.
Competing interests
The author reports no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Pan, W., Wang, Y. et al. MicroRNA-32-5p attenuates cerebral ischemia/reperfusion injuries by modulating the phosphatase and tensin homologous protein. Metab Brain Dis 36, 2495–2504 (2021). https://doi.org/10.1007/s11011-021-00744-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00744-1