Abstract
Several studies have examined neonatal diabetes, a rare disease characterized by hyperglycemia and low insulin levels that is usually diagnosed in the first 6 month of life. Recently, the effects of diabetes on the brain have received considerable attention. In addition, hyperglycemia may perturb brain function and might be associated with neuronal death in adult rats. However, few studies have investigated the damaging effects of neonatal hyperglycemia on the rat brain during central nervous system (CNS) development, particularly the mechanisms involved in the disease. Thus, in the present work, we investigated whether neonatal hyperglycemia induced by streptozotocin (STZ) promoted cell death and altered the levels of proteins involved in survival/death pathways in the rat brain. Cell death was assessed using FluoroJade C (FJC) staining and the expression of the p38 mitogen-activated protein kinase (p38), phosphorylated-c-Jun amino-terminal kinase (p-JNK), c-Jun amino-terminal kinase (JNK), protein kinase B (Akt), phosphorylated-protein kinase B (p-Akt), glycogen synthase kinase-3β (Gsk3β), B-cell lymphoma 2 (Bcl2) and Bcl2-associated X protein (Bax) protein were measured by Western blotting. The main results of this study showed that the metabolic alterations observed in diabetic rats (hyperglycemia and hypoinsulinemia) increased p38 expression and decreased p-Akt expression, suggesting that cell survival was altered and cell death was induced, which was confirmed by FJC staining. Therefore, the metabolic conditions observed during neonatal hyperglycemia may contribute to the harmful effect of diabetes on the CNS in a crucial phase of postnatal neuronal development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a common and severe chronic disease characterized by a high blood glucose concentration (hyperglycemia) that occurs either when the pancreatic beta cells do not produce enough insulin or when the body has a defect in insulin action or both, consequently resulting in impaired glucose uptake and storage and reduced utilization of glucose for energy (Wild et al. 2004; Quinn 2001; NCD Risk Factor Collaboration (NCD-RisC) 2016).
Currently, many clinical studies of pediatric patients have assessed neonatal diabetes, a rare condition in preterm babies with low birth weights (Srinivasan et al. 2004; Hays et al. 2006; Flanagan et al. 2007; Suzuki et al. 2007; Aguilar-Bryan and Bryan 2008; Flechtner et al. 2008; Greeley et al. 2010; Sagili et al. 2015; Nansseu et al. 2016). Neonatal diabetes has been linked to numerous complications, such as premature retinopathy, sepsis, severe intraventricular hemorrhage and increased early mortality and morbidity. In the literature, several case reports describe the symptoms, etiology and consequences of hyperglycemia in patients (Hays et al. 2006; Ertl et al. 2006; Garg et al. 2003; Kao et al. 2006). These studies have provided a better understanding of the genes involved in the mechanisms that control glucose homeostasis (Aguilar-Bryan and Bryan 2008). Few studies have investigated the damaging effects of neonatal hyperglycemia on the rat brain during central nervous system (CNS) development. Therefore, a study of the effects of neonatal diabetes on the rat brain may contribute to a better elucidation of these mechanisms.
In addition, the effects of diabetes on the CNS have received considerable attention. According to a recent experimental study with type 1 diabetic rats, neurological damage is a diabetic complication. The diabetic brain exhibits neurological alterations in structure, neurotransmitters, electrophysiology, cognitive function, neuronal density and apoptotic activity (Li and Sima 2004).
Moreover, many proteins respond to the diabetes status. The mitogen-activated protein kinase (MAPK) signaling pathway has been correlated with hyperglycemia-induced injury (Venkatachalam et al. 2008; Soetikno et al. 2012; Evans et al. 2002). Proteins of the MAPK family are important signal transduction mediators and play critical roles in cellular processes, such as cell growth and proliferation, differentiation, survival and apoptosis (Kyosseva 2004; Kyriakis and Avruch 2012). The p38 and JNK proteins are members of the MAPK superfamily that are activated by stress signals and promote many cellular responses, depending on the cell type and stimulus (Hommes et al. 2003; Heidenreich and Kummer 1996; Juretic et al. 2001; Sabapathy 2012). The p38 and JNK signaling pathway is involved in stress-induced apoptosis in many cell lines (Kummer et al. 1997; Dhanasekaran and Reddy 2008). A study of diabetic rats showed activation of p38 and JNK. In addition, the inhibition of both proteins ameliorated cell death in the dorsal root ganglion and sciatic nerve (Cavaletti et al. 2007).
Protein phosphorylation plays an important role in intracellular signal transduction activated by several extracellular stimuli, including insulin and plasma glucose levels. Protein kinase B (PKB/Akt) is a key protein in signal transduction pathways and is activated in response to growth factors or insulin. Akt is thought to contribute to several cellular functions, including nutrient metabolism, cell growth and apoptosis (Hanada et al. 2004; Scheid and Woodgett 2001). Gsk3 is a serine/threonine kinase that is involved in diabetes and neurodegenerative diseases. Downregulation of insulin signaling increases the activity and level of the Gsk3 protein in the skeletal muscle of mice with type 2 diabetes and in the adipose tissue of obese diabetic mice, probably due to both abnormal insulin signaling and hyperglycemia (Nikoulina et al. 2000; Eldar-Finkelman et al. 1999). Gsk3β is also involved in cell survival and apoptosis (Miyashita et al. 2009). Chronically increased glucose levels significantly downregulate Akt expression in retinal glial cells, potentially contribute to high glucose-induced apoptosis (Xi et al. 2005).
Cells under stress activate cell survival and cell death signaling pathways. Cell death signaling converges on the mitochondria, a process that is regulated by the activities of pro- and anti-apoptotic B-cell lymphoma 2 (Bcl2) family proteins. The important roles of Bcl-2 family proteins in regulating apoptosis have been studied for the past decade. Bcl2 proteins family play important roles in stimulating or blocking apoptosis during neuronal development and in response to injury, since they regulate the mitochondrial transmembrane passage of cytochrome C and activate downstream caspase proteins (Anilkumar and Prehn 2014; Youle and Strasser 2008). Therefore, the Bcl2 family acts as key modulators of the intrinsic (mitochondrial) apoptosis pathway (Youle and Strasser 2008). Moreover, a high Bax/Bcl2 ratio promotes increased apoptotic activity (Oltvai et al. 1993).
In summary, neonatal diabetes is a rare metabolic disease characterized by hyperglycemia that occurs in preterm babies and most studies of this condition focus on its etiology, symptoms, and treatments. On the other hand, few studies of neonatal rats have focused on the impact of this disease on the CNS. Based on previous findings, diabetes may lead to neuronal alterations. Thus, the mechanisms by which neonatal hyperglycemia and hypoinsulinemia cause brain damage remain poorly understood. Therefore, the present study aimed to investigate whether neonatal hyperglycemia promoted alterations in the expression of proteins involved in balancing cell survival and death and induced neuronal damage in a crucial period of postnatal brain development.
Materials and methods
Materials
All chemicals reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). Streptozotocin (STZ) solutions were freshly prepared with saline (70% of the final volume) and 20 mM sodium phosphate buffer pH 7.4 (30% of the final volume).
Animals
In this work, we utilized the NIH Guide for the Care and Use of Laboratory Animals (NIH publication # 80–23, revised 1996) as a guide for all experiments. For these experiments, we used five-day-old Wistar rats bred in the Animal House of Universidade Federal do Rio Grande do Sul, and the sample size was calculated based on 80% power at the 0.05 level of significance (MiniTab®). We used both male and female rats because they have not yet developed secondary sexual characteristics at the age in which they were used in the experiments. Animals were housed with dams until sacrifice, with the exception of a brief period of starvation. Dams had free access to water and a 20% (w/w) protein commercial chow (Germani, Porto Alegre, RS, Brazil). Rats were maintained in a room with 12:12 h light/dark cycle (lights on 7:00–19:00 h) and controlled temperature (22 ± 1 °C).
Streptozotocin-induced neonatal hyperglycemia
The STZ-induced neonatal hyperglycemia model was described previously (Rosa et al. 2015). Briefly, 5-day-old Wistar rats were submitted to eight hours of starvation. The neonatal hyperglycemia model was induced by a single intraperitoneal (i.p.) injection of STZ (100 mg/Kg body weight). Controls received vehicle (saline and buffer) instead. Ten hours after the STZ injection, rats received an i.p. injection of glucose (2 mg/g body weight). Finally, rats were sacrificed five days after the STZ injection. Glycemia was assessed by caudal puncture daily. Rats were sacrificed by decapitation, the brains were immediately removed, the olfactory bulb, pons, and medulla were discarded, and the whole brain was utilized for Western blot analyses. For the histochemical analyses and insulin dosage (plasma) experiments, rats were anesthetized (90 mg/Kg Ketamine and 10 mg/Kg xylazine, i.p.) and perfused with saline solution.
Western blot analysis
The brain was homogenized in buffer (20 mmol/L Tris, 150 mmol/L NaCl, 5 mmol/L EDTA, 10% glycerol, 20 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 μL/mL aprotinin and 10 μL/mL leupeptin). The homogenate was centrifuged at 1000 x g for 10 min at 0–4 °C to remove the nuclei and cell debris and the supernatants were utilized for the Western blot assay. We separated one hundred micrograms of protein by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a discontinuous system using a 12% (w/v) separating gel and stacking gel (Laemmli 1970). The proteins separated were electrophoretically transferred to nitrocellulose membranes utilizing a buffer containing 150 mmol/L glycine, 20 mmol/L Tris, pH 8.2, and 20% (v/v) methanol, and 0.1% (w/v) SDS, in a cooled Bio-Rad Trans-Blot unit. Afterwards, membranes were incubated with non-fat milk in Tris buffer for one hour to block non-specific protein-binding sites. Membranes were processed for immunodetection using the antibodies described in Table 1 and signals were revealed using chemiluminescence. We utilized an image densitometer (Imagemaster VDS CI, Amersham Biosciences Europe, IT) to quantitatively analyze the autoradiographs produced. A standard molecular weight marker was used as a reference to determine the molecular weights of the bands (RPN 800 rainbow full range, Bio-Rad, CA, USA).
The tubulin mRNA is expressed at lower levels in peripheral nerves from diabetic patients and animals (McLean 1997). In addition, insulin influences the expression of the GAPDH protein in other tissues, such as adipose tissues and the liver (Alexander et al. 1988). Therefore, the STZ-induced diabetic model presented hyperglycemia and hypoinsulinemia, both of which are capable of influencing housekeeping gene expression, such as tubulin, GAPDH and structural cytoskeletal proteins. Since the levels of housekeeping proteins should be influenced by the treatment or the protocol used in this study, their utilization as a normalizer could compromise the quantification and analysis of the data. Therefore, we used the Ponceau method (Klein et al. 1995), which is not influenced by the STZ diabetic model.
Histochemical processing for FluoroJade C staining
FluoroJade C (FJC) is a fluorochrome used to detect neurons that are undergoing death (Schmued et al. 2005). For this technique, animals were anesthetized and transcardially perfused with saline, and brain tissues were fixed in buffered formalin. Following fixation, tissues were dehydrated in a graded ethanol series and embedded in paraffin. Brain tissues were serially sectioned into 6 μm coronal sections. Slides were sequentially rinsed with 70% ethanol and distilled water for 2 min each and then incubated with a 0.06% potassium permanganate solution for 10 min. Slides were then incubated with a 0.0001% solution of FJC (Histo-Chem, Jefferson, AR, USA) dissolved in 0.1% acetic acid vehicle for 10 min. The slides were then rinsed with distilled water three times for 1 min each. The excess water was drained, and the air-dried slides were then cleared in xylene for 1 min and then coverslipped after addition of Permount media. Using a fluorescence microscope (4000X magnification, Zeiss microscope, Axiostar Plus, Jena, Germany), FJC-positive cells were identified by green fluorescence in the brain. The number of positive cells per slide was quantified by an observer who was blinded to the groups.
Protein determination
Protein concentrations in brain homogenates were determined using bovine serum albumin as a standard (LOWRY et al. 1951).
Statistical analysis
Data are expressed as means ± SEM and were compared using Student’s t-test. All analyses were performed using the GraphPad Prism 5 software on a PC-compatible computer. A value of p < 0.05 was considered statistically significant.
Results
The present study investigated whether neonatal hyperglycemia caused neuronal death using FJC staining, and subsequently investigated the effects of both neonatal hyperglycemia and hypoinsulinemia on the expression of a series of proteins involved in balancing survival and death in the brain.
As proof that the model is valid, a significant increase in glycemia in the neonatal diabetes group (222 ± 29 mg/dL) was observed compared to the controls (122 ± 3 mg/dL) p < 0.01, n = 10. The diabetic animals presented reduced insulin plasma levels (approximately five-fold lower) compared to control animals, p < 0.01, n = 10 (data not shown). Furthermore, the diabetic animals showed reduced body weights (21 ± 0.9 g) compared to control animals (17 ± 0.4 g) p < 0.001, n = 10.
Firstly, rats with STZ-induced diabetes exhibited a diminished brain weight (182 ± 17 mg) compared to the control group (270 ± 15 mg) p < 0.01, n = 10. Therefore, we evaluated the effect of neonatal hyperglycemia on neuronal cell death using FJC staining. Figure 1a depicts a representative image from the control group, in which few cells in each slide were positive for FJC (0.75 ± 0.5) compared to the diabetic group. Figure 1b shows a representative image from the hyperglycemic group, where a 3-fold increase in the number of dying cells per slide was observed (2.25 ± 0.95, p < 0.05), revealing an increase in cell death throughout the tissue. Figure 1c shows the results from the quantitative analysis of both groups. Therefore, cell death occurred in the hyperglycemic rat brain, and we proposed to verify which pathways were involved in neuronal cell death.
Effect of STZ-induced neonatal hyperglycemia on cell death, as analyzed by FluoroJade C (FJC) staining. a Representative image of a brain section from a control rat; b representative image of a brain section from a hyperglycemic rat showing an FJC-positive cell (arrow). c Quantification of the number of FJC-positive cells per slide.*p < 0.05 (Student’s t test)
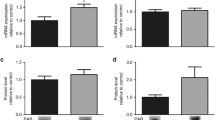
Thus, we investigated the effect of STZ-induced neonatal hyperglycemia on the expression of Bcl2 family proteins, which have complex roles in regulating cell death by apoptosis. As shown in Fig. 2a, neonatal hyperglycemia was not able to alter the expression of the pro-apoptotic protein Bax [t(5) = 0.77; p = 0.47]. However, as shown in Fig. 2b, neonatal hyperglycemia increased the expression of the anti-apoptotic protein Bcl2 [t(5) = 3.893; p = 0.0115].
Next, this study evaluated the effect of neonatal hyperglycemia on the expression of the p-JNK, JNK and p38 proteins, which are involved in the cell death process. Neonatal hyperglycemia did not alter p-JNK (Fig. 3a), JNK (Fig. 3b) and p-JNK/JNK ratio (Fig. 3c) protein expression, respectively (Fig. 3a) [t(7) = 0.6219; p = 0.5537];[t(6) = 2.301; p = 0.0611]; [t(70 = 1.514; p = 0.1738]. However, the expression of the p38 protein was significantly increased in the hyperglycemic group compared to the control group (Fig. 3d) [t(5) = 3.261; p = 0.0224].
Western blot analyses of STZ-induced neonatal hyperglycemia using a p-JNK antibody (a), JNK antibody (b), p-JNK⁄JNK (c) and p38 antibody (d). Results are presented as the means ± SEM (n = 6–7) for independent experiments performed in duplicate; *p < 0.05 and **p < 0.01 compared to the control (Student’s t test)
We also studied the effect of neonatal hyperglycemia on the expression of the Akt and Gsk3β proteins. The expression of the total Akt protein and p-AKT⁄AKT ratio were not altered by neonatal hyperglycemia compared to the control group, respectively (Fig. 4b and c) [t(6) = 1.952; p = 0.0988]; [t(6) = 0.007; p = 0.9941]. However, levels of the p-Akt protein were decreased in the hyperglycemic group compared to the control group (Fig. 4a) [t(6) = 2.671; p = 0.0370]. Furthermore, as shown in Fig. 4d, neonatal hyperglycemia did not alter levels of the Gsk3β protein [t(6) = 0.3430; p = 0.7433].
Western blot analyses of STZ-induced neonatal hyperglycemia using a p-Akt antibody (a), Akt antibody (b) p-AKT⁄AKT (c) and Gsk3β antibody (d). Results are presented as the means ± SEM (n = 7) for independent experiments performed in duplicate; *p < 0.05 and **p < 0.01 compared to the control (Student’s t test)
Discussion
Neonatal diabetes is a rare condition, characterized by hyperglycemia diagnosed before 6 months of life (Aguilar-Bryan and Bryan 2008). Hyperglycemia may induce neuronal damage due to the excessive glucose levels (Tomlinson and Gardiner 2008) and may consequently promote alterations in proteins involved in neuronal survival/death (Kummer et al. 1997; Xi et al. 2005; Yang et al. 2016; Chen et al. 2014). However, a few studies investigate the effects of hyperglycemia in a brain of diabetic neonate rats.
Therefore, in the present work, neonatal hyperglycemia increased the expression of the p38 protein but did not alter the pJNK, JNK and pJNK/JNK ratio protein levels in the brain tissue. In fact, a recent study showed that hyperglycemia activates JNK in endothelial cells leading to apoptosis (Ho et al. 2000). Besides that, in these cells, JNK inhibition blocked hyperglycemia-induced apoptosis (Ho et al. 2000). In our study it is possible that the short period of hyperglycemia induction (five days) could explain the absence of JNK alteration in the brain tissue. However, we cannot exclude the possibility that JNK expression would increase after an extended treatment. In relation to p38, since insulin negatively regulates the expression of these protein in the brain (Kummer et al. 1997), both hypoinsulinemia and hyperglycemia could be responsible for the increase in p38 expression in the brain of diabetic rats, as was previously described (Chen et al. 2014). In fact, according to studies of STZ-treated rats, diabetes significantly increases p38 expression in several brain structures (Cavaletti et al. 2007; Ramakrishnan et al. 2005), and these alterations are reversed by insulin administration to rats (Ramakrishnan et al. 2005). Based on these results, insulin promotes neuronal survival by inhibiting a cell death pathway regulated by p38 (Xia et al. 1995; Ichijo et al. 1997). In view of that, in the present study, insulin deprivation and neonatal hyperglycemia resulted in an increased expression of p38, which is an important regulator of neuronal apoptosis (Xia et al. 1995, Ichijo et al. 1997).
In terms of neuronal survival pathways, the effects of insulin are mediated by the activation of phosphoinositide 3-kinase (PI3K)/Akt signaling pathway (Duarte et al. 2008). In fact, the activation of Akt pathway induced by insulin avoided serum glucose deprivation-induced apoptosis in cells (rat amacrine cells and in the R28 retinal cell line) (Barber et al. 2001; Politi et al. 2001) and inhibited ganglion cell apoptosis after optic nerve transection (Kermer et al. 2000). Besides that, Akt activity is significantly reduced by the high glucose levels observed in the brains of non-malformed diabetic embryos (Kruse et al. 2012). In our study, the diabetic rats presented a decreased in p-Akt levels in the brain tissue when compared with control. Since insulin activates the PI3K/Akt pathway, the brains of diabetic rats may have showed decreased p-Akt levels due to hypoinsulinemia. Consistent with our results, chronically elevated glucose decreased Akt activity in retinal glial Muller cells in vitro and promoted Akt dephosphorylation in vivo (STZ-induced diabetic rats) in a previous study. In addition, the downregulation of Akt-mediated survival signaling is partially responsible for apoptosis induced by high glucose levels (Xi et al. 2005). Therefore, in our study, the Akt-mediated survival signaling downregulation and the p38-mediated death signaling upregulation probably contributed to the glucose-induced neuronal cell death, which was verified by FluroroJade C staining.
Another protein involved in insulin signaling and PI3K/Akt pathway is Gsk3β. In our study, Gsk3β expression was not altered by hyperglycemia during the neonatal period. However, insulin-deficient diabetes significantly decreases the phosphorylation of the insulin receptor, suggesting that Gsk3β activity is increased by phosphorylation (Thomas et al. 2013).
Since apoptotic cell death has consistently been shown to play a significant role in neurotoxicity of diabetes (Zhang et al. 2010; Ye et al. 2011), in the present study, we used FluroroJade C staining to evaluate neuronal cell death. A greater number of FJC-positive cells was observed in the diabetic rats, which indicates an increase in cell death. This result is consistent with studies performed in cell cultures that showed a decrease in brain cell viability induced by high glucose levels (Yang et al. 2016; Yang et al. 2014; Aminzadeh et al. 2014; Liu et al. 2013; Afrazi et al. 2014). Besides the induction of pro-apoptotic proteins expression (Ho et al. 2000), hyperglycemia was recently shown to induce neuronal cell death also due to extracellular ROS generation in the mitochondria via Nox, suggesting that this condition may promote oxidative stress and brain damage (Yang et al. 2016). In addition, ROS produced during oxidative stress activate various signaling pathways, including MAPKs (Torres and Forman 2003; McCubrey et al. 2006; Cargnello and Roux 2011). Therefore, oxidative stress may also contribute to the neurological effects induced by hyperglycemia during this crucial period of postnatal brain development (Rosa et al. 2015).
In order to evaluate proteins related with apoptosis, Blc2 and Bax expression were measured in the brain tissue. Bcl2 plays a role in cellular survival and has also been correlated with the regulation of redox state. In relation to oxidative stress, Bcl2 overexpression protects the cells suppressing lipid peroxidation (Hockenbery et al. 1993), increasing total reduced glutathione and NADPH levels (Esposti et al. 1999; Ellerby et al. 1996; Kane et al. 1993) and increasing the levels of catalase, glutathione reductase and peroxidase (Ellerby et al. 1996). Bcl2 family regulates apoptosis by modulating cytochrome C release and their downstream effects (Green and Reed 1998; Hengartner 2000). Although, in our study, hyperglycemia and hypoinsulinemia reduced cell survival, the increased expression of the anti-apoptotic Bcl2 protein could represent a counter-regulatory response to cell death. Bax expression, however, was not altered in this model. In contrast to our results, studies have showed diminished levels of Bcl2 in neuronal cell cultures exposed to high glucose (Ye et al. 2011; Yang et al. 2014). Another study of adult STZ-induced hyperglycemic rats showed that Bax expression was increased and Bcl2 protein expression was significantly reduced in the cortex and hippocampus (Wu et al. 2012). A possible explanation may be that these results were observed in adult rats, whereas our study utilized neonatal rats. Moreover, neuronal cell death represents a continuous process, and these findings reflect the expression of proteins related to cell survival and cell death at one time point. In addition, Caspase 3 expression was not altered in STZ-induced diabetic adult rats (Guo et al. 2014) and rat embryonic brain in some studies (Kruse et al. 2012). Besides that, it is possible that hyperglycemia-induced neuronal cell death observed in our study was not mediated by the mitochondrial pathway. However, other pathways that also trigger neuronal cell death, such as autophagy, could not be excluded.
Finally, as outlined in Fig. 5, the STZ-induced neonatal hyperglycemia model produces a similar metabolic condition observed in patients with neonatal diabetes, namely, hyperglycemia and hypoinsulinemia. Neonatal diabetes increases p38 and decreases p-Akt levels, both of which may disrupt neuronal survival and proliferation. Finally, neuronal cell death occurs in the brains tissue of rats with neonatal diabetes, as evidenced by FJC staining. Based on these results, we postulate that a counter-regulatory response could explain the increased Bcl2 protein expression observed in subjects with neonatal diabetes, although a significant difference in Bax protein expression was not observed. These effects may suggest possible therapeutic targets and are relevant for a better understanding of this important pathological condition.
Overview of the principal effects of hyperglycemia and hypoinsulinemia on the brains of rats with neonatal hyperglycemia. Hyperglycemia and hypoinsulinemia increased the expression of the p38 protein and decreased the levels of the p-Akt protein, both of which could disrupt neuronal survival and proliferation. Neuronal cell death was verified by FJC staining. Finally, a counter-regulatory response could explain the increased Bcl2 protein expression. p38 - p38 mitogen activated protein kinase; Akt - protein kinase B; Bcl2 - B cell lymphoma 2. The figure was produced using Servier Medical Art (www.servier.com)
References
Global report on diabetes
Afrazi S, Esmaeili-Mahani S, Sheibani V, Abbasnejad M (2014) Neurosteroid allopregnanolone attenuates high glucose-induced apoptosis and prevents experimental diabetic neuropathic pain: in vitro and in vivo studies. J Steroid Biochem Mol Biol 139:98–103. https://doi.org/10.1016/j.jsbmb.2013.10.010
Aguilar-Bryan L, Bryan J (2008) Neonatal diabetes mellitus. Endocr Rev 29(3):265–291. https://doi.org/10.1210/er.2007-0029
Alexander MC, Lomanto M, Nasrin N, Ramaika C (1988) Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proc Natl Acad Sci U S A 85(14):5092–5096. https://doi.org/10.1073/pnas.85.14.5092
Aminzadeh A, Dehpour AR, Safa M, Mirzamohammadi S, Sharifi AM (2014) Investigating the protective effect of lithium against high glucose-induced neurotoxicity in PC12 cells: involvements of ROS, JNK and P38 MAPKs, and apoptotic mitochondria pathway. Cell Mol Neurobiol 34(8):1143–1150. https://doi.org/10.1007/s10571-014-0089-y
Anilkumar U, Prehn JH (2014) Anti-apoptotic BCL-2 family proteins in acute neural injury. Front Cell Neurosci 8:281. https://doi.org/10.3389/fncel.2014.00281
Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW (2001) Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem 276(35):32814–32821. https://doi.org/10.1074/jbc.M104738200
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75(1):50–83. https://doi.org/10.1128/MMBR.00031-10
Cavaletti G, Miloso M, Nicolini G, Scuteri A, Tredici G (2007) Emerging role of mitogen-activated protein kinases in peripheral neuropathies. J Peripher Nerv Syst 12(3):175–194. https://doi.org/10.1111/j.1529-8027.2007.00138.x
Chen J, Guo R, Yan H, Tian L, You Q, Li S, Huang R, Wu K (2014) Naringin inhibits ROS-activated MAPK pathway in high glucose-induced injuries in H9c2 cardiac cells. Basic Clin Pharmacol Toxicol 114(4):293–304. https://doi.org/10.1111/bcpt.12153
Dhanasekaran DN, Reddy EP (2008) JNK signaling in apoptosis. Oncogene 27(48):6245–6251. https://doi.org/10.1038/onc.2008.301
Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC (2008) Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta 1783(6):994–1002. https://doi.org/10.1016/j.bbamcr.2008.02.016
Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG (1999) Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes 48(8):1662–1666. https://doi.org/10.2337/diabetes.48.8.1662
Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE (1996) Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. J Neurochem 67(3):1259–1267
Ertl T, Gyarmati J, Gaál V, Szabó I (2006) Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biol Neonate 89(1):56–59. https://doi.org/10.1159/000088199
Esposti MD, Hatzinisiriou I, McLennan H, Ralph S (1999) Bcl-2 and mitochondrial oxygen radicals. New approaches with reactive oxygen species-sensitive probes. J Biol Chem 274(42):29831–29837. https://doi.org/10.1074/jbc.274.42.29831
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23(5):599–622. https://doi.org/10.1210/er.2001-0039
Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT (2007) Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 56(7):1930–1937. https://doi.org/10.2337/db07-0043
Flechtner I, Vaxillaire M, Cavé H, Scharfmann R, Froguel P, Polak M (2008) Neonatal hyperglycaemia and abnormal development of the pancreas. Best Pract Res Clin Endocrinol Metab 22(1):17–40. https://doi.org/10.1016/j.beem.2007.08.003
Garg R, Agthe AG, Donohue PK, Lehmann CU (2003) Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol 23(3):186–194. https://doi.org/10.1038/sj.jp.7210879
Greeley SA, Tucker SE, Worrell HI, Skowron KB, Bell GI, Philipson LH (2010) Update in neonatal diabetes. Curr Opin Endocrinol Diabetes Obes 17(1):13–19. https://doi.org/10.1097/MED.0b013e328334f158
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281(5381):1309–1312. https://doi.org/10.1126/science.281.5381.1309
Guo YJ, Wang SH, Yuan Y, Li FF, Ye KP, Huang Y, Xia WQ, Zhou Y (2014) Vulnerability for apoptosis in the hippocampal dentate gyrus of STZ-induced diabetic rats with cognitive impairment. J Endocrinol Invest 37(1):87–96. https://doi.org/10.1007/s40618-013-0030-0
Hanada M, Feng J, Hemmings BA (2004) Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta 1697(1–2):3–16. https://doi.org/10.1016/j.bbapap.2003.11.009
Hays SP, Smith EO, Sunehag AL (2006) Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics 118(5):1811–1818. https://doi.org/10.1542/peds.2006-0628
Heidenreich KA, Kummer JL (1996) Inhibition of p38 mitogen-activated protein kinase by insulin in cultured fetal neurons. J Biol Chem 271(17):9891–9894. https://doi.org/10.1074/jbc.271.17.9891
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407(6805):770–776. https://doi.org/10.1038/35037710.
Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY (2000) High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation 101(22):2618–2624. https://doi.org/10.1161/01.CIR.101.22.2618
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ (1993) Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75(2):241–251. https://doi.org/10.1016/0092-8674(93)80066-N
Hommes DW, Peppelenbosch MP, van Deventer SJ (2003) Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 52(1):144–151. https://doi.org/10.1136/gut.52.1.144
Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275(5296):90–94. https://doi.org/10.1126/science.275.5296.90
Juretic N, Santibáñez JF, Hurtado C, Martínez J (2001) ERK 1,2 and p38 pathways are involved in the proliferative stimuli mediated by urokinase in osteoblastic SaOS-2 cell line. J Cell Biochem 83(1):92–98. https://doi.org/10.1002/jcb.1211
Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE (1993) Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262(5137):1274–1277. https://doi.org/10.1126/science.8235659
Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA (2006) Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol 26(12):730–736. https://doi.org/10.1038/sj.jp.7211593
Kermer P, Klöcker N, Labes M, Bähr M (2000) Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 in vivo. J Neurosci 20(2):2–8
Klein D, Kern RM, Sokol RZ (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36(1):59–66
Kruse MS, Barutta J, Vega MC, Coirini H (2012) Down regulation of the proliferation and apoptotic pathways in the embryonic brain of diabetic rats. Cell Mol Neurobiol 32(6):1031–1037. https://doi.org/10.1007/s10571-012-9820-8
Kummer JL, Rao PK, Heidenreich KA (1997) Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem 272(33):20490–20494. https://doi.org/10.1074/jbc.272.33.20490
Kyosseva SV (2004) Mitogen-activated protein kinase signaling. Int Rev Neurobiol 59:201–220. https://doi.org/10.1016/S0074-7742(04)59008-6
Kyriakis JM, Avruch J (2012) Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 92(2):689–737. https://doi.org/10.1152/physrev.00028.2011
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Li ZG, Sima AA (2004) C-peptide and central nervous system complications in diabetes. Exp Diabesity Res 5(1):79–90. https://doi.org/10.1080/15438600490424550
Liu D, Zhang H, Gu W, Liu Y, Zhang M (2013) Neuroprotective effects of ginsenoside Rb1 on high glucose-induced neurotoxicity in primary cultured rat hippocampal neurons. PLoS One 8(11):e79399. https://doi.org/10.1371/journal.pone.0079399
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
McCubrey JA, Lahair MM, Franklin RA (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 8(9–10):1775–1789. https://doi.org/10.1089/ars.2006.8.1775
McLean WG (1997) The role of axonal cytoskeleton in diabetic neuropathy. Neurochem Res 22(8):951–956. https://doi.org/10.1023/A:1022466624223
Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawa H, Hayashi Y, Hamada J, Minamoto T (2009) Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res 15(3):887–897. https://doi.org/10.1158/1078-0432.CCR-08-0760
Nansseu JR, Ngo-Um SS, Balti EV (2016) Incidence, prevalence and genetic determinants of neonatal diabetes mellitus: a systematic review and meta-analysis protocol. Syst Rev 5(1):188. https://doi.org/10.1186/s13643-016-0369-3
NCD Risk Factor Collaboration (NCD-RisC) (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387(10027):1513–1530. https://doi.org/10.1016/S0140-6736(16)00618-8
Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR (2000) Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 49(2):263–271. https://doi.org/10.2337/diabetes.49.2.263
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74(4):609–619. https://doi.org/10.1016/0092-8674(93)90509-O
Politi LE, Rotstein NP, Salvador G, Giusto NM, Insua MF (2001) Insulin-like growth factor-I is a potential trophic factor for amacrine cells. J Neurochem 76(4):1199–1211. https://doi.org/10.1046/j.1471-4159.2001.00128.x
Quinn L (2001) Type 2 diabetes: epidemiology, pathophysiology, and diagnosis. Nurs Clin North Am 36(2):175–192 v
Ramakrishnan R, Kempuraj D, Prabhakaran K, Jayakumar AR, Devi RS, Suthanthirarajan N, Namasivayam A (2005) A short-term diabetes induced changes of catecholamines and p38-MAPK in discrete areas of rat brain. Life Sci 77(15):1825–1835. https://doi.org/10.1016/j.lfs.2004.12.038
Rosa AP, Jacques CE, de Souza LO, Bitencourt F, Mazzola PN, Coelho JG, Mescka CP, Dutra-Filho CS (2015) Neonatal hyperglycemia induces oxidative stress in the rat brain: the role of pentose phosphate pathway enzymes and NADPH oxidase. Mol Cell Biochem 403(1–2):159–167. https://doi.org/10.1007/s11010-015-2346-x
Sabapathy K (2012) Role of the JNK pathway in human diseases. Prog Mol Biol Transl Sci 106:145–169. https://doi.org/10.1016/B978-0-12-396456-4.00013-4
Sagili H, Kamalanathan S, Sahoo J, Lakshminarayanan S, Rani R, Jayalakshmi D, Kumar KT (2015) Comparison of different criteria for diagnosis of gestational diabetes mellitus. Indian J Endocrinol Metab 19(6):824–828. https://doi.org/10.4103/2230-8210.167550
Scheid MP, Woodgett JR (2001) PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2(10):760–768. https://doi.org/10.1038/35096067
Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035(1):24–31. https://doi.org/10.1016/j.brainres.2004.11.054
Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata M, Takagi R, Watanabe K (2012) Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC-MAPK signaling pathway. Eur J Pharm Sci 47(3):604–614. https://doi.org/10.1016/j.ejps.2012.04.018
Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V (2004) Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med 5(4):329–336. https://doi.org/10.1097/01.PCC.0000128607.68261.7C
Suzuki S, Makita Y, Mukai T, Matsuo K, Ueda O, Fujieda K (2007) Molecular basis of neonatal diabetes in Japanese patients. J Clin Endocrinol Metab 92(10):3979–3985. https://doi.org/10.1210/jc.2007-0486
Thomas J, Garg ML, Smith DW (2013) Altered expression of histone and synaptic plasticity associated genes in the hippocampus of streptozotocin-induced diabetic mice. Metab Brain Dis 28(4):613–618. https://doi.org/10.1007/s11011-013-9418-y
Tomlinson DR, Gardiner NJ (2008) Glucose neurotoxicity. Nat Rev Neurosci 9(1):36–45. https://doi.org/10.1038/nrn2294
Torres M, Forman HJ (2003) Redox signaling and the MAP kinase pathways. Biofactors 17(1–4):287–296. https://doi.org/10.1002/biof.5520170128
Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B (2008) Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol 294(5):H2078–H2087. https://doi.org/10.1152/ajpheart.01363.2007
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053. https://doi.org/10.2337/diacare.27.5.1047
Wu KJ, Chen YF, Tsai HY, Wu CR, Wood WG (2012) Guizhi-Fuling-wan, a traditional Chinese herbal medicine, ameliorates memory deficits and neuronal apoptosis in the Streptozotocin-induced hyperglycemic rodents via the decrease of Bax/Bcl2 ratio and Caspase-3 expression. Evid Based Complement Alternat Med 2012:656150–656111. https://doi.org/10.1155/2012/656150
Xi X, Gao L, Hatala DA, Smith DG, Codispoti MC, Gong B, Kern TS, Zhang JZ (2005) Chronically elevated glucose-induced apoptosis is mediated by inactivation of Akt in cultured Müller cells. Biochem Biophys Res Commun 326(3):548–553. https://doi.org/10.1016/j.bbrc.2004.11.064
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326–1331. https://doi.org/10.1126/science.270.5240.1326
Yang RH, Lin J, Hou XH, Cao R, Yu F, Liu HQ, Ji AL, Xu XN, Zhang L, Wang F (2014) Effect of docosahexaenoic acid on hippocampal neurons in high-glucose condition: involvement of PI3K/AKT/nuclear factor-κB-mediated inflammatory pathways. Neuroscience 274:218–228. https://doi.org/10.1016/j.neuroscience.2014.05.042
Yang CM, Lin CC, Hsieh HL (2016) High-glucose-derived oxidative stress-dependent Heme Oxygenase-1 expression from astrocytes contributes to the neuronal apoptosis. Mol Neurobiol 54(1):470–483. https://doi.org/10.1007/s12035-015-9666-4
Ye L, Wang F, Yang RH (2011) Diabetes impairs learning performance and affects the mitochondrial function of hippocampal pyramidal neurons. Brain Res 1411:57–64. https://doi.org/10.1016/j.brainres.2011.07.011
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59. https://doi.org/10.1038/nrm2308
Zhang T, Jia W, Sun X (2010) 3-n-butylphthalide (NBP) reduces apoptosis and enhances vascular endothelial growth factor (VEGF) up-regulation in diabetic rats. Neurol Res 32(4):390–396. https://doi.org/10.1179/016164110X12670144526264
Acknowledgements
This work was supported by research grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Núcleos de Excelência (PRONEX), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosa, A.P., Mescka, C.P., Catarino, F.M. et al. Neonatal hyperglycemia induces cell death in the rat brain. Metab Brain Dis 33, 333–342 (2018). https://doi.org/10.1007/s11011-017-0170-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0170-6