Abstract
Therapeutic hypothermia (TH) is a promising neuroprotective agent for treating stroke. However, its clinical application was limited by the impractical duration. Icariin (ICA) were reported to have therapeutic effect on cerebral ischemia. In this research, our aim was to investigate whether the combination of TH and ICA had better neuroprotective effects on ischemic stroke. An ischemia-reperfusion rat model was established and treated with mild hypothermia, ICA or JSH-23 (inhibitor of NF-κB). Thermistor probe, 2′3’5′-triphenyl tetrazolium chloride (TTC), 5/12-score system, and ELISA were used to detect temperature (rectum, cortex, striatum), infarct volume, neurological deficit, and cerebral cell death of these rats. The expressions of tumor necrosis factor (TNF)-α, Interleukin- 6 (IL-6), nuclear factor-kappa B (NF-κB), nuclear factor erythroid2-related factor (Nrf2), peroxisome proliferator activated receptor gamma (PPARα), PPARγ, Janus kinase 2 (JAK2), p-JAK2, signal transducers and activators of transduction-3 (STAT3), and p-STAT3 were detected by Western blot or q-PCR. Mild hypothermia, ICA, and JSH-23 reduced the cerebral infarct volume, neurological deficit, cerebral cell death of rats, downregulated the expressions of TNF-α, IL-6, C-Caspase 3 and Bax, and the activation of PPARs/Nrf2/NF-κB and JAK2/STAT3 pathways, but elevated the expression of Bcl-2. ICA promoted the effect of mild hypothermia on infarct volume, neurological deficit, and cerebral cell death. Moreover, ICA also enhanced the regulatory effect of mild hypothermia on apoptosis/inflammation factors expressions and activation of PPARs/Nrf2/NF-κB and JAK2/STAT3 pathways. ICA could promote mild hypothermia-induced neuroprotection by inhibiting the activation of NF-κB through the PPARs/Nrf2/NF-κB and JAK2/STAT3/NF-κB pathways in experimental stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is characterized by high morbidity, disability, and mortality (Hankey 2017). Stroke can be divided into ischemic stroke and hemorrhagic stroke, and the proportion of ischemic stroke is more than 80% (Hankey 2017; Minnerup et al. 2012). Ischemic stroke is caused by the blockage of blood vessels entering into the cerebrum and decrease of blood volume in the whole body, therey resulting in decreased intracranial blood flow, damaged nervous system caused by cerebral ischemia and hypoxia (Favate and Younger 2016). Studies have shown that about 50% of patients can spontaneously restore blood supply after cerebral ischemia, but some patients will develop aggravated tissue damage, and even irreversible damage, which refers to cerebral ischemia-reperfusion injury (CIRI) (Frieler et al. 2017; Wu et al. 2017). Therefore, CIRI is the main complication of the ischemic stroke, stroke petients with CIRI are always accompanied by cerebral inflammation, neurological deficits and limb disorders (Frieler et al. 2017; Gao et al. 2015; Wu et al. 2017). At present, tissue plasminogen activator thrombolytic therapy is the only method approved by the Food and Drug Administration (FDA) for the treatment of acute ischemic stroke (Ginsberg 2008; Minnerup et al. 2012). However, due to the limited treatment time, fewer than 5% of stroke patients can benefit from it. Although some promising neuroprotective agents have been developed, most of them showed poor clinical outcomes in clinical trials (Patel and McMullen 2017).
Therapeutic hypothermia (TH) has shown promising effects in animal experiments and clinical studies (Yenari and Han 2012). The Copenhagen Stroke Study proved that 1 °C rise in the body temperature could independently predict a 30% increase in long-term mortality risk (Kammersgaard et al. 2002). An et al. also confirmed that TH can minimize infarct volume by 90% in ischemic rodent models (An et al. 2017). However, delayed induction of hypothermia limits its clinical application (Neimark et al. 2008), which encouranged us to explore new methods to increase the neuroprotective effect and clinical practicability of TH without prolonging the duration and extent of hypothermia treatment.
Icariin (ICA) is a phytoestrogens and flavonoid compounds extracted from Epimedium and is widely used in China as a tranditional Chinese Herbal Medicine (Zheng et al. 2020). Modern pharmacological research has proven that ICA could promote bone growth, cardiovascular protection, immune regulation, and has anti-tumor and anti-inflammatory effects (Aljehani et al. 2020; Li et al. 2020; Wang et al. 2020a). In addition, the neuroprotection of ICA is also discovered (Wang et al. 2020b; Zhang et al. 2020a) to have neuroprotection after cerebral ischemia (Liu et al. 2018a; Wang et al. 2020b). However, whether ICA could increase the neuroprotective effect and clinical practicability of mild hypothermia in CIRI was not clear. Therefore, in this study, we first established a rat middle cerebral artery occlusion (MCAO) model. Then, we focused on exploring whether the combination of TH and ICA had better neuroprotective effects on MCAO rats, and investigated the potential mechanisms under the neuroprotective effects.
Materials and methods
Ethics statement
Animal study in this research was approved by the Committee of Experimental Animals of the Second Affiliated Hospital of Hainan Medical University (Z2019010604N). All animal experiments were performed strictly following the guidelines of China Council on Animal Care and Use. All the experiments involving animals were performed in the Second Affiliated Hospital of Hainan Medical University.
Subjects
A total of 150 adult male Sprague-Dawley rats (250–300 g, SLAC Laboratory Animal Technology Co., Shanghai, China) were used in this research. All experimental animals were fed in the SPF animal environment and given 12 h dark/12 h light cycle. After 5 days of adaptive feeding, the animals were randomly divided into the following six groups (n = 25): Sham group: the animals underwent surgery without infarct and received intragastric administration of normal saline (S0817, Sigma, St. Louis, Missouri, USA) once a day for 28 day. Model group: the rats were given right middle cerebral artery occlusion (MCAO) surgery and intragastric administration of normal saline once a day for 28 day. Model + mild hypothermia group: the rats underwent MCAO surgery and mild hypothermia treatment and then intragastric administration of normal saline once a day for 28 day. Model + ICA group: the rats underwent MCAO sugery and then intragastric administration of 60 mg/kg/d ICA (I1286, Sigma) once a day for 28 day. Model + mild hypothermia + ICA group: the rats underwent MCAO sugery and mild hypothermia treatment and then received intragastric administration of 60 mg/kg/d ICA once a day for 28 day. Model + JSH-23 group, the rats underwent MCAO sugery 3 h after intraperitoneal injection of 5 mg/kg JSH-23 (NF-κB inhibitor; J4455, Sigma).
Rat MCAO model establishment
Rat MCAO model was established according to a previous research (Liu et al. 2018a; Zhao et al. 2018). In brief, after the rats were anestheszed with 1.5% enflurane (Liu et al. 2018b), the left common carotid artery, external carotid artery, and internal carotid artery were exposed and separated through a median neck incision. To occlude the origin of the middle cerebral artery, the MCAO bolt (1623, Cinontech, Beijing, China) was inserted into the internal carotid artery lumen via the external carotid artery lumen until a slight resistance. After blocking for 2 h, the bolt was pulled out for reperfusion. And then the mice in each group were given saline, ICA, mild hypothermia, or JSH-23 treatment respectively. During the operation, the spontaneous respiration was kept and the rectal temperature was maintained at 37 °C.
Mild hypothermia treatment
The induction of mild hypothermia was performed as previously reported (Liu et al. 2018b). In brief, during the 2 h of ischemia, the skull of the rat was drilled (2.0 mm deep) from the outside of bregma and further implanted with a brain temperature probe (No. 2013082512, Thermometer, Taiwan, China). For hypothermia treatment, the rats were kept in cold condition (4 °C) and the isolated cortical temperature was kept continuously at 33 ± 1 °C. Meanwhile, the anal temperature was maintained at 37 ± 1 °C. In addition, the temperatures of cortex, striatum, and rectal were recorded every 10 min until the rectal temperature of the rats was restored to 36 °C.
Cerebral infarct volume detection
Twenty four h after reperfusion, the detection of cerebral infarct detection was performed using 2′3’5′-triphenyl tetrazolium chloride (TTC) staining (Zhao et al. 2018). In brief, the six rats in each group were was anestheszed with 1.5% enflurane and further sacrificed by cervical dislocation. Then the brain tissue was taken out. After the brain tissue section, coronal brain slices with a 2 mm thickness were treated with TTC (S19026, Yuanye, Shanghai, China) at 37 °C for 20 min. Then 10% formalin solution (P804537, Macklin) was used to fix the brain tissue sections. Finally, infarct volume was calculated using Image Proplus 6.0 (Fu et al. 2013; Geng et al. 2015).
Neurological deficits evaluation
Twenty four hour after the drug treatment, the degree of neurological deficits of the rats were evaluated by the modified 5-score system and 12-point scoring scale as the previously described (Cai et al. 2017).
Cell death detection
A cell death detection ELISA kit (11,544,675,001, Sigma) was used to examine the apoptosis through measuring the amount of cytoplasmic histone-associated DNA fragments generated by apoptosis using a photometric enzyme immunoassay. In brief, brain sample (10 mg) was incubated in citric acid solution (GZ02618, Hongji, Neimenggu, China) mixed with 0.5% Tween-20 (W070–1-1, Bioroyee, Beijing, China) and then centrifuged for 15 min at 2000×g. The supernatant was diluted by incubation buffer. The absorbance of incubation supernatant was measured at 450 nm by a microplate reader (Infinite M200 PRO, Tecan Austria GmbH, Austria).
mRNA expression detection
Quantitative real-time polymerase chain reaction (q-PCR) assays were used to detect the expression level of mRNA in rat brain samples. In brief, after the mRNA in the tissue was isolated using a TRIzol reagent (15,596, Invitrogen, MA, USA), PrimeScript RT kit (RR037A, Takara, Dalian, China) was used to reverse-transcribe RNA into cDNA. Finally, gene expression was determined by q-PCR assays using Verso 1-step RT-qPCR Kit (A15300, Thermo Scientific, MA, USA) in ABI 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA) according to the instructions. mRNA was quantified by the 2-△△CT method (Schmittgen and Livak 2008). All the primer sequences were showed in Table 1.
Protein expression detection
Western bolt assays were performed to detect protein expression levels in rat brain samples. Total protein from the brain samples was isolated by RIPA lysis buffer (P0013B, Beyotime, Shanghai, China), and the nuclear protein and cytoplasmic protein from the samples were also isolated by the kit (P0028, Beyotime). Then a BCA assay kit (23,250, Pierce, MA, USA) was applied to detect protein concentrations. Finally, the protein (25 μg) was separated by SDS-PAGE gels (P0052A, Beyotime) and further transferred to PVDF membranes (FFP24, Beyotime), which were then blocked with 5% skimmed milk for 2 h. Then, the membrane was incubated with the following relative primary antibodies at 4 °C overnight: TNF-α (1:1000, ab255275, Abcam, Cambridge, UK), IL-6 (1:1000, ab6672, Abcam), C-Caspase 3 (1:1000, ab49822, Abcam), Caspase 3 (1:1000, ab13847, Abcam), Bax (1:1000, ab32503, Abcam), Bcl-2 (1:1000, ab182858, Abcam), NF-κB (1:2000, ab16502, Abcam), Nrf2 (1:3000, ab92946, Abcam), Lamin B (1:3500, ab32535, Abcam), PPARα (1:1000, ab227074, Abcam), PPARγ (1:2000, ab272718, Abcam) p-JAK2 (1:1000, 120kD, ab32101, Abcam), JAK2 (1:1000, 120kD, ab108596, Abcam), p-STAT3 (1:1000, 88kD, ab76315, Abcam), STAT3 (1:1000, 88kD, ab119352, Abcam), and GAPDH (1:1000, 36kD, ab8245, Abcam). The next day, mouse IgG secondary antibody (1:5000, ab205719, Abcam) and rabbit IgG secondary antibody (1:5000, ab205718, Abcam) were incubated with the membranes for 1 h. Finally, after the ECL detection solution (34,078, Thermo Scientific, MA, USA) was used to incubate the membranes, the protein signal was detected by Image Lab™ Software (Bio-Rad, Hercules, CA, USA) .
Statistical analysis
One-way ANOVA were applied to analyze the data involving in this study by SPSS software (version 18.0). Bonferroni served as post-hoc tests. The statistical data were expressed as Mean ± standard deviation. All experiments were conducted three times. Statistically, a significant result was labeled by P < 0.05.
Results
ICA reduced the time for low physical temperature to reach target temperature during the mild hypothermia treatment in MCAO rats
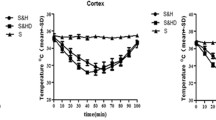
In Sham, Model, Model + ICA, and Model + JSH-23 groups, rat temperatures in the rectum (Fig. 1a), cortex (Fig. 1b), and striatum (Fig. 1c) were maintained within a normal range. In Model + mild hypothermia group, rectum temperature (37.3 °C), cortex temperature (35 °C), and striatum temperature (36.2 °C) were decreased to 33.0 °C, 31.5 °C and 33 °C, respectively, within 60 min, and gradually returned to 36 °C (rectum), 35 °C (cortex), and 36 °C (striatum) at 100 min. In Model + mild hypothermia + ICA group, rectum temperature (36.5 °C), cortex temperature (34 °C) and striatum temperature (36 °C) were rapidly decreased to the lowest degree at 32.5 °C, 31 °C and 32 °C, respectively, within 40 min (20 min faster than Model + mild hypothermia group), and also gradually returning to 36 °C (rectum), 35 °C (cortex), and 36 °C (striatum) at 100 min. Therefore, Model + mild hypothermia + ICA group showed a faster cooling rate than Model + mild hypothermia and Model +ICA groups.
ICA shortened the time for the physical low temperature to reach target temperature during the mild hypothermia treatment in MCAO rats. a The rectum temperature was measured through the thermistor probe in each group. b The reduction of cortex temperature was measured through the thermistor probe in each group. c The reduction of striatum temperature was measured through the thermistor probe in each group. Sham group: rats underwent surgery without infarct and received intragastric administration of normal saline; Model group: rats were given right MCAO surgery and received intragastric administration of normal saline; Model + mild hypothermia group: rats underwent MCAO surgery and mild hypothermia treatment and received intragastric administration of normal saline; Model + ICA group: rats underwent MCAO sugery and received intragastric administration of ICA; Model + mild hypothermia + ICA group, the rats underwent MCAO sugery and mild hypothermia treatment and received intragastric administration of ICA; Model + JSH-23 group, rats underwent MCAO sugery and intraperitoneal injection of JSH-23. (ICA: Icariin, MCAO: middle cerebral artery occlusion)
ICA enhanced the inhibitory effect of mild hypothermia on infarct, neurological deficit, and cerebral cell death in MCAO rats
As shown in Fig. 2a and b, at 24 h of reperfusion, the infarct volume in the Model group was remarkably higher than Sham group (P < 0.01). In Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups, the infarct volume was lower than that in Model group (P < 0.01), while in Model + mild hypothermia + ICA group, the infarct volume was further lower than that in Model + mild hypothermia (P < 0.01) and Model + ICA group (P < 0.01). For neurological deficit, as exhibited in Fig. 2c and d, the 5-scoring and 12-scoring in Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups were lower than that in Model group (P < 0.05 or P < 0.01), while in Model + mild hypothermia + ICA group, the 5-scoring and 12-scoring were both lower than that in Model + mild hypothermia and Model + ICA groups (P < 0.01). These results indicated that ICA enhanced the effect of mild hypothermia on infarct and neurological deficits in MCAO rats. To further confirm the results, we detected the cell death level in MCAO rat brain using a cell death detection ELISA assay. As shown in Fig. 2e, the relative cell death value in Model group was significantly increased compared with Sham group (P < 0.01). The cell death in Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups was lower than that in Model group (P < 0.01), while in Model + mild hypothermia + ICA group, the cell death was both lower than that in Model + mild hypothermia and Model +ICA groups (P < 0.01).
ICA enhanced the inhibitory effect of mild hypothermia on infarct, neurological deficit, and cerebral cell death in MCAO rats. a, b Cerebral infarct volume was detected by TTC histology. c-d Neurological deficits evaluation was determined by the modified 5-score system and 12-point scoring scale. e Cerebral cell death was detected by ELISA. (△△P < 0.01, △△△P < 0.001, vs. Sham; **P < 0.01, ***P < 0.001, vs. Model; ##P < 0.01, ###P < 0.001, vs. Model + mild hypothermia; ^^^P < 0.001, vs. Model + ICA). (ICA: Icariin, MCAO: middle cerebral artery occlusion)
ICA enhanced the effect of mild hypothermia on inflammation-related factors expression in MCAO rats
To further confirm the previous results in the current study at molecular level, we conducted a series of western blot and q-PCR assays. As shown in Fig. 3a-c, the protein expression levels of TNF-α and IL-6 in Model group were remarkably higher than Sham group (P < 0.01). In Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups, the expression levels of TNF-α and IL-6 were down-regulated by treatment with mild hypothermia, ICA, and JSH-23, as compared with Model group (P < 0.01). The expression levels were both further down-regulated in Model + mild hypothermia + ICA group compared with Model + mild hypothermia (P < 0.05) and Model + ICA group (P < 0.01). As shown in Fig. 3d, e, the TNF-α and IL-6 mRNA expression levels had the same tendency to its protein expression, which further suggested that ICA enhanced the effect of mild hypothermia on TNF-α and IL-6 expression in MCAO rats.
ICA enhanced the effect of mild hypothermia on TNF-α and IL-6 expressions in MCAO rats. a-c The protein expressions of TNF-α and IL-6 in MCAO rat brain tissues were detected by western blot assays, GAPDH served as an internal control. (D, E) The mRNA expressions of TNF-α and IL-6 in MCAO rat brain tissues were detected by q-PCR, GAPDH served as an internal control. (△△△P < 0.001, vs. Sham; **P < 0.01, ***P < 0.01, vs. Model; ##P < 0.01, ###P < 0.01, vs. Model + mild hypothermia; ^^^P < 0.01, vs. Model + ICA). (ICA: Icariin, MCAO: middle cerebral artery occlusion)
ICA enhanced the effect of mild hypothermia on apoptosis-related factors expression in MCAO rats
As shown in Fig. 4a-e, the Caspase 3 expression showed no changes among these groups, and the expression of C-Caspase 3 and Bax in Model group were remarkably higher than Sham group (P < 0.01). In Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups, the expression of C-Caspase 3 and Bax were down-regulated by treatment with mild hypothermia, ICA, or JSH-23 compared with Model group (P < 0.05 or P < 0.01). However, the expression levels were both further inhibited in Model + mild hypothermia + ICA group when compared with Model + mild hypothermia and Model + ICA group (P < 0.05 or P < 0.01). The expression level of Bcl-2 in Model group was remarkably lower than that in Sham group (P < 0.01). In Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups, the expression level of Bcl-2 was up-regulated by treatment with mild hypothermia, ICA, and JSH-23 when compared with Model group (P < 0.01). The expression level of Bcl-2 was further up-regulated in Model + mild hypothermia + ICA group when compared with Model + mild hypothermia (P < 0.05) and Model + ICA group (P < 0.01). As shown in Fig. 4f-h, the mRNA expression levels of Caspase 3, Bax, and Bcl-2 had the same tendency as its protein expression, which further suggested that ICA enhanced the effect of mild hypothermia on C-Caspase 3, Bax, and Bcl-2 expression in MCAO rats.
ICA enhanced the effect of mild hypothermia on C-Caspase 3, Bax, and Bcl-2 expressions in MCAO rats. a-e The protein expressions of C-Caspase 3, Caspase 3, Bax, and Bcl-2 in MCAO rat brain tissues were detected by western blot assays, GAPDH served as an internal control. f-h The mRNA expressions of Caspase 3, Bax, and Bcl-2 in MCAO rat brain tissues were detected by q-PCR, GAPDH served as an internal control. (△△△P < 0.001, vs. Sham; **P < 0.01, ***P < 0.001, vs. Model; ###P < 0.001, vs. Model + mild hypothermia; ^P < 0.05, ^^P < 0.01, ^^^P < 0.01, vs. Model + ICA). (ICA: Icariin, MCAO: middle cerebral artery occlusion)
ICA enhanced the inhibitory effect of mild hypothermia on activation of PPARs/Nrf2/NF-κB in MCAO rats
Then we detected the activation of PPARs/Nrf2/NF-κB, as exhibited in Fig. 5a-c, the expression of nucleus NF-κB in Model group was remarkably higher than those in Sham group (P < 0.01). In Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups, the expression levels of nucleus NF-κB were down-regulated by treatment with mild hypothermia, ICA, or JSH-23 when compared with Model group (P < 0.01). However, the expression of nucleus NF-κB was further down-regulated in Model + mild hypothermia + ICA group when compared with Model + mild hypothermia group (P < 0.05). The expression of nucleus Nrf2 in Model group were remarkably higher than those in Sham group (P < 0.01), and it was further up-regulated in Model + mild hypothermia and Model + ICA groups when compared with Model group (P < 0.001). In addition, the expression of nucleus Nrf2 were further up-regulated in Model + mild hypothermia + ICA group when compared with Model + mild hypothermia and Model + ICA group (P < 0.01). Meanwhile, the expressions of cytoplasm NF-κB and Nrf2 was also evaluated (Fig. 5d-f), and it showed an opposite tendency to the NF-κB and Nrf2 expressed in nucleus. Moreover, the expressions of PPARα and PPARγ was determined, as shown in Fig. 5g-i, the expression of PPARγ was down-regulated in Model group as compared to the Sham group (P < 0.05), while in the Model + mild hypothermia and Model + ICA group, the expressions of PPARα and PPARγ were both up-regulated when compared with the Model group (P < 0.05). In addition, the expressions of PPARα and PPARγ were further up-regulated in Model + mild hypothermia + ICA group when compared with the Model + mild hypothermia or Model + ICA group (P < 0.05). These results suggested that ICA enhanced the inhibitory effect of mild hypothermia on activation of PPARs/Nrf2/NF-κB in MCAO rats.
ICA enhanced the inhibitory effect of mild hypothermia on activation of PPARs/Nrf2/NF-κB in MCAO rats. a-c The expressions of nucleus NF-κB and Nrf2 in MCAO rat braintissues were detected by western blot assays, Lamin B served as an internal control. d-f The expressions of cytoplasm NF-κB and Nrf2 in MCAO rat brain tissues were detected by Western blot assays, GAPDH served as an internal control. g-i The expressions of PPARα and PPARγ in MCAO rat brain tissues were detected by Western blot assays, GAPDH served as an internal control. (△P < 0.05, △△△P < 0.001, vs. Sham; *P < 0.05, **P < 0.01, ***P < 0.001, vs. Model; #P < 0.05,##P < 0.01, ###P < 0.001, vs. Model + mild hypothermia; ^^P < 0.01, ^^^P < 0.01, vs. Model + ICA). (ICA: Icariin, MCAO: middle cerebral artery occlusion)
ICA enhanced the inhibitory effect of mild hypothermia on JAK2/STAT3 pathway activation in MCAO rats
To further confirm investigated whether the treatment of ICA and mild hypothermia had an effect on the activation of JAK2/STAT3 pathway, we further detected these proteins expression. As shown in Fig. 6a-e, the expression levels of JAK2 and STAT3 showed no changes among these groups (Fig. 6c, e), and the expression levels of p-JAK2 and p-STAT3 in Model group were remarkably higher than those in Sham group (P < 0.01). In Model + mild hypothermia, Model + ICA, and Model + JSH-23 groups, the expression levels of p-JAK2 and p-STAT3 were down-regulated by treatment with mild hypothermia, ICA, or JSH-23 when compared with Model group (P < 0.01). However, the expression levels were further decreased in Model + mild hypothermia + ICA group when compared with Model + mild hypothermia and Model + ICA group (P < 0.05). Considering that no difference was found in the expressions of JAK2 and STAT3, we found the ratios of p-JAK2/JAK2 and p-STAT3/STAT3 showed the same tendency to the expressions of p-JAK2 and p-STAT3 among groups (Fig. 6f, g), which further suggested that ICA enhanced the effect of mild hypothermia on the activation of JAK2/STAT3 pathway.
ICA enhanced the inhibitory effect of mild hypothermia on JAK2/STAT3 pathway activation in MCAO rats. a-e The expressions of p-JAK2, JAK2, p-STAT3, and STAT3 in MCAO rat brain tissues were detected by western blot assays, GAPDH served as an internal control. f The ratio of p-JAK2/JAK2 was analyzed based on the Western blot results. g The ratio of p-STAT3/STAT3 was analyzed based on the Western blot results. (△△△P < 0.001, vs. Sham; **P < 0.01, ***P < 0.001, vs. Model; #P < 0.05,##P < 0.01, ###P < 0.001, vs. Model + mild hypothermia; ^^^P < 0.001, vs. Model + ICA). (ICA: Icariin, MCAO: middle cerebral artery occlusion)
Discussion
In the current study, our purpose was to investigate whether the combination of TH and ICA had a better neuroprotective effect on experimental stroke, and to investigate its potential mechanisms. After we established the MCAO rat model and treated it with mild hypothermia, ICA, or JSH-23, we detected the temperature (rectum, cortex, and striatum), infarct volume, and neurological deficit of these rats. The results showed that combined with ICA and mild hypothermia, ICA shortened the time required for the physical low temperature to reach target temperature (35 °C) and also enhanced the protective effect of mild hypothermia on infarct and neurological deficit. In addition, we further confirmed that the mechanisms of these neuroprotective effects might be related to the expression of inflammatory factors (TNF-α, IL-6) and apoptotic factors (C-Caspase 3, Bax, Bcl-2), which were regulated by PPARs/Nrf2/NF-κB and JAK2/STAT3/NF-κB signaling pathways.
Research on effective protective measures and drug treatment to reduce CIRI after cerebral ischemia has become a hot topic in medical research in recent years. In clinical, there is mild hypothermia (34.5 ~ 36.5 °C), moderate hypothermia (34.5 ~ 32.0 °C), marked hypothermia (28.0 ~ 32.0 °C) and profound hypothermia (< 28.0 °C) (Li et al. 2017). Since 1987, there is large number of animal and clinical trials have found that mild hypothermia has obvious neuroprotective effect on cerebral ischemia (An et al. 2017; Busto et al. 1987; Li et al. 2017; Yenari and Han 2012). In this study, we also proved that mild hypothermia could decrease infarct volume and has a neuroprotective effect on CIRI. However, time is required to reach the target temperature of mild hypothermia after mild hypothermia treatment (Neimark et al. 2008). The delayed induction of hypothermia becomes the limitation of mild hypothermia clinical application, which encouranged us to explore new methods to increase the neuroprotective effect and clinical practicability of mild hypothermia without prolonging the duration and extent of hypothermia treatment.
Although studies have reported that ICA could promote bone growth, cardiovascular protection, immune regulation, and has anti-tumor and anti-inflammatory effects (Aljehani et al. 2020; Li et al. 2020; Wang et al. 2020a). ICA is also proven to have the neuroprotective effects on the injury of cerebral ischemia (Liu et al. 2018a; Wang et al. 2020b). However, whether ICA could increase the neuroprotective effect and clinical practicability of mild hypothermia in CIRI was not clear. In this research, for the first time, we found that combination with ICA and mild hypothermia, the time for physiological hypothermia to reach the target temperature was 20 min earlier than for mild hypothermia used alone, and that ICA also enhanced the protective effect of mild hypothermia on infarct and neurological deficit. These findings indicated that ICA could become potential therapeutic agents for increasing the neuroprotective effect and clinical practicability of mild hypothermia without prolonging the duration and extent of hypothermia treatment in clinical.
During the process of ischemic stroke, cell apoptosis and inflammation are the main major regulatory factors (An et al. 2017; Li et al. 2017). Research also reported that the progression of cell apoptosis and inflammation plays important roles in CIRI, for example, Hu et al. showed that cell apoptosis is remarkably increased in cerebral ischemia and CIRI (Hu et al. 2017), and Gao et al. also reported that there was excessive apoptosis and inflammation generation during the CIRI (Gao et al. 2017). Consistent with previous research, in this study, we also proved that the expression of TNF-α, IL-6, Bax, C-Caspase 3 were significantly up-regulated and Bcl-2 was down-regulated in MCAO rats indicating that the apoptosis and inflammation was enhanced during CIRI. We further found that mild hypothermia and ICA both reduced the cell apoptosis and inflammation. Moreover, after treatment with ICA and the mild hypothermia, ICA could enhance the inhibitory effect of mild hypothermia on cell apoptosis and inflammation.
PPARs (PPARα, PPARβ, and PPARγ) could inhibit neuronal death and inflammation in ischemic brain injury (Li et al. 2019). PPARs has anti-inflammatory and anti-oxidant effects on a rat model of Parkinson’s disease (Barbiero et al. 2014). In addition, overexpression of PPARβ/δ exerts a protective effect on ischemia/reperfusion-induced myocardial injury (Burkart et al. 2007). PPARγ reduced proinflammatory cytokine release by inhibiting the NF-κB (Sauer 2015). Nrf2 could protect against the inflammatory response, and is the up-stream of NF-κB and the activation of Nrf2 inhibits the NF-κB (Li et al. 2019; Xiong et al. 2016). It was proved that PPARs/Nrf2/NF-κB signaling pathway regulates the inflammation and apoptosis in ischemic stroke (Li et al. 2019). The activation of NF-κB promotes the proinflammatroy (including TNF-α and IL-6) and regulates the expressions of apoptosis-related factors (including Bax, C-Caspase 3, and Bcl-2) in ischemic stroke (Li et al. 2019). Previous research discovered that the activation of NF-κB and Nrf2 were promoted, while the PPARγ expression was down-regulated in MCAO rats (Li et al. 2019). Consistently, the same phenomena were discovered in our research. We found that the ICA and the mild hypothermia inhibited the the activation of NF-κB but promoted the activation of Nrf2 and PPARs expressions in MCAO rats. Moreover, ICA enhanced the effect of mild hypothermia on PPARs/Nrf2/NF-κB pathway. Therefore, we speculated that ICA and mild hypothermia affected the MCAO rat by regulating the PPARs/Nrf2/NF-κB pathway.
It is also proven that the activation of NF-κB is influenced by the expression of STAT3. The STAT3/NF-κB pathway is associated with the epithelial-mesenchymal transition (EMT) progression of CSCs, and could also enhance the cisplatin sensitivity of tumor cells (Kuo et al. 2017). JAK/STAT pathway is vital for the development and function of innate and adaptive immunities (Coskun et al. 2013; Kisseleva et al. 2002), moreover, cytokine and oxidative stress induce JAK2 phosphorylation and STAT3 transcripition factor activation, thereby promoting inflammation-relatived gene expression (Banerjee et al. 2017). In addition, JAK2/STAT3 pathway is discovered to be abnormally activated in stoke (Hu et al. 2017; Li et al. 2015; Tang et al. 2018). JAK2/STAT3/NF-κB pathway plays a regulatory role in myocardial ischemia-reperfusion injury in rats. Ganoderic acid A alleviates myocardial ischemia-reperfusion injury by regulating JAK2/STAT3/NF-κB pathway in rats (Zhang et al. 2020b). Mesenchymal stromal cell-derived extracellular vesicles protected MCAO-injured rats possibly through regulating the AMPK and JAK2/STAT3/NF-κB signaling pathways (Han et al. 2020). Therefore, we examined whether the JAK2/STAT3/NF-κB participated in the effect of ICA and mild hypothermia on the MCAO rat. After the JAK2/STAT3 pathway was activated in MCAO rat, we found that the ICA and mild hypothermia further decreased the activated JAK2/STAT3 pathway, and that ICA enhanced the effect of mild hypothermia on JAK2/STAT3 pathway.
To further verify that ICA and mild hypothermia affected the MCAO rat through regulating the PPARs/Nrf2/NF-κB and JAK2/STAT3/NF-κB pathways, the inhibitor of NF-κB (JSH-23) was used. To our delight, JSH-23 treatment could reduce MCAO rat cerebral infarct volume and neurological deficit, furthermore, the JSH-23 also inhibited the activation of NF-κB and JAK2/STAT3 pathways, showing that PPARs/Nrf2/NF-κB and JAK2/STAT3/NF-κB pathways were invovled in the ICA and mild hypothermia in affecting the MCAO rat.
In conclusion, this research reveals that ICA could enhance mild hypothermia-induced neuroprotection by inhibiting the activation of NF-κB through the PPARs/Nrf2/NF-κB and JAK2/STAT3/NF-κB pathways in experimental stroke, which might be a therapeutic agent for increasing the neuroprotective effect and clinical practicability of mild hypothermia without prolonging the duration and extent of hypothermia treatment in clinical practice.
Data Availability
The analysed data sets generated during the study are available from the corresponding author on reasonable request.
References
Aljehani AA, Albadr NA, Eid BG, Abdel-Naim AB (2020) Icariin enhances AMP-activated protein kinase and prevents high fructose and high salt-induced metabolic syndrome in rats. Saudi Pharm J 28:1309–1316. https://doi.org/10.1016/j.jsps.2020.08.021
An H et al (2017) Phenothiazines enhance mild hypothermia-induced Neuroprotection via PI3K/Akt regulation in experimental stroke. Sci Rep 7:7469. https://doi.org/10.1038/s41598-017-06752-5
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM (2017) JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 77:521–546. https://doi.org/10.1007/s40265-017-0701-9
Barbiero JK et al (2014) PPAR-α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson's disease. Prog Neuro-Psychopharmacol Biol Psychiatry 53:35–44. https://doi.org/10.1016/j.pnpbp.2014.02.009
Burkart EM et al (2007) Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117:3930–3939. https://doi.org/10.1172/jci32578
Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD (1987) Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 7:729–738. https://doi.org/10.1038/jcbfm.1987.127
Cai L et al (2017) Combining Normobaric oxygen with ethanol or hypothermia prevents brain damage from thromboembolic stroke via PKC-Akt-NOX modulation. Mol Neurobiol 54:1263–1277. https://doi.org/10.1007/s12035-016-9695-7
Coskun M, Salem M, Pedersen J, Nielsen OH (2013) Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res 76:1–8. https://doi.org/10.1016/j.phrs.2013.06.007
Favate AS, Younger DS (2016) Epidemiology of ischemic stroke. Neurol Clin 34:967–980. https://doi.org/10.1016/j.ncl.2016.06.013
Frieler RA et al (2017) Genetic neutrophil deficiency ameliorates cerebral ischemia-reperfusion injury. Exp Neurol 298:104–111. https://doi.org/10.1016/j.expneurol.2017.08.016
Fu P, Peng C, Ding JY, Asmaro K, Sullivan JM, Guthikonda M, Ding Y (2013) Acute administration of ethanol reduces apoptosis following ischemic stroke in rats. Neurosci Res 76:93–97. https://doi.org/10.1016/j.neures.2013.02.011
Gao HJ et al (2015) Ligustrazine monomer against cerebral ischemia/reperfusion injury. Neural Regen Res 10:832–840. https://doi.org/10.4103/1673-5374.156991
Gao XJ, Xie GN, Liu L, Fu ZJ, Zhang ZW, Teng LZ (2017) Sesamol attenuates oxidative stress, apoptosis and inflammation in focal cerebral ischemia/reperfusion injury. Exp Ther Med 14:841–847. https://doi.org/10.3892/etm.2017.4550
Geng X et al (2015) Ethanol and normobaric oxygen: novel approach in modulating pyruvate dehydrogenase complex after severe transient and permanent ischemic stroke. Stroke 46:492–499. https://doi.org/10.1161/STROKEAHA.114.006994
Ginsberg MD (2008) Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 55:363–389. https://doi.org/10.1016/j.neuropharm.2007.12.007
Han M et al (2020) Neuroprotective effect of Mesenchymal stromal cell-derived extracellular vesicles against cerebral ischemia-reperfusion-induced neural functional injury: a pivotal role for AMPK and JAK2/STAT3/NF-κB signaling pathway modulation. Drug Des Devel Ther 14:2865–2876. https://doi.org/10.2147/dddt.s248892
Hankey GJ (2017) Stroke. Lancet 389:641–654. https://doi.org/10.1016/s0140-6736(16)30962-x
Hu GQ, Du X, Li YJ, Gao XQ, Chen BQ, Yu L (2017) Inhibition of cerebral ischemia/reperfusion injury-induced apoptosis: nicotiflorin and JAK2/STAT3 pathway. Neural Regen Res 12:96–102. https://doi.org/10.4103/1673-5374.198992
Kammersgaard LP et al (2002) Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke 33:1759–1762. https://doi.org/10.1161/01.str.0000019910.90280.f1
Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW (2002) Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285:1–24. https://doi.org/10.1016/s0378-1119(02)00398-0
Kuo WY, Hwu L, Wu CY, Lee JS, Chang CW, Liu RS (2017) STAT3/NF-kappaB-regulated Lentiviral TK/GCV suicide gene therapy for Cisplatin-resistant triple-negative breast cancer. Theranostics 7:647–663. https://doi.org/10.7150/thno.16827
Li L, Li H, Li M (2015) Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med 8:14985–14991
Li C, Dong Y, Chen D, Xie Z, Zhang Y (2017) Mild Hypothermia Attenuates the Anesthetic Isoflurane-Induced Cytotoxicity. Front Cell Neurosci 11:15. https://doi.org/10.3389/fncel.2017.00015
Li Q et al (2019) Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARgamma/Nrf2/NF-kappaB signaling pathway. Int Immunopharmacol 66:309–316. https://doi.org/10.1016/j.intimp.2018.11.044
Li XL, Xu F, Lin FH, Ai LZ, Zhao YJ, Bi XL, Sui L, Zhang Y (2020) A Naringin- and Icariin-contained herbal formula, gushukang, ameliorated aged osteoporosis of aged mice with high calcium intake. Am J Chin Med 48:1671–1691. https://doi.org/10.1142/s0192415x20500834
Liu D, Ye Y, Xu L, Yuan W, Zhang Q (2018a) Icariin and mesenchymal stem cells synergistically promote angiogenesis and neurogenesis after cerebral ischemia via PI3K and ERK1/2 pathways. Biomed Pharmacother 108:663–669. https://doi.org/10.1016/j.biopha.2018.09.071
Liu LQ et al (2018b) Brain-selective mild hypothermia promotes long-term white matter integrity after ischemic stroke in mice. CNS Neurosci Ther 24:1275–1285. https://doi.org/10.1111/cns.13061
Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C (2012) Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13:11753–11772. https://doi.org/10.3390/ijms130911753
Neimark MA, Konstas AA, Choi JH, Laine AF, Pile-Spellman J (2008) Brain cooling maintenance with cooling cap following induction with intracarotid cold saline infusion: a quantitative model. J Theor Biol 253:333–344. https://doi.org/10.1016/j.jtbi.2008.03.025
Patel RAG, McMullen PW (2017) Neuroprotection in the Treatment of Acute Ischemic Stroke. Prog Cardiovasc Dis 59:542–548. https://doi.org/10.1016/j.pcad.2017.04.005
Sauer S (2015) Ligands for the nuclear peroxisome proliferator-activated receptor gamma. Trends Pharmacol Sci 36:688–704. https://doi.org/10.1016/j.tips.2015.06.010
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Tang Y, Tong X, Li Y, Jiang G, Yu M, Chen Y, Dong S (2018) JAK2/STAT3 pathway is involved in the protective effects of epidermal growth factor receptor activation against cerebral ischemia/reperfusion injury in rats. Neurosci Lett 662:219–226. https://doi.org/10.1016/j.neulet.2017.10.037
Wang F, Yang Z, He W, Song Q, Wang K, Zhou Y (2020a) Effects of icariin on the proliferation and osteogenic differentiation of human amniotic mesenchymal stem cells. J Orthop Surg Res 15:578. https://doi.org/10.1186/s13018-020-02076-9
Wang M, Rong Y, Luo L (2020b) Neuroprotective effects of icariin in neonatal hypoxia-ischemic brain damage via its anti-apoptotic property. Childs Nerv Syst. https://doi.org/10.1007/s00381-020-04690-8
Wu R et al (2017) TREM2 protects against cerebral ischemia/reperfusion injury. Mol Brain:10–20. https://doi.org/10.1186/s13041-017-0296-9
Xiong D, Deng Y, Huang B, Yin C, Liu B, Shi J, Gong Q (2016) Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-kappaB PPARalpha and PPARgamma in rats. Int Immunopharmacol 30:157–162. https://doi.org/10.1016/j.intimp.2015.11.035
Yenari MA, Han HS (2012) Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 13:267–278. https://doi.org/10.1038/nrn3174
Zhang T, Dong K, Xiao L, Li G, Zhang Z (2020a) Effects of Co-Administration of Icariin and Panax notoginseng Saponins on Intestinal Microbiota and Hippocampal Protein Expression in a Mouse Model of Alzheimer's Disease. Neuropsychiatr Dis Treat 16:2169–2179. https://doi.org/10.2147/ndt.s253972
Zhang Y et al (2020b) Ganoderic acid a alleviates myocardial ischemia-reperfusion injury in rats by regulating JAK2/STAT3/NF-κB pathway. Int Immunopharmacol 84:106543. https://doi.org/10.1016/j.intimp.2020.106543
Zhao K et al (2018) Combination of mild therapeutic hypothermia and adipose-derived stem cells for ischemic brain injury. Neural Regen Res 13:1759–1770. https://doi.org/10.4103/1673-5374.238617
Zheng X, Zhang L, Jiang W, Abasubong KP, Zhang C, Zhang D, Li X, Jiang G, Chi C, Liu W (2020) Effects of dietary icariin supplementation on the ovary development-related transcriptome of Chinese mitten crab (Eriocheir sinensis). Comp Biochem Physiol Part D Genomics Proteomics 37:100756. https://doi.org/10.1016/j.cbd.2020.100756
Funding
This work was supported by the Medical and Health Research Project of Hainan Province [Grant Number 18A200002]; the Scientific ResearchProject of Colleges and Universities in Hainan Province [Grant Number Hnky2018–50]; the Research Project of the Second Affiliated Hospital of Hainan Medical University [Grant Number 2019–10]; the Academic Innovation Project for Youth Talents of Hainan Association for Science and Technology [Grant Number QCXM201813].
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design: MD, BC.
Data acquisition, data analysis and interpretation: XW, CG, HY.
Drafting the article or critically revising it for important intellectual content: MD, BC.
Final approval of the version to be published: All authors.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal study in this research was approved by the Committee of Experimental Animals of the Second Affiliated Hospital of Hainan Medical University (Z2019010604N). All animal experiments were performed strictly in light of the guidelines of China Council on Animal Care and Use. All experiments with animals were performed in the Second Affiliated Hospital of Hainan Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, M., Chen, B., Wang, X. et al. Icariin enhance mild hypothermia-induced neuroprotection via inhibiting the activation of NF-κB in experimental ischemic stroke. Metab Brain Dis 36, 1779–1790 (2021). https://doi.org/10.1007/s11011-021-00731-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00731-6