Abstract
Zinc plays an important role in neuronal signaling and neurotransmission. However, dyshomeostasis of this metal or its accumulation in the brain has been linked with neurological disorders such as Alzheimer’s disease and Parkinson’s disease. In this study, the neuroprotective effects of Ecklonia maxima (KPM), Gracilaria gracilis (GCL), Ulva lactuca (ULT) and Gelidium pristoides (MNP) in Zn –induced neurotoxicity in HT-22 cells was examined. Cells were treated with Zinc sulphate and/or aqueous - ethanol extracts and cell viability, apoptosis, acetylcholinesterase activity, including some antioxidant enzymes (catalase and superoxide dismutase activity) and glutathione (GSH) levels were determined. Malondialdehyde and nitric oxide levels produced in the Zn and/or seaweed extract treated cells were also determined. Prior treatment with the seaweed extracts improved cell viability and inhibited Zn – induced cell death. Acetylcholinesterase activity was significantly high in Zn treated cells compared to the control. Pre-treatment with the seaweed extracts triggered a decrease in acetylcholinesterase activity in Zn – treated cells. Furthermore, treatment with Zn caused a significant reduction in GSH levels as well as a decrease in superoxide dismutase and catalase activities. In contrast, the seaweed extract increased antioxidant enzyme activities and GSH levels. An increase in malondialdehyde and nitric oxide levels was also reversed after treatment with the seaweed extracts. These results suggest that the seaweed extracts improved cholinergic transmission disrupted by Zn – induced neurotoxicity and protected the cells against oxidative damage and neuroinflammation. The neuroprotective effects of the seaweed extracts could be linked to their bioactive constituents. Hence these seaweeds are potential sources of active ingredients with neuroprotective potentials and could be used for the development of functional foods and/or nutraceuticals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegeneration is a complex pathological process that is associated with loss of neuronal function and progression of oxidative injury to brain cells (Cannon and Greenamyre 2011). Metals has been reported to play a role in the pathogenesis of neurodegenerative conditions such as Alzheimer’s disease (AD), Prion disease and Parkinson’s disease (PD) (Gaeta and Hider 2005). Although metal ions such as Fe, Zn and Cu play important roles in brain and memory functions, accumulation or depletion of these metals may trigger pathological features associated with AD, PD etc (Duce and Bush 2010; Olasehinde et al. 2017). Zn is an essential metal ion which is abundant in the brain and participates in neuronal signaling and neurotransmission (Sheline et al. 2000). Zn contributes to the regulation of spatial learning and memory and also maintain the integrity of cell membrane. However, elevated levels of Zn induce neuronal death (Berry and Toms 2006). Furthermore, extracellular levels of Zn may rise abnormally hence inducing neurotoxicity in cortical neurons (Sheline et al. 2000). There are indications that accumulation of Zn ions triggers the deposition of beta-amyloid plaques by disrupting the amyloid processing precursor pathway which leads to cognitive dysfunction and neurodegeneration in AD (Wang et al. 2010). Thus the use of neuroprotective agents alongside with metal chelators could be an effective therapeutic approach for the treatment of metal – induced neurotoxicity. Drugs that have been developed for the management of some neurological diseases such as AD and PD include cholinesterase inhibitors and N-methyl-D-aspartate receptor (NMDA) antagonists but do not exhibit metal chelating activity and may not be effective.
The use of naturally-occurring bioactive compounds from some marine organisms have proven to be an alternative approach. Seaweeds-based foods have shown great nutritional benefits (Cornish et al. 2017). Commercial applications of some seaweeds can be found in the production of functional foods, fertilizers, agar and animal feed (Suganthy et al. 2010; Klnc et al. 2013; Wells et al. 2017). Macroalgal-derived proteins and peptides are being developed as nutraceuticals and functional foods for therapeutic purpose (Tanna and Mishra 2019). Furthermore, active components of some seaweeds are also being explored as functional ingredients in dietary supplements and therapeutic agents while some are still under clinical trials (Tanna and Mishra 2018). Marine algal compounds exert multiple actions on different therapeutic targets associated with AD (Barbosa et al. 2014; Alghazwi et al. 2016). Many of these seaweeds are rich sources of biologically active compounds which include phlorotannins, sulfated polysaccharides, carotenoids, sterols (Alghazwi et al. 2016; Olasehinde et al. 2019a). Seaweeds have been reported to exert neuroprotective effects against glutamate – induced neurotoxicity in neuronal cells (Kim et al. 2016). Yang et al. (2015) also reported that phloroglucinol attenuated beta-amyloid – induced neurotoxicity in rats’ brain. Phlorotannin isolated from Ecklonia maxima exhibited neuroprotective effects in SH-SY5H cells treated with beta-amyloid (Wang et al. 2018). Gracilaria gracilis and Ulva reticulata also exhibited cholinesterase inhibitory effects (Suganthy et al. 2009, 2010). Despite several reports on the neuroprotective effects of seaweeds, there is paucity of evidence on their therapeutic role on metal – induced neurodegeneration. In this study the neuroprotective effects of aqueous – ethanol extracts of Ecklonia maxima (brown algae), Ulva lactuca (green algae), Gelidium pristoides (red algae) and Gracilaria gracilis (red algae) against Zn – induced neurotoxicity in hippocampal neuronal (HT-22) cells was examined via their effects on antioxidant enzymes, apoptosis and cholinesterases.
Materials and methods
Materials
Acetylcholine iodide, MTT, Griess reagent, 5,5′-dithiobisnitrobenzoic acid (DTNB), Epinephrine, trichloroacetic acid, Dulbecco’s phosphate buffered saline and fetal bovine serum were obtained from Sigma Aldrich (St Louis, USA). Zinc sulphate was sourced from Merck (Germany) while dubelcco’s modified eagle medium and penicillin-streptomycin were sourced from Life technologies (UK). BCA protein assay kit was obtained from Thermoscientific
Collection of seaweeds and identification
Ecklonia maxima (KPM) was obtained from kelpak products (Pty) Ltd., South Africa, Gelidium pristoides (MNP) was collected from Port Alfred (33°36.16’S, 26°53.983′E) while Gracilaria gracilis (GCL) and Ulva lactuca (ULV) were obtained from Wild Coast Abalone East London, Eastern Cape, South Africa and were identified by Dr. Paul-Pierre Steyn at the Department of Botany, Nelson Mandela University, South Africa.
Aqueous-ethanol extraction
The Seaweeds (100 g) were soaked in the extraction solvent (ethanol: water and 1 N HCl (1:1, v/v) in the ratio 1:20 w/v for 24 h and thereafter filtered using filter paper, and the filtrate was reduced under pressure until about 90% of the solvent had been evaporated (Oboh et al. 2016). The concentrate obtained was then lyophilized and stored at 4 °C for further analysis.
UHPLC-ESI-QTOF-MS analysis
Analysis of the phytochemicals present in the seaweed extracts was determined following the method of Kalinski et al. (2019) with slight modification. The UHPLC (Thermo Fisher Scientific, Sunnyvale, CA, USA) used in this study was equipped with C18 (2.1 × 100 mm, 2.2 um) (Acclaim RSLC 120). The flow rate was set at 0.300 mL/min using mixtures of water and acetonitrile, containing 0.1% formic acid (FA). The analysis was done in LC-MS/MS mode and the MS analyses were performed on a Bruker Compact QToF mass spectrometer using an electrospray ionization probe (Bruker, Bremen, Germany). The mobile phase consists of water (A) and acetonitrile (B) with 0.1% formic acid and was set to follow a step gradient which include; 90% A and 10% B (0–5 min), 60% A and 40% B (5–15 min), 60% A and 40% B (15–20 min), 30% A and 70% B (20–25 min) 30%A and 70%B (25–30), 100% B (30–35) and 100% (35–40 min). Minimum intensity of the MS was set at 5000 counts, collision energy 40 eV with 5 precursors. The data obtained was converted to mzXML format using Bruker Compass Software (Bruker, Germany). The files generated were subjected to MZmine 2 (2.36 version). Masses were detected from the raw data using the mass detection module and noise level was set at 15.00. Then the chromatogram builder icon was used to build a chromatogram for each mass. The minimum times span (retention time) was set at 0.030 while the minimum height (peak) was set at 25.000. The m/z tolerance was set at 0.04 Da or 5.0 ppm. After the peaks were obtained, local minimum search algorithm was used for chromatogram deconvolution. The threshod was set at 65% while the minimum relative height was 5.0%. Minimum retention time, relative height, absolute height and ration of peak were set at 0.030 min, 5.0% 50.00 and 2 respectively. The peak duration range was also set between 0.00 and 2.00 min. After this isotope grouping was done to search for peak lists within the peak with same isotope patterns using the isotope grouper. The peaks were then filtered and de-isotoped. The peaks were identified using the custom search database module. m/z tolerance was set at 0.04 Da or 5.0 ppm while retention time tolerance was set at 0.07 min. The search online database was used to search for similar identities using different online databases on mzmine including pubchem, kebb, metaCyc, and Hmdb
Cell culture and treatment
HT-22 cells, an immortalized mouse hippocampal neuron cell line were obtained from Salk institute San Diego USA. Cells were maintained in normal conditions Dubelcco medium FBS (10%), penicillin (100 U/mL,) and streptomycin 100 μg/mL, CO2 (5%), 37 °C. Cells were plated in 96 or 24 well plates after they had grown to about 60–70% confluence. The cells were placed into the following groups: Control (without Zn or extract); Zinc (50 uM); Zn (50 uM) + KPM (0.5 mg/mL); Zn (50 uM) + KPM (1 mg/mL) Zn (50 uM) + GCL (0.5 mg/mL); F: Zn (50 uM) + GCL (1.0 mg/mL); Zn (50 uM) + ULT (0.5 mg/mL); G: Zn (50 uM) + ULT (1 mg/mL); G: Zn (50 uM) + MNP (0.5 mg/mL); G: Zn (50 uM) + ULT (1 mg/mL). Distilled water was used as the solvent control.
Cell viability assay
MTT was used to determine percentage cell viability in treated and untreated neuronal cells. Cells (100 μL/well) were pre-incubated in 96-well plate at 37 °C in a humidified atmosphere at 5% CO2. After 24 h incubation, cells were treated appropriately as shown in the grouping above and incubated for another 18 h. The medium was removed and cells were washed with cold phosphate-buffered saline. Fresh medium was added to each well alongside with MTT (20 μL, 1 mg/mL) and was incubated for 4 h. The mixture was aspirated after the incubation period and 100 μL of dimethyl sulfoxide was added to dissolve the formazan crystals formed. A microplate reader was used to measure the absorbance of the solution at 570 nm and percentage cell viability was calculated for each group.
Apoptotic assay
The induction of apoptosis in the treated and untreated cells were investigated qualitatively using the acridine orange/ethidium bromide (AO/EB) dual staining method. Briefly, cells were seeded in 24-well plates at a cell density of 1.5 × 105/well and incubated at 37 °C in 5% CO2 for 24 h to allow cells to attach. Thereafter, spent medium was removed and replenished with 0.4 mL of complete medium. ZnSO4 and/or seaweed extract were place in the wells appropriately in triplicates. The cells were incubated for 18 h at 37 °C in 5% CO2 humidified environment. Untreated cells (without ZnSO4 and/or seaweed extracts) was used a positive control. After incubation the medium was removed, and wells were rinsed with cold PBS (100 μL). Cells were stained with 12 μL of acridine orange/ethidium bromide dye mixture (1:1 v/v of 100 mg/ml acridine orange and 100 mg/ml ethidium bromide) prepared in phosphate buffered saline. After 5 mins, the stained cells were viewed with a fluorescence microscope (Olympus) fitted with a CC12 fluorescent camera (Olympus Co., Japan).
Preparation of homogenates
HT-22 cells were seeded and treated with seaweed extracts as described above. After incubation for 18 h, cells were detached and centrifuged at 2500 rpm for 10 min. The supernatant was decanted and stored in different vials at -20 °C for further experiment. The protein content of the homogenates were determined using BCA protein assay kit.
Estimation of glutathione content
Reduced glutathione (GSH) levels in the homogenates were determined using the method of Ellman (1959). The samples were deproteinize with an equal volume of 10% trichloroacetic acid and then centrifuged at 3500 rpm for 5 min at 25 °C. Thereafter, 100 μL of the supernatant was pipetted into a 96 well plate. DTNB (50 μL) was added to the mixture allowed to stand for 5 min. A yellow derivative (5′-thio-2-nitrobenzoic acid [DTNB]) formed was measured at 415 nm in a microplate reader. The GSH level was then extrapolated from the standard curve of plotted GSH concentrations.
Determination of catalase activity
Catalase activity in the homogenates was determined based on the measurement of decreased absorbance of test samples owing to H2O2 decomposition (Aebi 1984). Two hundred and forty microlitre (240 μL) of 50 mM sodium phosphate buffer (pH 7.0) was mixed with 10 μL of the samples, then 100 μL of 2 M H2O2 was added to the mixture. Absorbance was read at 240 nm for 3 min at 1 min interval.
Determination of superoxide dismutase activity
Superoxide dismutase activity in the homogenate was determined via the method of (Misra and Fridovich 1972). The mechanism of the reaction is based on the measurement of inhibition of adrenaline which undergoes autooxidation. The homogenates were mixed with carbonate buffer (200 μL) after which adrenaline (17 μL) was added to the solution. The wavelength of the microplate reader was set at 570 nm and absorbance was measured for two minutes at 15 s interval. The activity of the enzyme portion of SOD necessary to inhibit (50%) adrenaline autooxidation.
Determination of nitric oxide (NO) levels
The NO produced in the cells were determined using Griess reagent. One hundred microliters of the samples or distilled water (blank) and was incubated with an equal volume of Griess reagent for 30 min at 25 °C in the dark (Sun et al. 2003).
Acetylcholinesterase activity assay
The method of Ellman et al. (1961) was used to determine acetylcholinesterase activity in the treated and untreated cells. The enzyme preparation (about 10–20 μg of protein) was pre-incubated for 2 min with the reaction medium which contains 150 μL of phosphate buffer (0.1 M, pH 7.8) and 50 μL of 5,5′-dithiobisnitrobenzoic acid (6.6 mM). Fifty microlitre of substrate (acetylcholine iodide, 0.05 mM) was added to the mixture to trigger the reaction. The yellow anion formed was measured at 412 nm at interval for 2 min and enzyme activity was calculated as micromoles AChE per min per milligram of protein.
Statistical analysis
The data obtained from triplicate (n = 3) experiments were expressed as mean ± standard error of mean (S.E.M.). Analysis involving statistical significance was analyzed using one-way (ANOVA) and post hoc Tukey’s test. In both analyses, differences at p < 0.05 were significantly different.
Results
Identification of compounds
LC-MS characterization of the seaweed extracts revealed the presence of phloroglucinol, catechin, epicatechin-2-glucoside, plastoquinone-1, vulgaxanthin, Biochanin A and 7,2,4 – trihydoxyisoflavanol were detected in KPM, GCL, MNP and ULT (Table 1). Moreover, ferulic acid was identified in ULT and MNP while 3,7-dimethyl quercetin was detected in KPM and GCL (Table 1). Dihydroxylbenzoic acid and 4-heptyloxybenzoic acid were also identified in GCL and MNP respectively while these two compounds were absent in KPM and ULT. Kaempferol 3 – (6 – acetylgalactoside) 7- rhamnoside and 2 – hydroxyl 3,7,4-trimethyl were present in KPM and MNP but were not detected in ULT and GCL. The major classes of compounds observed in the seaweed extracts include phlorotannins, flavonoids and phenolic acids.
Effect of Zn and seaweed extracts on cell viability
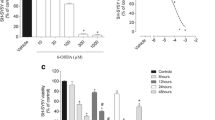
The effect of ZnSO4 and seaweed extracts on cell viability of HT-22 cells was measured using MTT assay. Figure 1a revealed the cytotoxicity of Zn which was dose dependent with concentration ranging from 50 to 500 μM. Zn significantly reduced the viability of HT-22 cells and 56.9% viability was achieved at 50 μM concentration. Further increase in the concentration of ZnSO4, caused marked reduction in percentage viability.
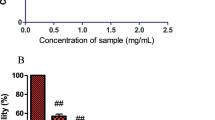
Figure 1b revealed the effect of aqueous – ethanol from the seaweeds on cell viability of HT-22 cells. At a lower concentration (0.5 mg/mL), the seaweed did not show cytotoxic effects. Cell viability of 101.9%, 97.1%, 95.4% and 95.0% was observed for KPM, GCL, ULT and MNP respectively. However, an increase in the concentration of the seaweed extracts led to decrease in cell viability (Fig. 1b). KPM, GCL, ULT and MNP exhibited cell viability of 82.0, 77.8, 77.7 and 66.8% respectively.
Effect of seaweed extracts against Zn – induced neuronal death
The effect of seaweed extracts (0.5 and 1.0 mg/mL) on Zn – induced neurotoxicity was also determined via MTT assay. The result revealed that 0.5 mg/mL of KPM, GCL. ULLT and MNP improved cell viability in Zn treated cells as shown in Fig. 2a. Moreover, KPM and MNP caused a significant increase in cell viability in cells treated with Zn compared to GCL and ULT. However, after treatment with 1.0 mg/mL of the seaweed extracts, a significant increase in cell viability was observed compared to cells treated with 0.5 mg/mL extracts as shown in Fig. 2b. There was no significant difference in percentage cell viability after treatment with 1.0 mg/mL of KPM, GCL, ULT and MNP in Zn treated cells, although the percentage cell viability of the extracts was significantly lower than the control (Fig. 2b).
Protective effect of seaweed extracts against zinc-induced neuronal damage in HT-22 cells. Cells were exposed to 50 μM of zinc sulphate in combination with/without the extracts (a) 0.5 mg/mL abd (b) 1 mg/mL for 24 h and cell viability was measured via MTT assay. All data are shown as the mean ± SEM; n = 3; #P < 0.05 vs. control; $P < 0.05 vs. Zn; &P < 0.05 KPM and MNP
To further investigate the effect of the seaweed extracts in Zn – induced neurotoxicity in HT – 22 cells, acridine orange and ethidium bromide staining method was used to measure the level of neuronal damage and/or cell death. The representative fluorescence micrographs in Fig. 3 revealed no occurrence of neuronal death in the control (vehicle) (Fig. 3a) compared to cells treated with Zn (Fig. 3b) which showed late apoptotic and necrotic cells as indicated by the yellow/orange condense chromatin and red necrotic cells. In Fig. 3c–f, cells treated with the extracts showed similar morphology as the control indicating a low apoptotic rate. The number of apoptotic cells was significantly low in cells treated with the seaweed extracts compared to Zn – treated cells. Necrotic cells were not observed in cells treated with seaweed extracts.
Representative fluorescent micrographs of dual acridine orange and ethidium bromide stained cells revealing morphological changes in HT-22 cells. a: Control (without Zn or extract); b: Zn alone (50 μM); c: Zn (50 μM) + KPM (0.5 mg/mL); d: Zn (50 μM) + GCL (0.5 mg/mL); e: Zn (50 μM) + ULT (0.5 mg/mL); f: Zn (50 μM) + MNP (0.5 mg/mL); g: Zn (50 μM) + KPM (1.0 mg/mL); h: Zn (50 μM) + GCL (1.0 mg/mL); i: Zn (50 μM) + ULT (1.0 mg/mL); j: Zn (50 uM) + MNP (1.0 mg/mL)
The fluorescence micrographs in Fig. 3g–j shows cells treated with the seaweed extracts prior to exposure to Zn. Protection against Zn – induced apoptosis was observed after treatment with 1 mg/mL of KPM, GCL, ULT and MNP. The percentage of apoptotic cells in cells treated with seaweed extracts and Zn were significantly low compared to treatment with Zn alone. Prior exposure of the cells to seaweed extracts reversed Zn – induced neuronal death and confer neuroprotection via significant reduction of apoptotic cells.
Seaweed extracts protects against Zn – induced oxidative damage to neuronal cells
Intracellular GSH levels was significantly low in Zn – treated cells compared to the control as shown in Fig. 4a. However, prior treatment with seaweed extracts triggered an increase in GSH levels after the cells were treated with Zn. The result also revealed that KPM and MNP showed significant increase in GSH levels in Zn – treated cells compared to GCL and ULT.
Effect of treatment with aqueous-ethanol extracts of seaweeds on GSH levels (a) and SOD activity (b) in Zinc-induced neuronal damage in HT-22 cells. All data are shown as the mean ± SEM; n = 3; #P < 0.05 vs Control; &P < 0.05 Zinc; *P < 0.05 vs. KPM (0.5 mg/mL); **P < 0.05 vs. GCL (0.5 mg/mL); $P < 0.05 vs. ULT (0.5 mg/mL); ^P < 0.05 vs. MNP (0.5 mg/mL)
Similar result was obtained for SOD activity in Zn – induced neuronal cells. Activity of SOD was significantly low after cells were treated with Zn compared to the control as depicted by Fig. 4b. However, in cells pretreated with seaweed extracts, SOD activity was observed to be significantly higher compared to cells treated with Zn alone. Treatment with 1 mg/mL with KPM and MNP triggered significantly higher SOD activity compared to GCL and ULT.
Furthermore, a significant reduction in CAT activity was also observed in Zn – treated cells compared to the control as depicted by Fig. 5a. This result shows possible activation of redox imbalance in the neuronal cells. The seaweed extracts elevated CAT activity in Zn treated cells. It was observed that treatment with 1 mg/mL of KPM and GCL reversed CAT activity in Zn – treated cells and was not significantly different from the control. Although ULT and MNP triggered an increase in CAT activity, their effect was significantly lower compared to KPM and GCL.
Effect of treatment with aqueous-ethanol extracts of seaweeds on CAT activity (a) and MDA levels (b) in Zinc-induced neuronal damage in HT-22 cells. All data are shown as the mean ± SEM; n = 3; #P < 0.05 vs Control; &P < 0.05 Zinc; *P < 0.05 vs. KPM (0.5 mg/mL); **P < 0.05 vs. GCL (0.5 mg/mL); $P < 0.05 vs. ULT (0.5 mg/mL); ^P < 0.05 vs. MNP (0.5 mg/mL)
A significant increase in malondialdehyde (MDA) levels was observed in Zn – treated cells compared to the control as shown in Fig. 5b. This result shows that Zn induced the production of MDA via lipid peroxidation reaction in the neuronal cells. Pretreatment with the seaweed extracts significantly reduced MDA levels in Zn – treated cells. Moreover, KPM and GCL caused significant reduction in MDA levels compared to ULT and MNP.
Effect of seaweed extracts on nitric oxide levels
Furthermore, the production of NO significantly increased after the cells were exposed to Zn at concentration of 50 μM compared to the control (Fig. 6). HT −22 cells pretreated with the seaweed extracts showed significant reduction in NO levels compared to the group of cells treated with Zn alone. There was no significant difference between the NO levels of cells treated with KPM and MNP. Moreover, NO levels observed in cells treated with ULT and GCL were significantly lower compared to KPM and MNP.
Effect of treatment with aqueous-ethanol extracts of seaweeds on NO levels in Zinc-induced neuronal damage in HT-22 cells. All data are shown as the mean ± SEM; n = 3; #P < 0.05 vs Control; &P < 0.05 Zinc; *P < 0.05 vs. KPM (0.5 mg/mL); **P < 0.05 vs. GCL (0.5 mg/mL); $P < 0.05 vs. ULT (0.5 mg/mL); ^P < 0.05 vs. MNP (0.5 mg/mL)
Modulatory effect of seaweed extract on acetylcholinesterase activity
Acetylcholinesterase activity in Zn – induced neuronal damage in HT-22 cells was measured using Ellman’s reagent. The result revealed that exposure to ZnSO4 (50 μM) significantly increased acetylcholinesterase activity in HT-22 cells as shown in Fig. 7. About fivefold increase in acetylcholinesterase activity was observed in Zn – treated cells compared to the control. However, prior exposure to seaweed extracts significantly reduced acetylcholinesterase activity in Zn treated cells. Treatment with 1 mg/mL of the seaweed extracts caused significant reduction in acetylcholinesterase activity compared to exposure of the cells to 0.5 mg/mL of the extracts. Moreover, no significant reduction was observed in cells treated with 1 mg/mL of KPM, GCL and MNP compared to ULT.
Effect of treatment with aqueous-ethanol extracts of seaweeds on acetylcholinesterase activities in Zinc-induced neuronal damage in HT-22 cells. All data are shown as the mean ± SEM; n = 3; #P < 0.05 vs Control; &P < 0.05 Zinc; *P < 0.05 vs. KPM (0.5 mg/mL); **P < 0.05 vs. GCL (0.5 mg/mL); $P < 0.05 vs. ULT (0.5 mg/mL); ^P < 0.05 vs. MNP (0.5 mg/mL)
Discussion
This study revealed the chemical constituents and neuroprotective effects of some seaweed extracts against Zn - induced neurotoxicity in HT-22 cells. A tentative identification of compounds present in the seaweed extracts was done based on correlation and similarity with mass spectral obtained from different databases. The results revealed that some phlorotannins, flavonoids and phenolic acids may be present in the seaweed extracts. Phloroglucinol was identified in all the seaweed extracts. It belongs to the class of compound known as phlorotannin and has been discovered to possess antioxidative and neuroprotective potentials (Yang et al. 2015). The LC-MS results revealed that the seaweed extracts may be rich in flavonoids as shown by the presence of epicatechin −3 glucoside, catechin and biochanin A, 5,7 dimethoxyflavone, 7,2,4 – trihydoxyisoflavanol. The observed presence of catechin correlates with the report of Quirós et al. (2010) which revealed the presence of this compound in brown seaweed extracts. Catechins have been identified to be potent neuroprotective agents while biochanin A has been reported to attenuate glutamate and beta-amyloid induced neurotoxicity in PC -12 cells (Tan et al. 2013; Tan and Kim 2016). Although flavonoids have been identified in seaweeds (Rajauria 2018), other flavonoids identified in KPM, GCL, ULT and MNP include 2 – hydroxyl, 3,6,7,4- tetramethyl quercetin, 5,7,3-trihydroxymethoxyflavanone, 7,2,4 – trihydoxyisoflavanol, 3,7-dimethyl quercetin, Dihydronaringenin –O- Sulphate, 5,7 dimethoxyflavone, 3,3,4,5,6,7,8-heptahydroxyflavone, Kaempferol 3 – (6 – acetylgalactoside) 7- Rhamnose and 4,5,7 – trimethoxyflavone (apigenin trimethyl ester). This study reports the presence of some of these flavonoids in brown, red or green seaweeds for the first time. Phenolic acids such as dihydroxybenzoic acid was identified in GCL while ferulic acid was detected in ULT and MNP. This is consistent with the report of Rajauria (2018) which revealed the presence of ferulic acid in Himanthalia elongata (Irish brown seaweed). Agregán et al. (2017) also reported the presence of dihydroxybenzoic acid derivatives in Ascophyllum nodosum. Vulgaxanthin was also identified in KPM, GCL and ULT. It is a group of betaxanthin and has been identified as a natural antioxidant. Antioxidants are used as an effective therapeutic strategy to alleviate some pathological features in some neurological disorders such as AD (Feng and Wang 2012). Our findings revealed that KPM, GCL, ULT and MNP could be excellent sources of antioxidants and may play potential role as neuroprotective agents against some of the pathological manifestations associated with neurodegeneration.
Metals such as zinc, iron and copper function as neurotoxicants and nutrients depending on their levels in the brain (Wright and Baccarelli 2007). These metals play important role in redox reactions which are required for cellular metabolism, mitochondrial function and as well participate in the synthesis of neurotransmitters (Chen et al. 2016). Although metals have been shown to exert some physiological effects in brain and memory function, however, disruption of these processes could cause depletion or accumulation of metals which may lead to neuronal damage (Farina et al. 2013). Neurodegeneration triggers disruption of neurotransmission, neuronal damage and/or death (Gorman 2008). In this study, Zn at different concentrations induced neurotoxicity dose dependently in HT-22 cells. The neurotoxic effect of Zn was further confirmed in the results of the apoptotic assay using acridine orange and ethidium bromide dual staining. Zn induced apoptosis in HT-22 cells which was revealed by the yellow/orange condensed chromatin and red stains which connotes late apoptosis and necrosis. The observed decrease in cell viability, late apoptotic and necrotic cells after treatment with Zn may be due to the production of reactive oxygen species which induces oxidative damage to neurons. Furthermore, accumulation of Zn ions has also been reported to disrupt the amyloid precursor protein processing pathway and trigger the deposition of beta-amyloid plaques which are toxic to the neurons (Wang et al. 2010). Previous report has also shown that elevated concentration of Zn may disrupt physiological levels of metal ions, enhance production of reactive oxygen species and induce AD-like pathological characteristics (Maynard et al. 2009). However, the seaweeds used in this study attenuated Zn – induced neurotoxicity as shown by the increase in cell viability and inhibition of apoptosis. The observed protective effects of the seaweed extracts against Zn – induced neuronal damage could be linked to the presence of phlorotannin, phenolic acids and flavonoids. Chemical compounds in these class of phytochemicals are known for their antioxidant activities and may prevent cell death mediated by excessive free radical production induced by Zn. The capacity of the phenolic compounds in the extracts to mitigate Zn – induced neuronal death could be linked to their radical scavenging and metal chelating activities. Previous report from our laboratory have shown that KPM, GCL, MNP and ULT exhibit potent radical scavenging and metal chelating activities (Olasehinde et al. 2019b). Furthermore, some of the compounds present in the extracts such as phloroglucinol, catechin and epicatechin have been reported to exhibit protective effects against free radical induced - cell death (Heo and Lee 2005; Park et al. 2019).
Antioxidant enzymes play an important role in the brain as defensive mechanism against oxidative damage and neuronal death (Uttara et al. 2009). The brain is highly susceptible to oxidative stress due to low antioxidant defense system and high levels of lipids. Its capacity to consume high levels of oxygen due to high rate of metabolic activities also makes it vulnerable to free radical attack (Oboh et al. 2013). Superoxide radicals produced in the brain are dismutated by superoxide dismutase to form hydrogen peroxide which is decomposed by catalase (Valko et al. 2007). In this study, treatment with Zn significantly reduced catalase and superoxide dismutase activities which suggests redox imbalance and free radical attack against the neuronal cells. Similarly, GSH levels was observed to be significantly lower than the control. However, the seaweed extracts triggered an increase in catalase and superoxide dismutase activities as well as GSH levels in Zn treated cells. The observed increase in catalase and superoxide dismutase activities as well as high GSH levels could be linked to the antioxidant capacity of the seaweed extracts. High levels of these antioxidant enzymes will scavenge and prevent the accumulation of free radicals, hence protecting the cells against oxidative damage and neurodegeneration. The presence of some phlorotannins and flavonoids in the seaweed extracts may contribute to the increase in catalase and superoxide dismutase activities as well as GSH levels observed after treatment with the seaweed extracts. The study of Ryu et al. (2013) revealed that phloroglucinol restored catalase activity and GSH levels in 6 – hydroxydopamine – treated rats. Phenolic compounds are known to mitigate redox imbalance and improve antioxidant defense mechanisms in the cells (He et al. 2017). The synergistic effects of the flavonoids (Kaempferol, apigenin, quercetin derivative, catechin, epicatechin – glucoside, biochanin A, trihydroxyisoflavanol, Trimethoxyflavone) and phenolic acid (ferulic acid) identified in the extracts may contribute to the improved antioxidant status of the cells after treatment with the seaweeds.
Some metals have been reported to induce lipid peroxidation via the initiation of the breakdown of hydrogen peroxide leading to the production of more toxic radical such as hydroxyl radicals (Valko et al. 2007). This radical attacks polyunsaturated fatty acids by abstracting an electron leading to the formation of a lipid peroxyl radical which induces lipid peroxidation. Malondialdehyde is formed as a product of lipid peroxidation and has been shown to be toxic and capable of causing oxidative damage to cells (Nam 2011). Excessive production of malondialdehyde was observed after treatment with Zn compared to the control which suggests metal – induced lipid peroxidation and oxidative damage to the neuronal cells. However, treatment with the seaweed extracts reduced malondialdehyde levels. The observed reduction in malondialdehyde levels could be linked to the presence of some antioxidants in the seaweed extracts. Compounds such as phloroglucinol, catechin and epicatechin −3 glucoside, ferulic acid have been reported to be potent antioxidants. The antioxidant activity of phloroglucinol via its capacity to reduce cellular oxidative stress markers in HepG2 cells was reported by Quéguineur et al. (2012). Simos et al. (2012) also reported the antioxidant activity of catechin and epicatechin via their modulatory effect on some antioxidant enzymes and malondialdehyde production.
Nitric oxide (NO) plays a significant role as a signaling molecule in the nervous system via activation of neuronal nitric oxide synthase, regulation of synaptic plasticity and neurotransmission (Džoljić et al. 2015). However, impairment in NO signaling has been linked with neurological disorders (Steinert et al. 2010). While physiological levels of NO exhibit neuroprotective effects, higher concentrations have been reported to initiate neurotoxic pathologies associated with neurodegenerative diseases. (Steinert et al. 2010). Wang et al. (2015) reported that NO – induced neuronal cell death via impaired cyclic-Guanosine monophosphate signaling. The effect of manganese on NO production and pro-inflammatory factors in dopaminergic neurons was also reported by Zhao et al. (2009). Our findings revealed that Zn – induced overproduction of NO compared to the control. The observed increase in NO levels could be due to oxidative burst caused by Zn – induced neurotoxicity. NO may react with superoxides to form peroxynitrites which may induce nitrosative stress, lipid oxidation, DNA fragmentation and neuroinflammation (Valko et al. 2007). Treatment with KPM, GCL, ULT and GLT reduced Zn – induced NO production. The observed decrease in NO levels caused by the seaweed extracts may prevent neuroinflammation and neuronal death.
Evidence has shown that metals may cause deleterious effects to the central nervous system and one of the ways by which they do so is to disrupt the flow of neurotransmission across the neurons which may lead to memory impairment (Chen et al. 2016). Acetylcholine is a neurotransmitter required for transmission of nerve impulse between cholinergic neurons (Colovic et al. 2013). Physiological levels of acetylcholine are regulated by acetylcholinesterase and any alteration in this enzyme may lead to depletion of acetylcholine, cholinergic deficit and cognitive decline (Ferreira-vieira et al. 2016; Garcia-Ayllon et al. 2011). There are indications that metal – induced neurodegeneration may impair cholinergic function via alteration of acetylcholinesterase activity (Carageorgiou et al. 2005). In this study, it was observed that Zn induced a significant increase in acetylcholinesterase activity compared to the control. This result correlates with the report of Richetti et al. (2011) which revealed that metals such as cadmium, zinc and lead altered acetylcholinesterase activity in Zebra fish. Babadi et al. (2014) also confirmed that manganese induced high acetylcholinesterase in rats’ brain. However, the seaweeds used in this study significantly reduced acetylcholinesterase activity in Zn – treated cells. This suggest that the seaweed extracts may attenuate cholinergic dysfunction caused by Zn – induced neurotoxicity via inhibition of acetylcholinesterase. There is an indication that the significant reduction in acetylcholinesterase activity observed in Zn – treated cells may be attributed to the enzyme inhibitory effects of the seaweed extracts. Our previous findings on aqueous-ethanol extracts of KPM, GCL, ULT and MNP revealed that these seaweed extracts exhibited inhibitory effects on acetylcholinesterase activity in vitro (Olasehinde et al. 2019b). Some biologically active compounds such as flavonoids and phlorotannins present in seaweeds are potent inhibitors of acetylcholinesterase (Alghazwi et al. 2016). Phloroglucinol has been reported to be a potent acetylcholinesterase inhibitor due to its tight binding to the amino acid residues of the enzyme via polar interactions (Burmaoglu et al. 2018). Furthermore, catechin also showed potent inhibitory effect on acetylcholinesterase activity as reported by Suganthy and Devi (2016) and has been identified as a potential drug to alleviate cholinergic deficit and neuronal damage. Other compounds such as ferulic acid, biochanin A, kaempferol, may also contribute to the reduction of acetylcholinesterase as they have been reported as a potent inhibitor of the enzyme (Biradar et al. 2014; Adefegha et al. 2016; Khan et al. 2018).
Conclusion
This study provides evidence that aqueous - ethanol extracts of KPM, GCL, ULT and MNP exhibit neuroprotective effect against Zn – induced neurotoxicity via inhibition of apoptosis, attenuation of cholinergic dysfunction, oxidative stress, and protection against neuroinflammation. LC-MS characterization of the seaweed extracts revealed the presence of phloroglucinol, catechin, epicatechin, biochanin A, vulgaxanthin and 7,2,4 – trihydoxyisoflavanol. The synergistic and/or additive effects of the compounds identified in the seaweed extracts may contribute to their neuroprotective effects. These findings suggest that these seaweeds may be good sources of bioactive compounds and could be considered for the development of functional foods, nutraceuticals or drugs for the management of Alzheimer’s disease. Although further investigations are required using other experimental models.
References
Adefegha SA, Omojokun OS, Oboh G, Fasakin O, Ogunsuyi O (2016) Modulatory effects of ferulic acid on cadmium-induced brain damage. J Evid Based Complementary Altern Med 21(4):NP56–NP61
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agregán R, Munekata PE, Franco D, Dominguez R, Carballo J, Lorenzo JM (2017) Phenolic compounds from three brown seaweed species using LC-DAD – ESI-MS / MS. Food Res Int 99:979–985
Alghazwi M, Kan YQ, Zhang W, Gai WP, Garson MJ, Smid S (2016) Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J Appl Phycol 28:3599–3616
Babadi Y, Sadeghi L, Kobra S, Malekirad A, Mohammad R (2014) The toxic effect of manganese on the acetylcholinesterase activity in rat brains. J Toxicol 2014:946372
Bao D, Wang J, Pang X, Liu H (2017) Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat Pheochromocytoma (PC-12) cells. Molecules 22:1122
Barbosa M, Valentão P, Andrade PB (2014) Bioactive compounds from macroalgae in the new millennium: implications for neurodegenerative diseases. Mar Drugs 12:4934–4972
Berry EV, Toms NJ (2006) Pyruvate and oxaloacetate limit zinc-induced oxidative HT-22 neuronal cell injury. Neurotox 27:1043–1051
Biradar SM, Joshi H, Chheda TK (2014) Biochanin-a ameliorates behavioural and neurochemical derangements in cognitive-deficit mice for the betterment of Alzheimer’s disease. Hum Exp Toxicol 33(4):369–382
Burmaoglu S, Yilmaz A, Taslimi P, Algul O, Kilic D, Gulcin I (2018) Synthesis and biological evaluation of phloroglucinol derivatives possessing α-glycosidase, acetylcholinesterase, butyrylcholinesterase , carbonic anhydrase inhibitory activity. Arch Pharm Chem Life Sci 351:1
Cannon JR, Greenamyre JT (2011) The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124(2):225–250
Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S (2005) Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc , calcium and L-cysteine co-administration. Basic Clin Pharmacol Toxicol 97:320–324
Chen P, Rahman M, Aschner M (2016) Metals and neurodegeneration. F1000Research 5:38
Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11:315–335
Cornish ML, Critchley AT, Mouritsen OG (2017) Consumption of seaweeds and the human brain. J Appl Phycol 29:2377–2398
Duce JA, Bush AI (2010) Biological metals and Alzheimer’s disease: implications for therapeutics and diagnostics. Prog Neurobiol 92:1–18
Džoljić E, Grabatinic I, Kostic V (2015) Why is nitric oxide important for our brain ? Funct Neurol 30:159–163
Ellman GL (1959) Tissue sulfurhydryl groupss. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Farina M, Avila DS, Da Rocha JBT, Aschner M (2013) Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int 62:575–594
Feng Y, Wang X (2012) Antioxidant therapies for Alzheimer’s disease. Oxidative Med Cell Longev 2012:472932
Ferreira-Vieira HM, Guimaraes IR, Silva FM, Ribeiro F (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115
Gaeta A, Hider RC (2005) The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol 146(8):1041–1059
Garcia-Ayllon M, Small DH, Avilla JJ (2011) Revisiting the role of acetylcholinesterase in Alzheimer ’ s disease : cross-talk with P-tau and β -amyloid. Front Mol Neurosci 4:1–9
Gorman AM (2008) Neuronal cell death in neurodegenerative diseases : recurring themes around protein handling. J Cell Mol Med 12:2263–2280
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44(2):532–553
Heo HJ, Lee CY (2005) Epicatechin and catechin in cocoa inhibit amyloid β protein induced apoptosis. J Agric Food Chem 53(5):1445–1448
Kalinski JC, Waterworth S, Siwe Noundou X, Jiwaji M, Parker-Nance S, Krause R, McPhail K, Dorrington R (2019) Molecular networking reveals two distinct Chemotypes in Pyrroloiminoquinone-producing Tsitsikamma favus sponges. Mar Drugs 17:60
Khan H, Amin S, Kamal MA, Patel S (2018) Flavonoids as acetylcholinesterase inhibitors: current therapeutic standing and future prospects. Biomed Pharmacother 101:860–870
Kim JJ, Kang YJ, Shin SA, Bak DH, Lee JW, Lee KB, Yoo YC, Kim DK, Lee BH, Kim DW, Lee J, Jo EK, Yuk JM (2016) PhlorofucofuroKPMol improves glutamate- induced neurotoxicity through modulation of oxidative stress-mediated mitochondrial dysfunction in PC12 cells. PLoS One 11:0163433
Klnc B, Cirik S, Turan G, Tekogul H, Koru E (2013) Seaweeds for food and industrial applications, Food Industry. IntechOpen. https://doi.org/10.5772/53172
Maynard CJ, Cappai R, Volitakis I, Laughton KM, Masters CL, Bush AI, Li QX (2009) Chronic exposure to high levels of zinc or copper has little effect on brain metal homeostasis or Aβ accumulation in transgenic APP-C100 mice. Cell Mol Neurobiol 29:757–767
Misra HP, Fridovich I (1972) The role of superoxide anion in autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Nam T (2011) Lipid peroxidation and its toxicological implications. Toxicol Res 27:1–6
Oboh G, Agunloye OM, Akinyemi AJ, Ademiluyi AO, Adefegha SA (2013) Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem Res 38:413–419
Oboh G, Adewuni TM, Ademosun AO, Olasehinde TA (2016) Sorghum stem extract modulates Na+/K+-ATPase, ecto-5′-nucleotidase, and acetylcholinesterase activities. Comp Clin Pathol 25:749–756
Olasehinde TA, Olaniran AO, Okoh AI (2017) Therapeutic potentials of microalgae in the treatment of Alzheimer’s disease. Molecules 22:480
Olasehinde TA, Mabinya LV, Olaniran AA, Okoh AI (2019a) Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact Carbohydr Diet Fibre:100182
Olasehinde TA, Olaniran AO, Okoh AI (2019b) Aqueous–ethanol extracts of some south African seaweeds inhibit beta-amyloid aggregation, cholinesterases, and beta-secretase activities in vitro. J Food Biochem:e12870
Park C, Cha HJ, Hong SH, Kim GY, Kim S, Kim HS, Kim BW, Jeon YJ, Choi YH (2019) Protective effect of Phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar Drugs 17(4):225
Quéguineur B, Goya L, Ramos S, Angeles M, Mateos R, Bravo L (2012) Phloroglucinol : antioxidant properties and effects on cellular oxidative markers in human HepG2 cell line. Food Chem Toxicol 50:2886–2893
Quirós AR, Lage-Yusty MA, Lopez-Hernandez J (2010) Determination of phenolic compounds in macroalgae for human consumption. Food Chem 121:634–638
Rajauria G (2018) Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J Pharm Biomed Anal 148:230–237
Richetti SK, Rosemberg DB, Ventura-Lima J, Monserrat JM, Bogo MR, Bonan CD (2011) Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotox 32:116–122
Ryu J, Zhang R, Hong BH, Yang EJ, Kang KA, Choi M, Kim KC, Noh S, Kim HS, Le N, Hyun JW, Kim H (2013) Phloroglucinol attenuates motor functional deficits in an animal model of Parkinson's disease by enhancing Nrf2 activity. PLoS One 8(8):e71178
Sheline CT, Behrens MM, Choi DW (2000) Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD + and inhibition of glycolysis. J Neurosci 20:3139–3146
Simos YV, Verginadis II, Toliopoulos IK, Evangelou AM (2012) Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity , in vivo. Redox Rep 17:181–186
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction and dementia. Neurosci 16:435–452
Suganthy N, Devi KP (2016) In vitro antioxidant and anti-cholinesterase activities of Rhizophora mucronata. Pharm Biol 54(1):118–129
Suganthy N, Karutha P, Pandima D (2009) Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: seaweeds inhabiting south Indian coastal areas (Hare Island, Gulf of Mannar). Nat Prod Res 23:355–369
Suganthy N, Karutha Pandian S, Devi K (2010) Neuroprotective effect of seaweeds inhabiting south Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci Lett 468:216–219
Sun J, Zhang X, Broderick M, Fein H (2003) Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 3:276–284
Tan JW, Kim MK (2016) Neuroprotective effects of Biochanin a against β-amyloid-induced neurotoxicity in PC12 cells via a mitochondrial-dependent apoptosis pathway. Molecules 21:548
Tan JW, Tham CL, Daud I, Lee SH, Kim MK (2013) Neuroprotective effects of Biochanin a against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem Res 38:512–518
Tanna B, Mishra A (2018) Metabolites unravel nutraceutical potential of edible seaweeds: an emerging source of functional food. Compr Rev Food Sci Food Saf 17(6):1613–1624
Tanna B, Mishra A (2019) Nutraceutical potential of seaweed polysaccharides: structure, bioactivity, safety, and toxicity. Compr Rev Food Sci Food Saf 18(3):817–831
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases : a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Wang CY, Wang T, Zheng W, Zhao BL, Danscher G, Chen YH, Wang ZY (2010) Zinc overload enhances APP cleavage and Aβ deposition in the Alzheimer mouse brain. PLoS One 5:1–12
Wang L, Hagemann TL, Kalwa H, Michel T, Messing A, Feany MB (2015) Neurodegeneration in Alexander disease. Nat Commun 6:1–13
Wang J, Zheng J, Huang C, Zhao J, Lin J, Zhou X, Naman B, Wang NWG, Wang Q, Yan X, Cui W, He S (2018) Eckmaxol, a Phlorotannin extracted from Ecklonia maxima, produces anti-β-amyloid oligomer neuroprotective effects possibly via directly acting on glycogen synthase kinase 3β. ACS Chem Neurosci 9:1349–1356
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Wright RO, Baccarelli A (2007) Metals and Neurotoxicology. J Nutr 137:2809–2813
Yang E, Ahn S, Ryu J, Choi M, Choi S (2015) Phloroglucinol attenuates the cognitive deficits of the 5XFAD mouse model of Alzheimer ’ s disease. PLoS One 10:1–20
Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J (2009) Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of Manganism 107. Toxicol Sci 107:156–164
Acknowledgements
Authors acknowledge the support of South African Medical Research Council and National Research Foundation of South Africa/The World Academy of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors here declare no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 279 kb)
Rights and permissions
About this article
Cite this article
Olasehinde, T.A., Olaniran, A.O. & Okoh, A.I. Neuroprotective effects of some seaweeds against Zn – induced neuronal damage in HT-22 cells via modulation of redox imbalance, inhibition of apoptosis and acetylcholinesterase activity. Metab Brain Dis 34, 1615–1627 (2019). https://doi.org/10.1007/s11011-019-00469-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00469-2