Abstract
Pyruvate kinase (PK) catalyzes the last irreversible reaction of glycolysis pathway, generating pyruvate and ATP, from Phosphoenol Pyruvate (PEP) and ADP precursors. In mammals, four different tissue-specific isoforms (M1, M2, L and R) of PK exist, which are translated from two genes (PKL and PKR). PKM2 is the highly expressed isoform of PK in cancers, which regulates the aerobic glycolysis via reprogramming cancer cell’s metabolic pathways to provide an anabolic advantage to the tumor cells. In addition to the established role of PKM2 in aerobic glycolysis of multiple cancer types, various recent findings have highlighted the non-metabolic functions of PKM2 in brain tumor development. Nuclear PKM2 acts as a co-activator and directly regulates gene transcription. PKM2 dependent transactivation of various oncogenic genes is instrumental in the progression and aggressiveness of Glioblastoma Multiforme (GBM). Also, PKM2 acts as a protein kinase in histone modification which regulates gene expression and tumorigenesis. Ongoing research has explored novel regulatory mechanisms of PKM2 and its association in GBM progression. This review enlists and summarizes the metabolic and non-metabolic roles of PKM2 at the cellular level, and its regulatory function highlights the importance of the nuclear functions of PKM2 in GBM progression, and an emerging role of PKM2 as novel cancer therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyruvate kinase (PK) is the rate-limiting enzyme in the cellular glycolysis pathway which is involved in the transfer of phosphate group from the phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP) and generate pyruvate and adenosine triphosphate (ATP) (Altenberg and Greulich 2004; Majumder et al. 2004). PK is expressed in four different isoforms showing organ-specific distribution in the liver - PKL, red blood cells – PKR and muscle -PKM1 and PKM2 (Harada et al. 1978; Mazurek 2011; Israelsen and Heiden 2015; Chaneton and Gottlieb 2012). All these isoforms differ in their kinetic and regulatory mechanisms. Here in this review, we are focusing on PKM2 isoform of PK. PKM2 is encoded by PKM (15q23) gene, which undergoes alternative splicing to produce PKM1 and PKM2 isoforms (Noguchi et al. 1987; Chaneton and Gottlieb 2012). PKM2 is expressed in differentiated tissues such as adipose tissue, lung, retina, pancreatic islets, and proliferating cells (normal proliferating cells, embryonic cells, adult stem cells and especially tumor cells) whereas PKM1 is expressed in all kind of tissues in which the supply of a large amount of energy is rapidly needed like brain and muscles (Reinacher et al. 1979). In some recent studies, the role of PKM1 was also reported in the enhancement of tumor growth by boosting Warburg effect, anabolic metabolism, and favouring malignancy (Allen and Locasale 2018; Morita et al. 2018). During embryogenesis, the PKM2 isoenzyme is progressively replaced by the respective tissue-specific isoenzyme. Conversely, during the tumorigenesis, the tissue-specific isoenzymes of PK, i.e. PK-L in the liver or PKM1 in the brain disappear, and the PKM2 isoenzyme is expressed (Hacker et al. 1998; Reinacher and Eigenbrodt 1981; Steinberg et al. 1999; Yamada and Noguchi 1999). Among the PK isoforms PKM2 possess a stable, less active dimeric and more active tetrameric forms. PKM2 has both metabolic and non-metabolic functions that depend upon its different forms. The tetrameric form of PKM2 favors metabolic functions as a part of normal cellular physiology, and this form of PKM2 is the most dominated active form which acts as a gateway for the production of pyruvate, and which is channeled inside the mitochondria and is utilized in tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) pathways (Gruning et al. 2011; Hitosugi et al. 2009).
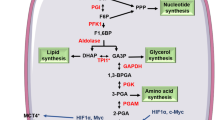
The dimeric form, which is the less active PKM2 form, favours the anabolic functions and supports the tumor formation in two ways. Firstly, the dimeric PKM2 is unable to catalyze the final step of glycolysis; thus, upstream intermediates start accumulating and as a result the glycolysis pathway is diverted to biosynthetic pathways. Thus, a large numbers of intermediate products, e.g. amino acids, nucleic acids and lipids are available for the proliferative cells (Hitosugi et al. 2009). The ratio of PKM2 dimer to tetramer state determines whether glucose metabolism in the cell is involved in the biosynthesis of nucleic acids, proteins, amino acids or form pyruvate, which eventually enter inside the mitochondria to participate in the energy metabolism (Wu and Le 2013). In the second way, dimeric PKM2 is translocated from the cytoplasm to the nucleus. In the nucleus, PKM2 (dimer) transcriptionally activates many genes which are involved in tumorigenesis (Gao et al. 2012; Wong et al. 2015). Awing to its role in activating genes involved in tumorigenesis, it is also expressed in various types of brain cancers, such as oligodendrogliomas, glioblastomas and ependymomas. Thus, the ratio of the active and less active form of PKM2 is the deciding factor for the glycolytic intermediates whether the cell will opt for glycolysis or pentose phosphate pathway [PPP] (Fig. 1) (Chaneton and Gottlieb 2012). Glioblastoma multiforme is the most common and aggressive type of primary brain tumor in adult and aged individuals. Approximately 82% of all cases of malignant gliomas are of GBM, which is characterized by extreme cell proliferation, angiogenesis and high resistance to apoptosis and chemoresistance (Omuro and DeAngelis 2013). All these characteristics of GBM are contributed by PKM2, and this review highlights the links between PKM2 and various kind of brain cancers, especially GBM.
PKM2 and its role in glycolysis: PKM2 in its tetrameric form involves in the last step of glycolysis and converts PEP into pyruvate. If PKM2 converts to a dimeric form, then its pyruvate kinase (PK) activity is lost and it will move in to the nucleus to activate oncogenes and all intermediates above the PEP, which results in accumulation of useful materials for the growth of cancerous cells
Structure of PKM2 and its mechanism of action
PKM2 is composed of 531 amino acids. A complete functional PKM2 protein consists of four monomeric subunits; each monomeric subunit further contains three domains, namely domain A, B and C. In between domain A and B, there is an active site where two substrates PEP and ADP bind along with K+ and Mg2+(Chaneton and Gottlieb 2012). PKM2 acts via transferring the phosphate group from PEP to ADP and forms pyruvate and ATP in a two-step mechanism. In the first step, the phosphate group is transferred from PEP to ADP and produce enolate and ATP. In the second step, the proton is added to enolate and form pyruvate (Seeholzer et al. 1991; Rose 1970; Kumar and Barth 2010). A-domain of PKM2 monomer holds Tyr 105, Lys 305 and Cys 358 whereas C-domain carries fructose 1,6-bisphosphate (FBP) binding site where FBP binds allosterically and regulate its transformation from monomer into a tetramer (Fig. 2). On the other hand, phosphorylation, acetylation and oxidation of Tyr 105, Lys 305 and Cys 358 respectively during the post-translational modification may stop the binding of FBP, resulting in keeping PKM2 in its dimeric form (Prakasam et al. 2018).
Domains of PKM2 and its post-translational modifications: Each PKM2 monomeric subunit contains three domains namely - domain A, B & C. In between the domain A & B, there is an active site present where the substrate PEP and ADP binding occurs along with the K+ and Mg2+. Domain - A of PKM2 monomer holds Tyr 105, Lys 305 and Cys 358 residues, whereas the C-terminal domain carries FBP binding site, where FBP binds allosterically and regulate its transformation from the monomeric state into the tetrameric state. On the other hand, PTMs such as phosphorylation, acetylation and oxidation of Tyr 105, Lys 305 and Cys 358 residues respectively regulate its FBP binding activity
Regulation of PKM2
Multiple factors are associated with the interconversion of different PKM2 forms. Metabolite from the glycolysis pathway, i.e. FBP, allosterically activates PKM2 (Anastasiou et al. 2011; Christofk et al. 2008b). FBP binds to the dimeric form of PKM2 and changes its conformation; thus, PKM2 dimer starts combining with other PKM2 dimer and form PKM2 tetramer (Fig. 3). Tetrameric PKM2 has a high affinity for PEP and favours pyruvate and ATP production compared to the dimeric form of PKM2 (Ashizawa et al. 1991; Christofk et al. 2008b). Fibroblast growth factor receptor type-1 favours the formation of the dimeric form of PKM2 by direct phosphorylation of PKM2 at its Tyr 105. This phosphorylation reduces the affinity of PKM2 dimer towards FBP; thus, PKM2 remains in a dimeric state, and thus its kinase activity is lost. The dimeric PKM2 enhances cancer cell proliferation via entering the nucleus to activate oncogenes or via diverting glycolytic intermediates to pentose phosphate pathway (PPP) which serves to provide amino acids and nucleotides for cancer cell proliferation (Gao et al. 2013; Hitosugi et al. 2009). P300 acetyltransferase is reported to cause acetylation of PKM2 at its Lys 433, which reduces the affinity of PKM2 toward FBP and inhibits the PKM2 tetramerization (Lv et al. 2013). Succinyl amino imidazole carboxyamide ribose-5-phosphate (SAICAR), an intermediate of purine biosynthesis activates kinase activity of PKM2. The binding of SAICAR and PKM2 may prove beneficial for the anabolic functions of the cell (Keller et al. 2012; Lunt et al. 2015). On the other hand, SAICAR binds to PKM2’s single nucleotide polymorphic (SNP) variant PKM2G415R and activates its kinase activity without forming a tetramer (Yan et al. 2016). This activated PKM2 dimer which is formed by SAICAR renders active role in cancer progression.
Intracellular levels of reactive oxygen species (ROS) causes the oxidation of PKM2 at Cys 358 which decreases the kinase activity of PKM2 via inhibiting the PKM2 tetramer formation; as a consequence, glycolysis halts at this step, where PKM2 works and all the accumulated intermediates of glycolysis are diverted towards anabolic processes for the growth and cellular division of cancer cell, but the exchange of Cys 358 with Ser may stop down the oxidation and impair the growth of cancer cells (Anastasiou et al. 2011). Another factor which regulates the PKM2 activity is O-GlcNAcylation. The O-GlcNAcylation of PKM2 on Thr 405 and Ser 406 may lead to the blockage of these two sites (Wang et al. 2017b). Consequently, the stability of PKM2 in the tetrameric state is reduced and PKM2 tetramer is dissembled to dimeric PKM2. This may reduce the aerobic glycolysis Warburg effect. The kinase activity of PKM2 is reduced, and its nuclear translocation facilitates cell proliferation (Wang et al. 2017a, b). PKM2 can be methylated by co-activator associated arginine methyltransferase (CARM1). In methylated form, PKM2 results in shifting the balance from oxidative phosphorylation to aerobic glycolysis. For OXPHOS reaction, it is essential to open the mitochondrial Ca2+ channel via the expression of inositol-1,4,5-triphosphate receptors (IP3Rs), but methylated PKM2 inactivates IP3Rs which reduces Ca2+ supply from the ER to the mitochondria and resulting in stoppage of OXPHOS. So, methylated PKM2 switches cellular metabolism towards aerobic glycolysis and enhances cancer proliferation (Liu et al. 2017). Nuclear translocation of PKM2 is regulated by the binding of Pin-1, which may cause the cis-trans isomerization of PKM2 and unmasks the nuclear localization signal (NLS) sequence. This unmasking results in NLS recognition via importin-α and translocates PKM2 to the nucleus (Lu and Hunter 2014). On the other hand, tetrameric PKM2 has masked NLS sequence, which is hidden. That’s why tetrameric PKM2 cannot cross the nuclear membrane (Presek et al. 1980). JNK1 (c-Jun N-terminal kinase-1), a pro-apoptotic kinase, causes the phosphorylation of PKM2 at Thr 365 position which boosts its kinase activity, but the activation of poly-ADP Ribose polymerase 14 (PARP-14), i.e. an anti-apoptotic protein inactivates JNK1, which subsequently leads to reduction in phosphorylation of PKM2 by JNK1. Consequently, PKM2᾿s kinase activity reduces, which is the prominent feature of Warburg effect and cancer cell survival because it upgrades aerobic glycolysis (Iansante et al. 2015; Barbarulo et al. 2013).
PKM2 role in metabolic reprogramming in Cancer cells
Aerobic glycolysis is the key pathway, which supplies energy to the cells. PK, which catalyzes the final step of glycolysis, has emerged as a potential regulator of this metabolic phenotype (Chaneton and Gottlieb 2012). Cancer cells rely on the Warburg effect of cellular metabolism. Cancer cells take up more glucose and produce more lactate and less ATP in the presence of oxygen, which we called aerobic glycolysis in contrast to the normal cell metabolism (Warburg 1956b). Cancer shows higher metabolic properties as compared to the normal cells, so, they need more energy supply and also need more biosynthetic intermediates. Both of these are supplied via the glycolytic pathway. PKM2, one of the isoforms of PK, is a key enzyme in the glycolytic pathway, which catalyzes the terminal step of glycolysis, whose active tetrameric form converts PEP into pyruvate. Inhibition of PKM2 leads to formation of glycolytic intermediates, which are utilized in enormous biosynthetic pathways (Mazurek 2011). Tumor cells require a huge supply of energy and biosynthetic intermediates for maintaining rapid cellular proliferation. Due to the high demand of nutrients in tumor cells, these cells’ metabolic activities are fundamentally different from those of normal differentiated cells (DeBerardinis et al. 2008a; Warburg 1956a, b) and require metabolic reprogramming to combat this metabolic flux. Metabolic activities in the tumor cells are oncogenic, which are further directed to support the anabolic growth of tumor cells (Ward and Thompson 2012). PKM2 possesses tyrosine kinase activity and modulates gene expression, contributing to tumorigenesis (Wong et al. 2013; Gao et al. 2012). Phosphotyrosine binding protein allosterically binds to the PKM2 and releases fructose 1–6-biphosphate. This may result in the inhibition of the metabolic function of PKM2 and divert its function towards the anabolic pathways, which are essential for the rapid growth in cancer cells (Christofk et al. 2008a). Thus for maintaining the high proliferation rate, PK activity is reduced through interaction with phosphotyrosine (p-Tyr) peptides, and PKM2 itself gets phosphorylated on Tyr 105 in response to various growth factors. These inhibitory events maintain the reduced glycolysis rate and divert the glycolytic intermediates toward the pentose phosphate pathways (Hitosugi et al. 2009). The shift of metabolic mechanism towards aerobic glycolysis is observed in various cancers, including highly aggressive glioblastoma multiforme (GBM), primary brain cancers (Marie and Shinjo 2011; Tennant et al. 2010).
Subcellular translocation and its role in Cancer cell survival
PKM2 is a multifunctional enzyme that translocates to noncanonical subcellular localizations such as nucleus and mitochondria in addition to its cytoplasmic localization (Amin et al. 2019). Under the oxidative stress condition, PKM2 translocates to the mitochondria. Mitochondrial PKM2 provides apoptosis escaping mechanism to the tumor cells via interacting and phosphorylating Bcl2, which is an anti-apoptotic factor. Mitochondrial PKM2 interacts with Bcl2 and phosphorylates it at the Thr 69, which inhibits the Cul3-based E3 ligase-mediated degradation of Bcl2. This makes glioblastoma resistant towards ROS mediated apoptosis. A chaperone protein, HSP90α1 plays a crucial role in this process, as its ATPase activity induces a conformational change in PKM2 and facilitates interaction between PKM2 and Bcl2 (Liang et al. 2016). Mitochondrial PKM2 is well established to be involved in the activation of ROS scavenging system. Increased ROS causes the oxidation of PKM2 at Cys 358, which is used to halt the glycolytic activity of PKM2. This inhibition pushes the glucose flux into PPP and indulges in the generation of an enormous amount of reducing potential (NADPH and H+), which causes the detoxification of ROS. This indicates the localization of PKM2 inside the mitochondria thus have a beneficial impact on the progression of cancer growth and ROS adaptation of cancer cells (Anastasiou et al. 2011).
In response to extracellular signals, PKM2 translocates to the nucleus where it functions as a transcriptional co-activator. Hypoxia and EGFR can induce the translocalization of PKM2 from the cytosol to the nucleus, where it regulates the gene expression of cyclin D, c-Myc and HIF1α (Luo et al. 2011; Yang et al. 2011). Metabolic stress is the key factor responsible for PKM2’s nuclear translocalization as a beneficial mechanism for cancer cell survival. In response to the metabolic stress, nuclear PKM2 interacts with octamer-binding transcription factor 4 (Oct4) and promotes the cancer stem cell population and metastasis (Yang et al. 2018). Nuclear PKM2’s role is well established in regulating the Warburg effect in the tumor cells. Monomeric PKM2 translocates to the nucleus via EGFR activation, where it functions as a histone kinase and upregulates the expression of c-Myc and cyclin D1. ERK1/2 phosphorylates PKM2, leading to PIN-dependent cis-trans isomerization and converting PKM2 tetramer to a monomer (Yang and Lu 2013). EGFR-activated ERK2 binds directly to the PKM2’s Ile 429/Leu 431 through the ERK2 docking groove and phosphorylates the PKM2 at Ser37. The resultant pPKM2 (Ser 37) recruits PIN1 for cis-trans isomerization of PKM2, promoting PKM2 binding to importin α5 and resulting in translocation to the nucleus (Yang et al. 2012c). EGFR activated PKM2 binds to the beta-catenin to regulate gene expression after being translocated into the nucleus (Yang et al. 2011). In the nucleus Lys 433 of PKM2 binds to c-Src-phosphorylated Tyr 333 of beta-catenin leading to the histone H3 acetylation and cyclin D1 expression (Yang et al. 2011). EGFR activation results in c-Src-mediated phosphorylation of Cdc25A, which dephosphorylates PKM2 at Ser 37, and promotes PKM2-dependent β-catenin transactivation and c-Myc-upregulated expression of the glycolytic genes viz. GLUT1, PKM2, LDHA, and Cdc25A. Cdc25A-mediated PKM2 dephosphorylation promotes the Warburg effect, cell proliferation, and brain tumorigenesis (Liang et al. 2016). In glioblastoma, EGF-induced expression of cyclin D and c-Myc via PKM2’s non-metabolic function via histone modification is instrumental in brain tumorigenesis (Venneti and Thompson 2013; Yang et al. 2012b). Replacement of wild-type PKM2 with a nuclear translocation-deficient mutant (Ser 37A1a) blocks the EGFR-promoted Warburg effect and brain tumor development in the mice. The levels of PKM2 Ser 37 phosphorylation has been correlated with the EGFR and ERK1/2 activity in human glioblastoma specimens (Yang et al. 2012c). These findings indicate that the role of nuclear functions of PKM2 in the Warburg effect and tumorigenesis. Estrogen increases the phosphorylation of PKM2 at Ser37; phosphorylated PKM2 is then translocated to the cytoplasm, regulating the expression of glycolytic genes (Lu et al. 2020). Jumonji C domain-containing protein 5 (JMJD5) and hypoxia positively regulates the nuclear translocation of PKM2. JMJD5 is upregulated by hypoxia and interacts directly with PKM2 to modulate metabolic flux and induces HIF1α mediated transactivation (Wang et al. 2014). Sirt6, sirtuin family member deacetylases PKM2 at the Lys 433 and results in PKM2 nuclear export via specific protein exportin 4. As a result of its nuclear export, PKM2 nuclear functions are abolished such as protein kinase and transactivation function (Bhardwaj and Das 2016). Beta-elemene (β-elemene), an approved drug for the complementary cancer therapy showed anti-metastatic activity in breast cancer via inhibiting the aerobic glycolysis as a result of blocked PKM2’s nuclear translocalization and suppression of PKM2’s kinase activity via transforming the dimeric form to tetrameric PKM2 (Pan et al. 2019). PKM2 dimer when enters inside the nucleus; it transfers the phosphate group from PEP to signal transducer and activator of transcription 3 (STAT3) at Tyr 705. This phosphorylation of STAT3 is involved in the formation of STAT3 dimer, which further activates the transcription of mitogen-activated protein kinase 5. It is important for tumor progression as well as for the malignancy. Excessive expression of PKM2 dimer causes prolonged activation of STAT3, which increase the survival rate as observed in the case of colorectal cancer (Yang et al. 2014). PKM2 may cause the stabilization of HIF-1α via enhancing occupancy of HRE by HIF-1α and P300. PKM2 may also transactivate HIF-1α for further activation of Glucose Transporter 1 (GLUT1) for glucose transportation inside the cell (Yang et al. 2012c), lactate dehydrogenase (LDHA) for the conversion of pyruvate to lactate (Yang et al. 2012c) and pyruvate dehydrogenase kinase1 (PDK1) (Huang et al. 2014) which halts the pyruvate dehydrogenase enzyme for stopping the movement of pyruvate inside the mitochondria for OXPHOS and TCA cycle (Fig. 4) (Luo et al. 2011). Subcellular translocalization of PKM2 not only protect the cancer cells against oxidative stress, but also regulates gene expression to promote rapid cell proliferation in cancer viz. GBM cells.
Nuclear role of PKM2: The dimeric PKM2 inside the nucleus causes dimerization of the transcription factor STAT3 by its phosphorylation, which further activates oncogenes. Activation of EGFR results in phoshorylation of PKM2 at the aa Lys433 which intern recruits the PKM2 in to the nucleus. The resultant activated EGFR further activates C-src and further phosphorylates the β-catenin at the aa Tyr 333 position. These two phosphorylations thus formed at the aa Lys 433 of PKM2 and Tyr 333 of β-catenin results in binding of PKM2 with the β-catenin, and triggering in nuclear accumulation and activation of tumor promoting genes
PKM2 association with Glioblastoma
In addition to the central regulator of cancer cell metabolism, various research findings established the additional function of PKM2 as protein kinase for gene transcription that appears to be instrumental in tumorigenesis. The PKM2 isoform of PK is preferentially upregulated in various cancer cells and allows cancer cells to adapt their metabolic needs as per the cellular microenvironment (Dombrauckas et al. 2005; Liu and Vander Heiden 2015). Glioma is the most common type of brain cancer. WHO has categorized the glioma by histopathological and clinical criteria as grade I to grade IV. The common subtypes of glioma are astrocytomas, oligodendrogliomas, and ependymomas (Gladson et al. 2010; Yan et al. 2009). The most common and biologically aggressive malignant glioma is glioblastoma multiforme (GBM), which is categorized by the WHO as grade IV glioma with hallmark features of uncontrolled cellular proliferation, diffused infiltration, propensity for necrosis, robust angiogenesis, intense resistance to apoptosis, and rampant genomic instability (Furnari et al. 2007). Glycolytic PKM2 is overexpressed in GBM, and also the isoform switching between the metabolic enzyme PKM1 and PKM2 observed in GBM. This phenomenon occurs only in the case of GBM and not in other cancers. It has been observed that there is an up-regulation of PKM2 expression in human glioma in a grade-specific manner, but this does not correlate with PK activity (Mukherjee et al. 2013). The metabolic reprogramming of PKM2 is essential for the glial cancer cells to utilize TCA cycle intermediates and OXPHOS in a different capacity for lipids, nucleotide biosynthesis towards cellular proliferation and progression (Vander et al. 2009; Agnihotri and Zadeh 2016; Ward and Thompson 2012; Deberardinis et al. 2008b). In addition to its metabolic function, PKM2 directly regulates the gene transcription. PKM2 also functions as a protein kinase, phosphorylates histone H3 and promote tumorigenesis. PKM2 directly bind to histone H3 and phosphorylates it at Thr 11 upon EGFR activation, which leads to dissociation of HDAC from CCND1 and c-Myc promoter region (Yang et al. 2012a, c).
Histone modification is required for subsequent gene transcription regulation through cyclin D1 and c-Myc (Yang et al. 2012b). PKM2 is also involved in the glioma differentiation via interaction with octamer-binding transcription factor 4(Oct4), which regulates the cell pluripotency and cell death (Morfouace et al. 2014). PKM2’s dual kinase activity has been attributed to its regulatory role in tumor growth (Mukherjee et al. 2016). It has the potential to regulate tumor growth through its binding with PTyr containing peptides. In glioblastoma cells, PKM2 interacts in the nucleus with the RNA-binding protein HuR to regulate HuR subcellular localization, p27 levels and cell cycle progression, thus resulting in glioma growth (Mukherjee et al. 2016). PKM2 regulates G1-S mediated cell cycle progression by controlling cyclin D1 expression, chromosomal segregation, and mitosis progression of tumor cells by binding to spindle checkpoint protein Bub3 during the mitosis (Jiang et al. 2014b).
P53 is a tumor suppressor, which regulates cell cycle progression and is involved in the apoptosis process. In a healthy cell, P53 level is low because it is continuously ubiquitinated and degraded by E3 ubiquitin-protein ligase MDM2 (Haupt et al. 1997; Ozaki and Nakagawara 2011). Upon DNA damage, ATM gets activated, which phosphorylates P53 at Ser 15; as a result, P53 rescue itself from MDM2 and cause cell cycle arrest via activating P21. In cancer or tumor condition, PKM2 inhibits the transactivation of P21 gene (i.e. tumor suppressor) by inhibiting the binding of P53 to the P21᾿s promoter. So, PKM2 is proved to be beneficial for the cell cycle progression and leads to non-stop G1 phase (Xia et al. 2016). Interaction of myosin2 and actin is responsible for the cytokinesis. Myosin2 is actually made up of heavy chain, light chain and regulatory light chain (RMLC). MLC2 is the isoform of RMLC. MLC2᾿s phosphorylation at Ser 15 results in binding of myosin2 with actin and form actomyosin complex, which causes cytokinesis. Aurora B phosphorylates PKM2 at Thr 45, which enhances its binding capacity with MLC2. Then, PKM2 phosphorylates MLC2 at Tyr 118. Now phosphorylated MLC2 is further attracted towards ROCK, which in turn mediates Ser 15 phosphorylation of MLC2. This phosphorylated MLC2 thus enhances the binding of actin and myosin, as mentioned above. In short, PKM2 is involved in this process of increased cell division (Jiang et al. 2014c); therefore, PKM2-dependent phosphorylation plays a key role in cancer cell proliferation.
Decreased PKM2 activity leads to building up of PEP. Then, the phosphate group from the PEP gets transferred to His 11 of phosphoglycerate mutase 1 (PGAM1); as a result of this, PGAM1 gets activated which further enhances glucose uptake inside the cancer cell and also increases glycolysis. The transfer of phosphate at His 11 takes place in a non-enzymatic process (Wiese and Hitosug 2018; Hitosugi et al. 2013). This activated PGAM1 catalyzes the conversion of 3-phosphogycerate (3PG) into 2-phosphoglycerate (2PG), and this 2PG is reported to be involved in the activation of 3-phosphoglycerate dehydrogenase (3-PHGDH) which further increases the production of Ser and other amino acids from 3-PG. So, the stoppage of glycolysis at PEP via PKM2 inactivation causes PPP activation and serine production pathway. Both of these pathways may amplify the supply of nucleotides and amino acids, respectively, which intensify the growth of GBM (Jiang et al. 2014a).
The interaction of JMJD5 with PKM2 results in blockage of its tetramerization; as a result, the dimeric form of PKM2 enters inside the nucleus and activates HIF-1α. This HIF-1α further activates VEGF, which may involve in the process of angiogenesis (Wang et al. 2014). PKM2 also plays an important role in mitosis and chromosome segregation because PKM2 binds with checkpoint protein Bub3. After its binding, PKM2 phosphorylates Bub3 at its Tyr 207 residue. This phosphorylation helps in the formation of Bub3-Bub1 complex, which may further recruit towards the kinetochore, where it interacts with Blinkin. This interaction is essential for kinetochore-microtubule attachment and chromosome segregation which may further help in tumor multiplication (Jiang et al. 2014b). In addition, the activated EGFR plays a key role in the nuclear translocation of PKM2 as studied in various cancer cell lines, including glioblastoma. EGFRvIII is the mutant version of EGFR. It is noted that EGFRvIII translocates and accumulates inside the nucleus, which helps in the formation of the tumor. EGFRvIII enters inside the Golgi body with the help of membrane protein syntaxin6. From Golgi body, it may undergo retrogressive movement into ER with the involvement of COP1; from ER, it may push inside the nucleus where it starts accumulating; and this EGFRvIII results in the nuclear translocation of PKM2 and phosphorylation of STAT3, which eventually results in cancer progression as described earlier (Zhang et al. 2019).
Long intergenic non-coding RNA-RoR (LIncRNA-RoR) has been reported to act as an inhibitor of glioblastoma, as it suppresses the expression of RICTOR which is an important element of mammalian target of rapamycin complex 2 (mTORC2). Consequently, it is proposed to be useful for the stoppage of mTORC2-Akt pathway, which may further reduce the expression of GLUT1, PKM2, hexokinase2 and LDHA. Collectively, LIncRNA-RoR may act as a road blocker for the path of aerobic glycolysis and glioblastoma progression (Li et al. 2018). Activation of EGFR results in c-src activation, which is further attributed for the phosphorylation of cdc25A at its Tyr 59 position and its activation. This activated cdc25A further dephosphorylates PKM2; resulting in enhanced binding of PKM2 and β-catenin. This dimer, in turn, activates c-Myc expression and consequently activation of GLUT1, LDHA and cdc25A. All these factors are known to promote the Warburg effect and tumorigenesis (Liang et al. 2016).
Fructose-1,6-bisphosphate (FBP) acts as a PKM2 modulator, as FBP binds to the allosteric site of PKM2 dimer and results in its tetramerization, but in the absence of FBP, PKM2 dimer found to be translocated to the nucleus, which may be beneficial for the proliferation of the cancer cells as well as for their metastasis. It is observed that PKM2 has phosphorylated Tyr binding capability through which it reduces the level of HuR in the cytoplasm, which regulates the translation of tumor suppressor protein p27. Thus, PKM2 is also indirectly involved in the downregulation of tumor suppressor protein p27, which subsequently lead to aggressiveness in GBM (Mukherjee et al. 2016).
PKM2 and brain cancers
Expression of PKM2 is not only observed in GBM, but also seen in many other types of brain cancers like meningiomas, oligodendrogliomas and ependymomas. This statement is justified by the detection of a large quantity of PKM2 in meningiomas (a tumor that arises from the meninges) but not in normal brain tissue (Veelen et al. 1977). It is also observed that meningiomas are associated with a high level of phosphatidylserine (PS) which is important for the kinase activity of PKM2. So, by reducing PS, the assets required to enable a cancerous phenotype are exhausted (Hatchell 2017). In oligodendrogliomas, a mutation in the chromosomal arm of PKM2 gene was observed which may prove to be beneficial for the growth of brain cancer cells by providing cellular energy metabolism or by enhancing cell migration (metastasis) and angiogenesis (Gladitz et al. 2018). The third type of brain cancer, that is ependymoma, shows incurable high malignancy rate in 45% of patients. It is characterized by Warburg phenotype, which correlates with an elevated expression of PKM2, HK2, PDK along with the high amount of lactate production (Mack et al. 2015). Medulloblastoma (MB), the most frequent brain malignancy of childhood, showed aberrant activation of the SHH pathway. Hedgehog activation induces transcription of PKM2. pharmacological targeting with the pyruvate kinase inhibitor dichloroacetate (DCA) efficiently represses MB growth in vitro and in vivo. (Magno et al. 2014).
PKM2 in cell proliferation and inhibition of apoptosis
In many human cancers, PKM2 expression is correlated with cell proliferation via metabolic reprogramming, including GBM. Cellular oxidative stress induces the expression of PKM2 studied in glioblastoma cells U87 MG and C6. PKM2 shows protective survival mechanism against the intracellular oxidative stress in glioblastoma cells and induces GBM cell proliferation (Cholia et al. 2018). Activation of epidermal growth factor receptor (EGFR) causes the phosphorylation of PKM2 at Lys 433, which recruits PKM2 inside the nucleus. Activated EGFR may also activate C-src which further phosphorylates β-catenin at Tyr 333. These two phosphorylations at Lys 433 of PKM2 and Tyr 333 of β-catenin causing binding of PKM2 with the β-catenin. Because of this, PKM2 and β-catenin accumulate inside the nucleus, which is important for different grades of glioma malignancy. EGFR-PKM2- β-catenin is an important axis for cell proliferation and tumorigenesis (Yang et al. 2011). Both of these interactions can also prove to be useful for cyclin D1 (CCND1) transcriptional activation (Lu 2012; Yang et al. 2011). Phosphorylated PKM2 binds to histone H3 and phosphorylates it at Thr 11 upon EGFR activation. This phosphorylation removes HDAC3 from CCND1 and c-Myc promoter region. Subsequently, CCND1 and c-Myc gets activated that is beneficial for the survival of cancer cell, increases its proliferation and enhances tumorigenesis, resulting in an increase in the glioma malignancy grades (Yang et al. 2012b; Jiang et al. 2019; Lu 2012; Yang et al. 2011). It is also reported that the vascular endothelial growth factor-A (VEGF-A) increases the blood supply to the developing tumor tissue by forming new blood vessels, i.e. angiogenesis. This VEGF-A can be activated by HIF-1α, further, this HIF-1α is activated by PKM2 as mentioned earlier. In short, it is this PKM2 which starts up the activation of HIF-1α that further activates VEGF-A. So, it can be said that PKM2 is involved in the tumor angiogenesis (Azoitei et al. 2016). For the survival and proliferation of cancer cell, angiogenesis is considered as an important factor. As PKM2 involve in the process of angiogenesis, it indirectly contributes to the growth and multiplication of cancer cells.
PKM2 promotes the growth of cancer cells by regulating apoptosis and cell migration. This has been observed by silencing PKM2 gene expression with ShRNA, which downregulates the PKM2-mRNA and ultimately reduces PKM2 activity. This silencing of PKM2 gene expression is associated with an increase in the number of apoptotic bodies in tumor cell population and induce caspases 3/7 mediated cell apoptosis. This may clearly indicate that PKM2 does not allow caspases 3/7 to activate and perform apoptosis (Suzuki et al. 2019). Similarly, another experiment performed with small interfering (si) RNA targeting the PKM2 gene, resulting in declined tumor cell survival and increased apoptosis in multiple cancer cell lines (Goldberg and Sharp 2012). Another way, which points out the role of PKM2 as anti-apoptotic factor is the interaction of PKM2 with Bcl-2. PKM2 translocates inside the mitochondria and phosphorylates Bcl-2 which protect Bcl-2 from Cul3-RBX1 ligase-mediated degradation and make glioma cells resistant toward apoptosis and Bcl-2 may directly involve in the inactivation or inhibition of various apoptotic proteins like Bax, cytochrome-C, caspases 9/3 etc. (Liu et al. 2020; Liang et al. 2017). Additionally, phosphorylation of PKM2 at Thr 328A resulting in increased glucose consumption and lactate production that is useful for the proliferation of cancer cells whereas Thr 328A mutant PKM2 significantly increased the caspase-3 activity and rate of apoptosis. As glucose consumption, lactate production, the activity of caspase-3 and apoptosis get affected in Thr 328A mutant; similarly, these processes mentioned above gets altered with PKM2 knockdown. Based on these evidences, we can say that PKM2 plays a powerful role in anti-apoptosis (Xu et al. 2017).
Under an oxidative stress response, PKM2 is translocated to the mitochondria and halts the apoptosis via interacting with Bcl2 and phosphorylates Bcl2, showing the essential role of PKM2 in ROS adaptation in glioma cells. An orthotopic xenograft model with phosphorylation deficient Bcl2 T69A mutation sensitizes glioma cells to oxidative stress-induced apoptosis and impairs brain cancer (Liang et al. 2017). These studies show the key role of PKM2 in cancer cell survival via regulating apoptosis and cancer cell growth.
PKM2 and Chemoresistance
Chemoresistance is the principal cause of treatment failure in all types of cancers, including brain cancer. Cancer cells develop preventive strategies to escape from advanced treatment regimes, and this adaptive mechanism(s) of cancer cells remain elusive. The key factors strongly linked to drug resistance in GBM are the glioblastoma tumor microenvironment and blood-brain barrier [BBB] (Haumann et al. 2020). Based on the previous research findings, PKM2 overexpression is the key factor playing the mechanistic role of chemoresistance to treatment regimes in various cancer, however, limited research findings are available in the brain cancer research studies related to PKM2 role in chemoresistance of GBM.
PKM2 is overexpressed in various tumors, and the inhibition of PKM2 helps to overcome chemoresistance in cancer cells. U87-MG glioblastoma cells express a higher expression of G protein-coupled receptor 55 (GPR55), and its function can be competitively inhibited with (R,R’)-4′-methoxy-1-naphthylfenoterol (MNF) by poorly defined signalling pathways. MNF significantly decreased the phospho-active forms of PKM2 and β-catenin. Inhibition of GPR55 activity produces antitumor effects via attenuation of the MEK/ERK and PI3K-AKT pathways leading to a reduction in the expression and function of MDR proteins (Singh et al. 2016). A recent report uncovers the role of PKM2 to promote double-strand break (DSB) repair in cancer cells and PKM2 role towards the resistance of cancer cells to DNA damaging treatment in vitro and in vivo. PKM2 knockdown resulted in an increase in the sensitivity against Ionization radiation as studied in U87, T98G and U251 GBM cells. Under oxidative stress conditions induced by radiation, ATM phosphorylates PKM2 at T328 and induces its nuclear accumulation which further assisted the homologous recombination (HR) mediated DNA DSB repair through phosphorylation of CtBP interacting protein (CtIP) on T126 to increase CtIP’s recruitment at DSBs and resection of DNA ends. Disrupting this interaction sensitizes cancer cells against various DNA damaging agents (Sizemore et al. 2018).
Chemoresistance role of PKM2 being highlighted during the radiotherapy in GBM. Upon radiotherapy, glioblastoma cells reprogram their glucose metabolism via increasing their glucose uptake and runs through hexose monophosphate shunt (HMS) pathway, which produces many anti-oxidants as byproducts, which keeps the cancer cells safe against the oxidative DNA damaged cell death. Cells usually reprogram glucose metabolism through Nrf2-PKM2 dependent manner (Bailleul et al. 2019). The role of PKM2 in chemoresistance is also justified by an experiment performed by Pan et al. in Pan et al. 2016. They analyzed the PKM2-mediated doxorubicin (DOX) resistance in cancer cells by examining the expression of miR-122, because its expression involves in the silencing mechanism of PKM2 mRNA. In DOX-resistant cells, they found the least expression of miR-122, whereas upregulation of miR-122 reversed the DOX resistance via the inhibition of PKM2 (Pan et al. 2016). Similarly, the cisplatin resistance is also associated with the over-expression of PKM2 (Wang et al. 2017a).
PKM2 as a novel cancer therapeutic target
Despite the major improvement in our current knowledge towards the molecular regulatory signalling pathways being implicated in the tumorigenesis, still, the therapeutic progress and median survival for the GBM patients remain poor. In the recent years, PKM2’s role in the tumor formation has been elucidated and indicates targeting the cancer cell metabolic pathways as an alternative approach along with current conventional cancer therapeutics may combat the tumor progression in GBM. PKM2 is a crucial regulator of cancer cell metabolism and is involved in the non-metabolic functions too. In dimeric form, PKM2 translocates to the nucleus and transactivates tumorigenesis related genes. Recently a lipophilic molecule [18F] DASA-23 has been reported as an activator of PKM2. It binds to the dimeric pocket of PKM2 and assist in tetramerization of PKM2. Tetrameric PKM2 is metabolically active form participating in the tumor growth via metabolic regulation instead of cell proliferation. [18F] DASA-23 is lipophilic in nature and can easily cross the BBB. Additionally, this molecule has longer half-life as compared to the previously reported molecule [11C] DASA-23. An additional advantage of [18F] DASA-23 attributed to its selective uptake by the cancer cells compared to very low uptake in the healthy brain tissue. Given minimal levels of PKM2 in the healthy brain, it is proposed that resulting in high levels of [18F] DASA-23 radiotracer uptake within the GBM tumor with low uptake in the healthy brain tissue may lead to protective effects (Beinat et al. 2019).
PKM2 also plays an important role in chemoresistance. Metabolically less active dimeric PKM2 causes accumulation of intermediates which are in turn further channelled into the (HMP pathway, which results in NADPH production. Further NADPH converts inactivated glutathione (GSH) into activated glutathione, which further converts ROS into neutral species. This dimeric PKM2’s indirect ROS neutralizing activity reduces the sensitivity of therapeutic drug, i.e. cisplatin against cancer treatment which highlighted the role of PKM2 therapeutics in cancer treatment management (Tamada et al. 2012).
Cancer cell’s hypoxic microenvironment increases ROS production, which further activates ataxia telengiectasia mutated (ATM). ATM is not only activated in response to the DNA DSBs, but it can also be activated via ROS. This ATM is called oxidative ATM or DNA damage independent ATM. Oxidative ATM causes activation of GLUT1 and PKM2 in cancer-associated fibroblast (CAF) present in the tumor microenvironment. As a result, glucose uptake and glycolysis are enhanced in CAFs, therefore resulting in more lactate formation. Further, lactate is expelled out by the CAF in the extracellular environment where it is redirected to the nearby cancer cells and it is converted into glucose, which is further rechanneled in glycolysis for the production of energy. Along with this recycling, this extracellular lactate decreases pH of the extracellular environment, which further enhances metastasis. So, oxidative ATM alternatively activates PKM2’s functions in cancer cell metabolism (Sun et al. 2019). During the oxidative stress condition, ATM phosphorylates PKM2 at its Thr 328; as a result, PKM2 starts accumulating inside the nucleus. This phosphorylated PKM2 further phosphorylates CtBP-interacting protein (CtIP) at its Thr 126 for repairing the DSBs. At the pThr 126-CtIP facilitates recruitment of CtIP at the DSBs, which may be beneficial for the growth of tumor tissue. If we block the ATM-CtIP-PKM2 axis as a therapeutic intervention, resulting in cancer cells being sensitive towards the DNA damage agents and poly ADP ribose polymerase (PARP) inhibition. So, it is well evident that PKM2 is crucial for brain tumor survival. Additionally, these facts support the utilization of PKM2-targeting strategies as a way to not only disrupt the cancer cell metabolism but also hinder the resistance to the genotoxic therapies (Li et al. 2016). Binding of PKM2 with Poly-ADP-ribose (PAR) is important for the localization of PKM2 inside the nucleus, but PARP inhibition prevents the localization of PKM2 inside the nucleus; as a result, PKM2 is unable to promote the expression of oncogenes. This may lead to halting the proliferation and brain tumor growth (Li et al. 2016). Parkin functions as a tumor suppressor. Its gene is located at a 6q25-q26 position on the chromosome, but this region is deleted in cancers such as glioblastoma, lung and ovarian. A glioblastoma study on mice reveals that parkin is a ubiquitin E3 ligase which binds to PKM2 and causes its ubiquitylation degradation; as a result, the glycolytic pathway is reduced which may further reduce the proliferation rate in glioblastoma patient. This ubiquitylation degradation of PKM2 by parkin may be utilize as a therapeutic target too (Liu et al. 2016). PKM2 is highly overexpressed in case of GBM and is involved in providing resistance from the apoptosis through maintaining the cancer stem cells. However, the interaction of PKM2 with Oct4 and dichloroacetate (DCA) reported increasing the sensitivity of glioma cells toward cell death and decrease in the effect of tumorigenicity. PKM2-Oct4 complex formation transfers high phosphorylated PKM2, i.e. dimeric form into a low phosphorylated PKM2, i.e. tetrameric form. On the other hand, DCA causes tetramer formation and enhances the Oct4 binding. So, the interaction of both DCA and Oct4 with the PKM2 can be taken under consideration as a therapeutic target, because these interactions may produce useful changes as mentioned above which may reduce the risk of glioma progression (Morfouace et al. 2014).
Additionally, a small molecular compound namely Dimethylaminomicheliolide (DMAMCL) binds to the monomeric form of PKM2 and converts it into the tetrameric form, which results into decrease in the lactate production, G-6-P and other intermediates of glycolysis. As a result, the substrates needed for the proliferation of cancer cells like lactate and the glycolytic intermediates are reduced with the stoppage of glycolysis. So, DMAMCL has been used in the clinical trials for recurrent GBM in Australia (Guo et al. 2019). Acetaldehyde dehydrogenase1 A3 (ALDH1A3) is reported to involve the activation of PKM2 and Hexokinase 2 (HK2), which stimulates glioblastoma progression, glucose consumption, lactate production, and halts apoptosis. On the suppression of ALDH1A3, the expression of both PKM2 and HK2 are ultimately diminished, which may attenuate the growth of GBM (Panasyuk et al. 2012; Nie et al. 2020; 2019). miRNA-338 binds to the untranslated region of PKM2 and stops its binding to the β-catenin thus act as glioma suppressor. In other words, miRNA stops down the expression of PKM2, which further reduces the chance of growth of glioblastoma. miRNA-338 is reported to be downregulated in the glioma patients (Han et al. 2017). Taken together, the multifaceted role of PKM2, directly or indirectly, in regulation/progression of GBM ascertains PKM2 to be a major therapeutic target against GBM.
Summary and conclusions
PKM2 is essential for the aerobic glycolysis in tumor cells. Over the past few years, the multifaceted role of PKM2 in tumorigenesis is elucidated, which includes cell cycle progression via trancriptionally activating several genes involved in cell cycle progression (nuclear function); cell death regulation via interacting with anti-apoptosis proteins (mitochondrial function) and regulating energetics to supply substrates for cancer cell survival. Besides the key role as a central regulator of GBM cell glycolysis, PKM2 processes key tumorigenesis functions as a protein kinase in the nucleus and is also involved in gene transcription and as a transcriptional co-activator of oncogenic signalling. Higher expression of PKM2 in GBM and regulation of oncogenic signalling thus provide the molecular evidence to advocates further PKM2 as a potential tumor therapeutic target to manage the aggressiveness of GBM. Knockdown of PKM2 results in substantial inhibition of glucose intake, accumulation of glycolytic intermediates, production of lactate, and downregulation in oncogenes’ activation in glioblastoma cells. Various research findings documented the key role of PKM2 in GBM thus suggesting it as a potential metabolic therapeutic target for the development of a new regime to manage the GBM.
References
Agnihotri S, Zadeh G (2016) Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncology 18:160–172. https://doi.org/10.1093/neuonc/nov125
Allen AE, Locasale JW (2018) Glucose metabolism in Cancer: the Saga of pyruvate kinase continues. Cancer Cell 33:337–339. https://doi.org/10.1016/j.ccell.2018.02.008
Altenberg B, Greulich KO (2004) Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 84:1014–1020. https://doi.org/10.1016/j.ygeno.2004.08.010
Amin S, Yang P, Li Z (2019) Pyruvate kinase M2: a multifarious enzyme in non-canonical localization to promote cancer progression. BBA - Rev Cancer 1871:331–341. https://doi.org/10.1016/j.bbcan.2019.02.003
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334:1278–1283. https://doi.org/10.1126/science.1211485
Ashizawa K, Willingham MC, Liang CM, Cheng SY (1991) In vivo regulation of monomer-tetramer conversion of pyruvate kinase subtype M2 by glucose is mediated via fructose 1,6-bisphosphate. J Biol Chem 266:16842–16846
Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T (2016) PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer 15:3. https://doi.org/10.1186/s12943-015-0490-2
Bailleul J, Yazal T, Sung D, Dao A, Palomera D, Sehgal A, Vlashi E (2019) Irradiation reprograms GBM metabolism towards an antioxidant profile that drives radiation resistance. Int J Radiat Oncol 105:165–166. https://doi.org/10.1016/j.ijrobp.2019.06.189
Barbarulo A, Iansante V, Chaidos A, Naresh K, Rahemtulla A, Franzoso G, Karadimitris A, Haskard DO, Papa S, Bubici C (2013) Poly(ADP-ribose) polymerase family member 14 (PARP14) is a novel effector of the JNK2-dependent pro-survival signal in multiple myeloma. Oncogene. 32:4231–4242. https://doi.org/10.1038/onc.2012.448
Beinat C, Patel CB, Xie Y, Gambhir SS (2019) Evaluation of glycolytic response to multiple classes of anti-glioblastoma drugs by noninvasive measurement of pyruvate kinase M2 using [18F]DASA-23. Mol Imaging Biol 22:124–133. https://doi.org/10.1007/s11307-019-01353-2
Bhardwaj A, Das S (2016) Sirt6, sirtuin family member deacetylase PKM2 at the lysine 433 residue and results in PKM2 nuclear export via specific exportin 4. As a result of this PKM2 nuclear export, PKM2 nuclear functions are abolished such as protein kinase and transactivation function. Proc Natl Acad Sci U S A 113:E538–E547. https://doi.org/10.1073/pnas.1520045113
Chaneton B, Gottlieb E (2012) Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci 37:309–316. https://doi.org/10.1016/j.tibs.2012.04.003
Cholia RP, Dhiman M, Kumar R, Mantha AK (2018) Oxidative stress stimulates invasive potential in rat C6 and human U-87 MG glioblastoma cells via activation and cross-talk between PKM2, ENPP2 and APE1 enzymes. Metab Brain Dis 33:1307–1326. https://doi.org/10.1007/s11011-018-0233-3
Christofk HR, Vander Heiden MG, Wu N, Asara JM, CantleyLC (2008a) Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452:181–186. https://doi.org/10.1038/nature06667
Christofk HR, Heiden MGV, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008b) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 452:230–233. https://doi.org/10.1038/nature06734
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008a) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7:11–20. https://doi.org/10.1016/j.cmet.2007.10.002
Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB (2008b) Brick by brick: metabolism and tumor cell growth. Curr Opin genet Dev 18:54–61. https://doi.org/10.1016/j.gde.2008.02.003
Dombrauckas JD, Santarsiero BD, Mesecar AD (2005) Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44:9417–9429. https://doi.org/10.1021/bi0474923
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710. https://doi.org/10.1101/gad.1596707
Gao X, Wang H, Jenny JY, Liu X, Liu XR (2012) Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45:598–609. https://doi.org/10.1016/j.molcel.2012.01.001
Gao X, Wang H, Yang JJ, Chen J, Jie J, Li L, Zhang Y, Liu ZR (2013) Reciprocal regulation of protein kinase and pyruvate kinase activities of pyruvate kinase M2 by growth signals. J Biol Chem 288:15971–15979. https://doi.org/10.1074/jbc.M112.448753
Gladitz J, Klink B, Seifert M (2018) Network-based analysis of oligodendrogliomas predicts novel cancer gene candidates within the region of the 1p/19q co-deletion. Acta Neuropathol Commun 6:49. https://doi.org/10.1186/s40478-018-0544-y
Gladson CL, Prayson RA, Liu W (2010) The pathobiology of Glioma tumors. Annu Rev Pathol 5:33–50 5:33–50. https://doi.org/10.1146/annurev-pathol-121808-102109
Goldberg MS, Sharp PA (2012) Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med 209:217–224. https://doi.org/10.1084/jem.20111487
Gruning NM, Rinnerthaler M, Bluemlein K, Mulleder M, Wamelink MMC, Lehrach H, Jakobs C, Breitenbach M, Ralser M (2011) Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab 14:415–427. https://doi.org/10.1016/j.cmet.2011.06.017
Guo J, Xue Q, Liu K, Ge W, Liu W, Wang J, Zhang M, Li Q, Cai D, Shan C, Zhang C, Liu X, Li J (2019) Dimethylaminomicheliolide (DMAMCL) suppresses the proliferation of Glioblastoma cells via targeting pyruvate kinase 2 (PKM2) and rewiring aerobic glycolysis. Front Oncol 9:993. https://doi.org/10.3389/fonc.2019.00993
Hacker HJ, Steinberg P, Bannasch P (1998) Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a cholinedeficient/DL-ethionine-supplemented diet. Carcinogenesis. 19:99–107. https://doi.org/10.1093/carcin/19.1.99
Han B, Meng X, Chen H, Chen L, Liu X, Wang H, Liu D, Gao F, Lin L, Ming J, Sun B, Yin S, Wang R, Wu P, Cai J, Jiang C (2017) Epigenetic silencing of miR-338 facilitates glioblastoma progression by de-repressing the pyruvate kinase M2-β-catenin axis. Aging (Albany NY) 9:1885-1897. Doi: https://doi.org/10.18632/aging.101271
Harada K, Saheki S, Wada K, Tanaka T (1978) Purification of four pyruvate kinase isozymes of rats by affinity elution chromatography. Biochim Biophys Acta 524:327–339. https://doi.org/10.1016/0005-2744(78)90169-9
Hatchell H (2017) The relationship between docohexanoic acid (DHA) and L-serine, providing an insight into the biochemistry of meningioma. Thesis, University of Central Lancashire
Haumann R, Videira JC, Kaspers GJL, Vuurden DGV, Hulleman E (2020) Overview of current drug delivery methods across the blood–brain barrier for the treatment of primary brain tumors. CNS Drugs 34:1121–1131. https://doi.org/10.1007/s40263-020-00766-w
Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387:296–299. https://doi.org/10.1038/387296a0
Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2(97):ra73. https://doi.org/10.1126/scisignal.2000431
Hitosugi T, Zhou L, Fan J, Elf S, Zhang L, Xie J, Wang Y, Gu TL, Aleckovic M, LeRoy G, Kang Y, Kang HB, Seo JH, Shan C, Jin P, Gong W, Lonial S, Arellano ML, Khoury HJ, Chen GZ, Shin DM, Khuri FR, Boggon TJ, Kang S, He C, Chen J (2013) Tyr26 phosphorylation of PGAM1 provides a metabolic advantage to tumours by stabilizing the active conformation. Nat Commun 4:1790. https://doi.org/10.1038/ncomms2759
Huang L, Yu Z, Zhang T, Zhao X, Huang G (2014) HSP40 interacts with pyruvate kinase M2 and regulates glycolysis and cell proliferation in tumor cells. PLoS One 9(3):e92949. https://doi.org/10.1371/journal.pone.0092949
Iansante V, Choy PM, Fung SW, Liu Y, Chai JG, Dyson J, Del Rio A, D'Santos C, Williams R, Chokshi S, Anders RA, Bubici C, Papa S (2015) PARP14 promotes the Warburg effect in hepatocellular carcinoma by inhibiting JNK1-dependent PKM2 phosphorylation and activation. NatCommun 6:7882. https://doi.org/10.1038/ncomms8882
Israelsen WJ, Heiden MGV (2015) Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol 43:43–51. https://doi.org/10.1016/j.semcdb.2015.08.004
Jiang X, Sun Q, Li H, Li K, Ren X (2014a) The role of phosphoglycerate mutase 1 in tumor aerobic glycolysis and its potential therapeutic implications. Int J Cancer 135:1991–1996. https://doi.org/10.1002/ijc.28637
Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, Wei C, Guo F, Chen Y, Lu Z (2014b) PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell 53:75–87. https://doi.org/10.1016/j.molcel.2013.11.001
Jiang Y, Wang Y, Wang T, Hawke DH, Yanhua LX, Zhou Q, Majumder S, Bi E, Liu DX, Huang S, Lu Z (2014c) PKM2 phosphorylates MLC2 and regulates cytokinesis of tumor cells. Nat Commun 5:5566. https://doi.org/10.1038/ncomms6566
Jiang X, Baucom C, Elliott RL (2019) Mitochondrial toxicity of azithromycin results in aerobic glycolysis and DNA damage of human mammary epithelia and fibroblasts. Antibiot (Basel) 8(3):Pii: E110. https://doi.org/10.3390/antibiotics8030110
Keller KE, Tan IS, Lee YS (2012) SAICAR stimulates pyruvate kinase isoform M2 and promotes Cancer cell survival in glucose -limited conditions. Science. 338:1069–1072. https://doi.org/10.1126/science.1224409
Kumar S, Barth A (2010) Phosphoenolpyruvate and Mg2+ binding to pyruvate kinase monitored by infrared spectroscopy. Biophys J 98:1931–1940. https://doi.org/10.1016/j.bpj.2009.12.4335
Li N, Feng L, Liu H, Wang J, Kasembeli M, Tran MK, Tweardy DJ, Lin SH, Chen J (2016) PARP inhibition suppresses growth of EGFR-mutant cancers by targeting nuclear PKM2. Cell Rep 15:843–856. https://doi.org/10.1016/j.celrep.2016.03.070
Li Y, He ZC, Liu Q, Zhou K, Shil Y, Yao XH, Zhang X, Kung FH, Bian XW, Ping YF (2018) Large Intergenic non-coding RNA-RoR inhibits aerobic glycolysis of Glioblastoma cells via Akt pathway. J Cancer 9:880–889. https://doi.org/10.7150/jca.20869
Liang J, Cao R, Zhang Y, Xia Y, Zheng Y, Li X, Wang L, Yang W, Lu Z (2016) PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. NatCommun 7:12431. https://doi.org/10.1038/ncomms12431
Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P, Li D, Li W, Yang W (2017) Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res 27:329–351. https://doi.org/10.1038/cr.2016.159
Liu VM, Vander Heiden MG (2015) The role of pyruvate kinase M2 in Cancer metabolism. Brain Pathol 25:781–783. https://doi.org/10.1111/bpa.12311
Liu K, Li F, Han H, Chen Y, Mao Z, Luo J, Zhao Y, Zheng B, Gu W, Zhao W (2016) Parkin regulates the activity of pyruvate kinase M2. J Biol Chem 291:10307–10317. https://doi.org/10.1074/jbc.M115.703066
Liu F, Ma F, Wang Y, Hao L, Zeng H, Jia C, Wang Y, Liu P, Ong IM, Li B, Chen G, Jiang J, Gong S, Li L, Xu W (2017) PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat Cell Biol 19:1358–1370. https://doi.org/10.1038/ncb3630
Liu T, Li S, Wu L, Yu Q, Li J, Feng J, Zhang J, Chen J, Zhou Y, Ji J, Chen K, Mao Y, Wang F, Dai W, Fan X, Wu J, Guo C (2020) Experimental study of hepatocellular carcinoma treatment by Shikonin through regulating PKM2. J Hepatocell Carcinoma 7:19–31. https://doi.org/10.2147/JHC.S237614
Lu Z (2012) Nonmetabolic functions of pyruvate kinase isoform M2 in controlling cell cycle progression and tumorigenesis. Chin J Cancer 31:5–7. https://doi.org/10.5732/cjc.011.10446
Lu Z, Hunter T (2014) Prolylisomerase pin-1 cancer. Cell Res 24:1033–1049. https://doi.org/10.1038/cr.2014.109
Lu Y, Liu X, Zhang E, Kopras EJ, Smith EP, Astreinidis A, Li C, Leung YK, Ho SM, Yu JJ (2020) Estrogen activates pyruvate kinase M2 and increases the growth of TSC2-deficient cells. PLoS One 15:e0228894. https://doi.org/10.1371/journal.pone.0228894
Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN, Hollern DP, Bellinger G, Dayton TL, Christen S, Elia I, Dinh AT, Stephanopoulos G, Manalis SR, Yaffe MB, Andrechek ER, Fendt SM, Vander Heiden MG (2015) Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell 57:95–107. https://doi.org/10.1016/j.molcel.2014.10.027
Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145:732–744. https://doi.org/10.1016/j.cell.2011.03.054
Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki N, Jiang Y, Zhou X, Li TT, Guan KL, Lei QY, Xiong Y (2013) Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell 52:340–352. https://doi.org/10.1016/j.molcel.2013.09.004
Mack SC, Agnihotri S, Bertrand KC, Wang X, Shih DJ, Witt H, Hill N, Zayne K, Barszczyk M, Ramaswamy V, Remke M, Thompson Y, Ryzhova M, Massimi L, Grajkowska W, Lach B, Gupta N, Weiss WA, Guha A, Hawkins C, Croul S, Rutka JT, Pfister SM, Korshunov A, Pekmezci M, Tihan T, Philips JJ, Jabado N, Zadeh G, Taylor MD (2015) Spinal MyxopapillaryEpendymomas demonstrate a Warburg phenotype. Clin Cancer Res 21:3750–3758. https://doi.org/10.1158/1078-0432.CCR-14-2650
Magno LD, Manzi D, D’Amico D, Coni S, Macone A, Infante P, Marcotullio LD, Smaele ED, Ferretti E, Screpanti I, Agostinelli E, Gulino A, Canettieri G (2014) Druggable glycolytic requirement for hedgehog-dependent neuronal and medulloblastoma growth. Cell Cycle 13(21):3404–3413. https://doi.org/10.4161/15384101.2014.952973
Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnel TJ, Golub TR, Loda M, Lane HA, Seller WR (2004) mTOR inhibition reverses Aktdependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10:594–601. https://doi.org/10.1038/nm1052
Marie SKN, Shinjo SMO (2011) Metabolism and brain Cancer. Clinics (Sao Paulo) 66:33–43. https://doi.org/10.1590/S1807-59322011001300005
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43:969–980. https://doi.org/10.1016/j.biocel.2010.02.005
Morfouace M, Lalier L, Oliver L, Cheray M, Pecqueur C, Cartron PF, Vallette FM (2014) Control of glioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis 5(1):e1036. https://doi.org/10.1038/cddis.2013.561
Morita M, Sato T, Nomura M, Sakamoto Y, InoueY TR, Ito S, Kurosawa K, Yamaguchi K, Sugiura Y, Takizaki H, Yamashita Y, Katakura R, Sato I, Kawai M, Okada Y, Watanabe H, Kondoh G, Matsumoto S, Kishimoto A, Obata M, Matsumoto M, Fukuhara T, Motohashi H, Suematsu M, Komatsu M, Nakayama KI, Watanabe T, Soga T, Shima H, Maemondo M, Tanuma N (2018) PKM1 confers metabolic advantages and promotes cell-autonomous tumor cell growth. Cancer Cell 33:355–367. https://doi.org/10.1016/j.ccell.2018.02.004
Mukherjee J, Phillips JJ, Zheng S, Wiencke J, Ronen SM, Pieper RO (2013) Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS One 8(2):e57610. https://doi.org/10.1371/journal.pone.0057610
Mukherjee J, Ohba S, See WL, Phillips JJ, Molinaro AM, Pieper RO (2016) PKM2 uses control of HuR localization to regulate p27 and cell cycle progression in human glioblastoma cells. Int J Cancer 139:99–111. https://doi.org/10.1002/ijc.30041
Ni W, Xia Y, Luo L, Wen F, Hu D, Bi Y, Qi J (2019) High expression of ALDH1A3 might independently influence poor progression free and overall survival in patients with glioma via maintaining glucose uptake and lactate production. Cell Biol Int 44:569–582. https://doi.org/10.1002/cbin.11257
Nie S, Qian X, Shi M, Li H, Peng C, Ding X, Zhang S, Zhang B, Xu G, Lv Y, Wang L, Friess H, Kong B, Zou X, Shen S (2020) ALDH1A3 accelerates pancreatic Cancer metastasis by promoting glucose metabolism. Front Oncol 10:915. https://doi.org/10.3389/fonc.2020.00915
Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T (1987) The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem 262:14366–14371
Omuro A, DeAngelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA 310:1842–1850. https://doi.org/10.1001/jama.2013.280319
Ozaki T, Nakagawara A (2011) Role of p53 in cell death and human cancers. Cancers (Basel) 3:994–1013. https://doi.org/10.3390/cancers3010994
Pan C, Wang X, Shi K, Zheng Y, Li J, Chen Y, Jin L, Pan Z (2016) MiR-122 reverses the doxorubicin-resistance in hepatocellular carcinoma cells through regulating the tumor metabolism. PLoS One 11:e0152090. https://doi.org/10.1371/journal.pone.0152090
Pan Y, Wang W, Huang S, Ni W, Wei Z, Cao Y, Yu S, Jia Q, Wu Y, Chai C, Zheng Q, Zhang L, Wang A, Sun Z, Huang S, Wang S, Chen W, Lu W (2019) Beta-elemene inhibits breast cancer metastasis through blocking pyruvate kinase M2 dimerization and nuclear translocation. J Cell Mol Med 23:6846–6858. https://doi.org/10.1111/jcmm.14568
Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, Pontoglio M, Ferré P, Scoazec JY, Birnbaum MJ, Ricci JE, Pende M (2012) PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun 3:672. https://doi.org/10.1038/ncomms1667
Prakasam G, Iqbal MA, Bamezai RNK, Mazurek S (2018) Posttranslational modifications of pyruvate kinase M2: tweaks that benefit Cancer. FrontOncol 8:22. https://doi.org/10.3389/fonc.2018.00022
Presek P, Glossmann H, Eigenbrodt E, Schoner W, Rubsamen H, Friis RR, Bauer H (1980) Similarities between a phosphoprotein (pp60src) associated protein kinase of rous sarcoma virus and cyclic adenosine 3′-5′-monophosphate-independent protein kinase that phosphorylates pyruvate kinase type M2. Cancer Res 40:1733–1741
Reinacher M, Eigenbrodt E (1981) Immunohistological demonstration of the same type of pyruvate kinase isoenzyme (M2-Pk) in tumors of chicken and rat. Virchows Arch B Cell Pathol Incl Mol Pathol 37:79–88. https://doi.org/10.1007/BF02892557
Reinacher M, Eigenbrodt E, Schering B, Schoner W (1979) Immunohistochemical localization of pyruvate kinase isoenzymes in chicken tissues. Histochemistry 64:145–161. https://doi.org/10.1007/BF00490095
Rose IA (1970) Stereochemistry of pyruvate kinase, pyruvate carboxylase, and malate enzyme reactions. J Biol Chem 245:6052–6056
Seeholzer SH, Jaworowski A, Rose IA (1991) Enolpyruvate: chemical determination as a pyruvate kinase intermediate. Biochemistry. 30:727–732. https://doi.org/10.1021/bi00217a022
Singh NS, Bernier M, Wainer IW (2016) Selective GPR55 antagonism reduces chemoresistance in cancer cells. Pharmacol Res 111:757–766. https://doi.org/10.1016/j.phrs.2016.07.013
Sizemore ST, Zhang M, Cho JH, Sizemore GM, Hurwitz B, Kaur B, Lehman NL, Ostrowski MC, Robe PA, Miao W, Wang Y, Chakravarti A, Xia F (2018) Pyruvate kinase M2 regulates homologous recombination-mediated DNA double-strand break repair. Cell Res 28:1090–1102. https://doi.org/10.1038/s41422-018-0086-7
Steinberg P, Klingelhoffer A, Schafer A, Wust G, Weisse G, Oesch F (1999) Expression of pyruvate kinase M2 in preneoplastic hepatic foci of N-nitrosomorpholinetreated rats. Virchows Arch 434:213–220. https://doi.org/10.1007/s004280050330
Sun K, Tang S, Hou Y, Xi L, Chen Y, Yin J, Peng M, Zhao M, Cui X, Liu M (2019) Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling. EBioMedicine 41:370–383. https://doi.org/10.1016/j.ebiom.2019.02.025
Suzuki A, Puri S, Leland P, Puri A, Moudgil T, Fox BA, Puri RK, Joshi BH (2019) Subcellular compartmentalization of PKM2 identifies anti-PKM2 therapy response in vitro and in vivo mouse model of human non-small-cell lung cancer. PLoS One 14:e0217131. https://doi.org/10.1371/journal.pone.0217131
Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, Suematsu M, Saya H (2012) Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res 72:1438–1448. https://doi.org/10.1158/0008-5472.CAN-11-3024
Tennant DA, Durán RV, Gottlieb E (2010) Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10:267–277. https://doi.org/10.1038/nrc2817
Vander HMG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033. https://doi.org/10.1126/science.1160809
Veelen CWV, Staal GE, Verbiest H, Vlug AM (1977) Alanine inhibition of pyruvate kinase in gliomas and meningiomas. A diagnostic tool in surgery for gliomas? Lancet 2:384–385. https://doi.org/10.1016/s0140-6736(77)90308-7
Venneti S, Thompson CB (2013) Metabolic modulation of epigenetics in gliomas. Brain Pathol 23:217–221. https://doi.org/10.1111/bpa.12022
Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin YS, Yang SF, Chen CC, Izumiya Y, Yu JS, Kung HJ, Wang WC (2014) JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α–mediated glucose metabolism. Proc Natl Acad Sci U S A 111:279–284
Wang X, Zhang F, Wu X (2017a) Inhibition of pyruvate kinase M2 markedly reduces Chemoresistance of advanced bladder Cancer to Cisplatin. Sci Rep 7:45983. https://doi.org/10.1038/srep45983
Wang Y, Liu J, Jin X, Zhang D, Li D, Hao F, Feng Y, Gu S, Meng F, Tian M, Zheng Y, Xin L, Zhang X, Han X, Aravind L, Wei M (2017b) O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci USA 114:13732–13737. https://doi.org/10.1073/pnas.1704145115
Warburg O (1956a) On respiratory impairment in cancer cells. Science. 124:269–270
Warburg O (1956b) On the origin of cancer cells. Science 123, 309–314. Science.123:309–314. doi: https://doi.org/10.1126/science.123.3191.309
Ward PS, Thompson CB (2012) Metabolic reprogramming: a Cancer Hallmark even Warburg did not anticipate. Cancer Cell 21:297–308. https://doi.org/10.1016/j.ccr.2012.02.014
Wiese EK, Hitosug T (2018) Tyrosine kinase signaling in Cancer metabolism: PKM2 paradox in the Warburg effect. Front Cell Dev Biol 6:79. https://doi.org/10.3389/fcell.2018.00079
Wong N, Melo JD, Tang D (2013) PKM2, a central point of regulation in Cancer metabolism. Int J Cell Biol 2013:242513–242511. https://doi.org/10.1155/2013/242513
Wong N, Ojo D, Yan J, Tang D (2015) PKM2 contributes to cancer metabolism. Cancer Lett 356:184–191. https://doi.org/10.1016/j.canlet.2014.01.031
Wu S, Le H (2013) Dual roles of PKM2 in cancer metabolism. ActaBiochimBiophys Sin (Shanghai) 45:27–35. https://doi.org/10.1093/abbs/gms106
Xia L, Wang XR, Wang XL, Liu SH, Ding XW, Chen GQ, Lu Y (2016) A novel role for pyruvate kinase M2 as a Corepressor for P53 during the DNA damage response in human tumor cells. J Biol Chem 291:26138–26150. https://doi.org/10.1074/jbc.M116.737056
Xu Q, Tu J, Dou C, Zhang J, Yang L, Liu X, Lei K, Liu Z, Wang Y, Li L, Bao H, Wang J, Tu K (2017) HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol Cancer 16:178. https://doi.org/10.1186/s12943-017-0748-y
Yamada K, Noguchi T (1999) Regulation of pyruvate kinase M gene expression. Biochem Biophys Res Commun 256:257–262. https://doi.org/10.1006/bbrc.1999.0228
Yan H, Bigner DD, Velculescu V, Parsons DW (2009) Mutant metabolic enzymes are at the origin of gliomas. Cancer Res 69:9157–9159. https://doi.org/10.1158/0008-5472.CAN-09-2650
Yan M, Chakravarthy S, Tokuda JM, Pollack L, Bowman GD, Lee YS (2016) SAICAR activates PKM2 in its dimeric form. Biochemistry. 55:4731–4736. https://doi.org/10.1021/acs.biochem.6b00658
Yang W, Lu Z (2013) Nuclear PKM2 regulates the Warburg effect. Cell Cycle 12:3154–3158. https://doi.org/10.4161/cc.26182
Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z (2011) Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480:118–122. https://doi.org/10.1038/nature10598
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L, You MJ, Koh MY, Cote G, Aldape K, Li Y, Verma IM, Chiao PJ, Lu Z (2012a) EGFR-induced and PKCεmonoubiquitylation-dependent NF-κB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell 48:771–784. https://doi.org/10.1016/j.molcel.2012.09.028
Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z (2012b) PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150:685–696. https://doi.org/10.1016/j.cell.2012.07.018
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z (2012c) ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Journal Nat Cell Biol 14:1295–1304. https://doi.org/10.1038/ncb2629
Yang P, Li Z, Fu R, Wu H, Li Z (2014) Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signaling. Cell Signal 26:1853–1862. https://doi.org/10.1016/j.cellsig.2014.03.020
Yang YC, Chien MH, Liu HY, Chang YC, Chen CK, Lee WJ, Kuo TC, Hsiao M, Hua KT, Cheng TY (2018) Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett 421:28–40. https://doi.org/10.1016/j.canlet.2018.01.075
Zhang M, Sun H, Den Y, Su M, We S, Wang P, Yu L, Liu J, Shen L, Guo J, Wang X, Han X, He Q (2019) COPI-mediated nuclear translocation of EGFRvIII promotes STAT3 phosphorylation and PKM2 nuclear localization. Int J Biol Sci 15:114–126. https://doi.org/10.7150/ijbs.28679
Data availability statement
Being a review article, this article does not contain any data or experimental results on the studies performed by any of the authors. Hence, authors claim none to share.
Funding
This work being supported to A.K.M. by the BSR-startup grant [F.20–39 (12)/2013 (BSR)], University Grants Commission (UGC), New Delhi, India, and Research Seed Money (RSM: CUPB/CC/14/OO/4507), Central University of Punjab, Bathinda, India. H.V., and S.K. thankfully acknowledges financial support in the form of junior research fellowship and senior research fellowship from the University Grants Commission (UGC-CSIR), New Delhi, India, respectively.
Author information
Authors and Affiliations
Contributions
H.V.; R.P.C.; conceived and provided major contribution in writing the manuscript; S.K. provided additional contribution while revising the manuscript. M.D. and A.K.M. conceptualized the work, interpreted, supervised and are the major contributors in writing the manuscript. Because of the limited focus of the article, many relevant and appropriate references could not be included, for which the authors apologize. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical concern
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Authors H.V.; R.P.C.; S.K.; M.D.; and A.K.M declares that none of them have no conflict of interest exists.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, H., Cholia, R.P., Kaur, S. et al. A short review on cross-link between pyruvate kinase (PKM2) and Glioblastoma Multiforme. Metab Brain Dis 36, 751–765 (2021). https://doi.org/10.1007/s11011-021-00690-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00690-y