Abstract
Hereditary tyrosinemia type I (HT1) is caused by mutations in the fumarylacetoacetate hydrolase (FAH) gene, the template for the final enzyme in the tyrosine catabolism pathway. If left untreated this deficiency of functional FAH leads to a buildup of toxic metabolites that can cause liver disease, kidney dysfunction and high mortality. The current treatment with the drug NTBC prevents the production of these metabolites and has consequently increased the survival rate in HT1 children. As a result of this increased survival, long term complications of HT1 are now being observed, including slower learning, impaired cognition and altered social behavior. We studied a mouse model of HT1 to gain insight into the effects of HT1 and treatment with NTBC on social behavior in mice. We showed that mice with HT1 display abnormal social behavior in that they spend more time in the absence of another mouse and do not discriminate between a novel mouse and an already familiar mouse. This altered behavior was due to HT1 and not treatment with NTBC. Quantification of cerebral cortex myelin in mice with HT1 showed a two to threefold increase in myelin expression. Our findings suggest that absence of FAH expression in the brain produces an altered brain biochemistry resulting in increased expression of myelin. This increase in myelination could lead to abnormal action potential velocity and altered neuronal connections that provide a mechanism for the altered learning, social behavior and cognitive issues recently seen in HT1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary tyrosinemia type I (HT1, OMIM 276700) is a rare metabolic disorder caused by a deficiency in fumarylacetoacetate hydrolase (FAH, EC 3.7.1.2), which can produce liver failure, hepatocellular carcinoma, renal tubulopathy, and a porphyria-like syndrome (de Laet et al. 2013). FAH is the final enzyme in the tyrosine catabolism pathway and is responsible for the conversion of fumarylacetoacetate (FAA) into acetoacetate and fumarate (Fig. 1). This absence of functional FAH causes a buildup of harmful metabolites such as FAA and malylacetoacetate (MAA), which are both rapidly converted into succinylacetone (SA). The damage caused by the buildup of this toxic metabolite affects the liver, kidneys, and nervous system (Lindblad et al. 1977; Mitchell et al. 1990). Recently, there have been reports of impaired cognition, working memory as well as decreased IQ in children with HT1 in comparison to healthy controls (Bendadi et al. 2014; de Laet et al. 2011; van Ginkel et al. 2016). The current treatment with NTBC prevents the symptoms associated with the buildup of these toxic metabolites (Fig. 1), yet it is unable to halt the neurocognitive impairments that are currently affecting children with HT1. Even though treatment with NTBC has drastically increased the survival rate in children with the disorder (Lindstedt et al. 1992), the resulting long-term cognitive effects of living with HT1 and NTBC treatment have yet to be determined.
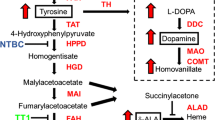
The tyrosine catabolism pathway and current theory of causes for the neurological effects of tyrosinemia. Tyrosine can be ingested from food, or synthesized by the hydroxylation of phenylalanine by phenylalanine hydroxylase (PAH, EC 1.14.16.1). The first step in the breakdown of tyrosine is conversion to 4-hydroxyphenylpyruvate by tyrosine aminotransferase (TAT, EC 2.6.1.5). The second step is conversion into homogentisate, by 4-hydroxyphenylpyruvate dioxygenase (HPD, EC 1.13.11.27) and this is the step that is inhibited by the pharmaceutical 2-[2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione (NTBC). NTBC prevents the breakdown of tyrosine causing hypertyrosinemia. Homogentisate is oxidized to malylacetoacetate by homogentisate dioxygenase (HGD, EC 1.13.11.5) and isomerized by malylacetoacetate isomerase (MAI, EC 5.2.1.2) to form fumarylacetoacetate. The final step of tyrosine metabolism involves the breakdown of fumarylacetoacetate into fumarate and acetoacetate, and is catalyzed by fumarylacetoacetate (FAH, 3.7.1.2). This is the enzyme mutated in HT1. Tyrosine also serves as the basic building block for dopamine and is hydroxylated to L-DOPA by tyrosine hydroxylase (TH, EC 1.14.16.2). In HT1 mutations render FAH non-functional causing malylacetoacetate (MAA) and fumarylacetoacetate (FAA) to accumulate and be converted to the metabolite succinylacetone which has deleterious effects on the liver, kidneys and inhibits myelin synthesis in the peripheral nervous system. FAH is expressed in the brain, but NTBC might show poor transport into the brain and hence may not be inhibiting tyrosine breakdown in the brain. Either increased tyrosine, dopamine, succinylacetone, FAA or other metabolites may be causing a neurological or biochemical change in the brain and are mechanistically causing the slower learning and altered behavior
Social behavior is a complex function that is believed to be integrated by the prefrontal cortex (PFC). Deficits in social behavior, such as those observed in autism spectrum disorder (ASD) and schizophrenia are thought to occur by an imbalance in the excitation/inhibition ratio within the cerebral cortex (Bicks et al. 2015). The maturation of the brain includes neuronal processes such as synapse and network formation, activity-dependent modulation and myelination (Ueda et al. 2015). Cognitive and social functions also rely on proper development and maintenance of myelin, which is a fatty substance made up of proteins and lipids that forms membranous sheets that cover axons (Nave and Werner 2014). These multiple layers of myelin envelop the axon creating electrical insulation for the neuronal impulses (Jahn et al. 2009). Any abnormality in myelination can therefore produce disastrous effects on electrical propagation and illicit neurological impairments. Changes in myelination levels have been proposed to be an overlooked mechanism of synaptic plasticity (Fields 2005; Zatorre et al. 2012) and (Fields 2005; Fields 2008; Nave 2010). Dysmyelination of white matter is now believed to be critical in cognition, learning and social behavior (Filley and Fields 2016) and disorders in white matter may be important in autism (Broek et al. 2014), depression and schizophrenia. Intriguingly, in mice models, either a decrease or an increase in myelination of the prefrontal cortex (PFC) is associated with social withdrawal and a lack of interest in social novelty phenotype (Desbonnet et al. 2014; Makinodan et al. 2012). Hence, it appears that myelin must be of an optimal thickness for normal physiological processes to occur or else dysfunction will result.
Much like humans, mice are a highly social species that display a propensity to engage in social interactions with other mice and show a preference for social novelty (Moy et al. 2004). Here we evaluate the effects of HT1 on social behavior, explorative activity and basic olfaction in mice. We also look for changes in cerebral cortex myelin levels and myelin gene related expression.
Materials and methods
Tyrosinemia type I mice
This Fah1R genetically modified mouse was originally created at Oak Ridge National Laboratories (Aponte et al. 2001). This results in no functional FAH protein expressed in the mouse. We obtained the Fah1RTyrC/RJ mice (Stock# 018129) from Jackson Labs (Bar Harbor, Maine, USA) and from now on we will refer to the Fah1R mice as Fahmut (Hillgartner et al. 2016). Each experimental cohort consisted of three groups, wild-type mice drinking water, wild-type mice drinking NTBC dissolved in water (7.5 mg/L) and HT1 mice Fahmut/Fahmut drinking NTBC dissolved in water (7.5 mg/L). The pregnant dams are treated with NTBC in their drinking water a few days after mating as the Fahmut/Fahmut experimental pups will die within 24 h if they do not receive NTBC from their mother’s breast milk. We tested two cohorts of mice from time separated breedings with a final total of 8–10 animals per group. Mice were weaned and separated at four weeks and behavioral testing was conducted when the mice were between 12 and 16 weeks of age. For more details on mouse breeding and experimental cohorts see Supplemental Methods.
Mice were housed on a 12 h reversed light/dark cycle, housed 2–5 mice per cage, with corn cob bedding (Harlan), fed a normal protein diet mouse chow (Teklad Global 18% Protein Rodent Diet which contained 0.6% tyrosine and 1% phenylalanine) and water ad libitum. NTBC was purchased from Yecuris Corporation (Tualatin, OR). Mouse houses and wheels as well as cotton nesting material were provided for enrichment purposes. The experimental tests were done in a specific order with the Open Field experiment being done first, followed by a 5–7 day break, then the Crawley three-chambered sociability test. All mouse care, breeding and experimental protocols were approved by the UAH IACUC committee.
Locomotion in an open field
Exploratory activity was evaluated in a 10 min test in a grey Perspex Open field 40 cm (L) by 40 cm (W) and (H) 35 cm (Ugo Basile, Varese, Italy). The parameters measured were total distance travelled, time spent in the center zone, number of entries into the center zone and average velocity.
The three-chambered Crawley sociability apparatus
The three-chambered sociability apparatus design was created by Moy et al. (2004), based on the earlier work of Brodkin et al. (2004). The apparatus is 60 (L) × 40 (W) × 22 (H) cm and contains three chambers, a left and right test chamber and a middle transitional chamber (Ugo Basile, Varese, Italy). A mouse can be placed in a cage in either of the test chambers (Cage internal diameter 7 cm, height 15 cm with bars 3 mm diameter and spaced by 7 mm) that allows a test mouse to have visual and sensory interaction with a stranger or familiar prisoner mouse. The stranger mice were unrelated mice of similar age and sex to the test animal that were not siblings and had never been housed together as cagemates. Mice were placed in the center chamber and allowed to explore for 5 min with the doors to the left and right chambers closed. The mouse was then placed in the transport box for 1 min and a stranger mouse and dummy mouse were randomly placed in the left or right chambers, respectively. The doors to the side chambers were then opened and the mouse was given 10 min to explore all three chambers. The mouse was then removed and placed in the transport box again for 1 min while the dummy mouse was replaced with a novel mouse and the previous mouse now became the familiar mouse. The mouse was replaced in the center chamber and given 10 min to explore the three chambers. An additional two cohorts of mice were used for this test as many mice had to be excluded. Exclusions occurred once a mouse discovered that it could climb on top of the prisoner cages and transition to walking around the top rim of the apparatus, voiding the experiment. The social data is analyzed by calculating the time that a mouse spends in each chamber or by calculating an Avoidance/Preference Score (Brodkin et al. 2004). In this analysis, the mice were given a score of +1 for every second they spent in the Dummy mouse side and −1 for every second spent in the Real mouse side. Any time spent in the center chamber was not included in this analysis. Hence a positive score would indicate a preference for the zone without a mouse and indicate social avoidance. A negative score would indicate avoidance of the zone without a mouse, hence would show social behavior. Conversely, in the social novelty experiment, a positive score would indicate a preference to time spent with the Novel mouse and indicate social novelty behavior. A negative score would indicate avoidance of the Novel mouse and more time spent with the Familiar mouse and lack of social novelty behavior. A score close to 0 would indicate an ambivalence of the mouse to spending time with the Novel mouse or Familiar mouse.
Plasma tyrosine assay
Mice were euthanized by exposure to CO2 in a confined chamber and blood was withdrawn by cardiac puncture. Blood was then transferred into a heparinized collection tube and centrifuged at 1500 x g for 5 min. Plasma was removed and frozen at −20 °C until needed. Tyrosine was measured using a colorimetric assay Kit No. K573 (BioVision Inc. (Milpitas, CA) according to manufacturer’s instructions. All plasma from NTBC treated mice was diluted 10 fold with distilled water before analysis. Samples were analyzed in triplicate and averaged before data analysis.
Brain histology and myelin staining
Mice were sacrificed and their brains were quickly removed, rinsed in ice cold PBS and fixed in 10% neutral buffered formalin for 4 days. Whole brains were sliced using a coronal brain slicer (Zivic Instruments, Pittsburgh, PA) into 3–4 mm sections acknowledging the bregma zero location. Tissue sections were then dehydrated and embedded in a paraffin block. The paraffin mounted thick brain slices were then sectioned into 20 μm slices using a rotary microtome Model 820 (American Optical, Buffalo, NY) and allowed to adhere to glass slides. The slides were dried overnight at 37 °C and then stained and mounted under coverslips.
Myelin was stained using the method of Kluver and Barrera (1953). Tissue was incubated with 0.1% Luxol Fast Blue, differentiated using 0.05% Lithium Carbonate and the cell nuclei were counterstained with 0.5% Cresyl Echt Violet (American MasterTech Lodi, CA). Brain slices were then photographed for analysis. Images for quantification were gathered from close to bregma −1. Luxol Fast Blue is a stoichiometric stain for myelin and can be used to quantify myelin in tissue sections (Scholtz 1977). Luxol Fast Blue staining was quantified using Image J software (Schneider et al. 2012). First background was subtracted, then pixel color intensity was averaged over a 250 μm square randomly selected from the cerebral cortex.
RT-PCR quantification of myelin associated gene expression
Mice were euthanized and brains removed and immediately placed in RNAlater® Stabilization Solution (Ambion, Carlsbad, CA). A thin wafer of cerebrum was removed from the pre-frontal cortex and frontal cortex using a razor blade. Tissue was homogenized in RNA isolation buffer and total tissue RNA was isolated using the Qiagen RNeasy® kit (catalog No. 74104). To make cDNA, about 1 μg of total cellular RNA was thawed on ice, and cDNA was synthesized according to the QuantiTect Reverse Transcription Kit protocol (Qiagen catalog No. 205311). The cDNA was then quantified using the Nanodrop spectrophotometer and frozen at −20 °C until use for real-time PCR analysis. Real-time PCR was performed using a 7500 Fast Real-time PCR system (Life technologies™, Carlsbad, CA). RT-PCR was set up in a 96-well plate in a reaction volume of 25 μL per well 2× Fast SYBR® Green DNA polymerase (catalog number 4385617). All oligos were purchased from Qiagen Inc. (Valencia, CA). Oligos were FAH, HPD, MBP, PLP1 and GAPDH. The threshold crossing point values (CT) were linearly correlated with the logarithmic value of the DNA amount. The slope of this line provided the PCR efficiency number for the gene under the given parameters (primers used and PCR-protocol). QuantiTect Primers FAH (QT01061739), HPD (QT00105581), MBP1 (QT00198478), PLP1 (QT00096096) and GAPDH (QT01658692) were used.
To determine the fold change of FAH, HPD, MBP and PLP1 expression over the reference gene GAPDH we used the quantification method and guidelines of Pfaffl (2001). The efficiencies of the primers for all three genes examined in this study was close to 100%, and we simplified our analysis and assigned E target a value of two. The reference gene GAPDH showed constant expression in the three different mouse genotypes, therefore no normalization was needed and a simplified equation could be used, Fold change over SDHA = (Etarget)ΔCt(ref-target). The threshold crossing RT-PCR cycle (Ct) for reference and target genes were obtained from the RT-PCR cycler software.
Data acquisition and analysis
Data were filmed, recorded and analyzed using Ethovision XT 9.0 Software (Noldus Information Technology Inc., Leesburg, VA). Mouse movement was tracked using the geometric animal center or three-point tracking using the nose, center and tail base. Mouse movement was detected using either static or dynamic subtraction with a data sampling of 30 times per second. Data were exported as a spreadsheet and plotted using Prism 6.0 software (GraphPad Software Inc., La Jolla, CA).
Statistical analysis
These data are presented in the results as mean ± SEM unless otherwise indicated. Three way comparisons were made using a one-way ANOVA, with P < 0.05 considered significant. Intergroup analyses were performed using Tukey’s multiple comparisons test, again with P < 0.05 considered significant. Two-way comparisons were made using a two-tailed Student’s t test with P < 0.05 considered significant. All data were plotted and statistics analyzed using Prism 6.0 software.
Results
Mouse plasma biochemistry and tissue localization of FAH gene expression
Plasma tyrosine levels were measured (Fig. 2a) and were 164.7 ± 21.4 μmol/L in WT-water mice (n = 5), and increased more than 10 fold when NTBC was added to the drinking water (P < 0.0001). Plasma tyrosine was 1846.0 ± 42.4 μmol/L (n = 3) in WT-NTBC mice and was 1905.0 ± 85.0 μmol/L (n = 3) in FAHmut-NTBC mice. There was no difference in the plasma tyrosine levels between the FAHmut disease mouse group and the WT-NTBC group of mice treated with NTBC in their drinking water (Fig. 2a, P = 0.5676). Succinylacetone was undetectable in the urine of WT-water, WT-NTBC or FAHmut-NTBC mice compared to a succinylacetone standard using GC-MS on trimethylsilane derivatized samples (data not shown). RT-PCT was used to test for the presence of FAH in liver, kidney and brain in WT-water mice (Fig. 2b). FAH was most highly expressed in liver about 30.07 ± 0.60 (n = 3) fold relative to GAPDH. This was about 50% higher than in the kidneys which was 20.23 ± 2.04 (n = 3). We were able to detect FAH in the cerebral cortex in mice and it was expressed at 1.18 ± 0.18 (n = 3) fold relative to GAPDH or about 25 fold less than in the liver. We looked for HPD (4-hydroxyphenylpyruvate dioxygenase) expression in the cerebral cortex. HPD is another enzyme in the tyrosine catabolism pathway and the target for NTBC (Fig. 1). HPD was expressed at 0.17 ± 0.02 (n = 3) relative to GAPDH. This was calculated from a raw threshold cross number (CT) average of 28.2 which is on the threshold of reliable expression detection, leading us to conclude that HPD may not be expressed in the cerebral cortex of mouse brain (Fig. 2b).
Mouse plasma biochemistry and FAH gene expression. a Plasma tyrosine levels were 164.7 ± 21.4 μmol/L in WT-water mice (n = 5). Plasma tyrosine was about 10 fold higher in both groups of NTBC treated mice (P < 0.0001). Plasma tyrosine was 1846.0 ± 42.4 μmol/L (n = 3) in WT-NTBC mice and 1905.0 ± 85.0 μmol/L (n = 3) in FAHmut-NTBC mice. b The FAH gene was most highly expressed in liver at about 30.07 ± 0.60 (n = 3) fold relative to GAPDH. The kidneys expressed at 20.23 ± 2.04 (n = 3) fold over GAPDH. FAH expression was detected in the cerebral cortex in mice and it was 1.18 ± 0.18 (n = 3) fold relative to GAPDH. HPD was expressed at 0.17 ± 0.02 (n = 3) relative to GAPDH in the mouse cerebral cortex
Mice with HT1 avoid social contact with other mice and do not discriminate between a novel and familiar mouse
Wild-type mice drinking water (WT-water) spent equal amounts of time in a chamber containing no mouse (dummy mouse) as the side containing a real mouse (Fig. 3a). WT-water mice spent 201.1 ± 20.07 s (n = 8) with the dummy mouse and 222 ± 16.67 s (n = 8) with the real mouse (P = 0.4356). The WT-NTBC mouse also did not discriminate between the two choices, spending 186.7 ± 22.46 s (n = 9) with the dummy mouse and 250.7 ± 35.51 s (n = 9) with the real mouse (P = 0.1468). However, the FAHmut-NTBC mice spent close to twice as much time in the empty chamber with the dummy mouse (Fig. 3a) 283.1 ± 24.75 s (n = 10) compared to the side containing the real mouse 148.1 ± 23.25 s (n = 10, P = 0.0009).
Mice with tyrosinemia type I are socially withdrawn and show no social novelty preference. a Wild-type mice drinking water (WT-water) and WT-mice drinking NTBC water (WT-NTBC) spent equal amounts of time in the chambers containing no mouse (dummy mouse) as the real mouse (P = 0.4356 and P = 0.1468, respectively). However, the FAHmut-NTBC mice spend close to twice as much time in the chamber without a mouse 283.1 ± 24.75 s (n = 10) compared to the side containing the real mouse 148.1 ± 23.25 s (n = 10, P = 0.0009). b In the social novelty test, both the WT-water and the WT-NTBC mice spent more time in the company of the novel mouse. WT-water mice spent 223.4 ± 16.58 s (n = 8) with the novel mouse and 157 ± 16.34 s (n = 8) with the familiar mouse (n = 8, P = 0.0147). The WT-NTBC mice spent 259 ± 20.58 s (n = 9) with the novel mouse and 153.7 ± 19.34 s (n = 9) with the familiar mouse (n = 10, P = 0.0018). The FAHmut-NTBC mice did not discriminate between novel and familiar mice and spent equal amounts of time with each mouse, 204 ± 12.51 s (n = 10) with the novel mouse and 195.3 ± 19.55 s (n = 10) with the familiar mouse (P = 0.7126). c An example of the trail taken by a FAHmut-NTBC mouse is shown as it spends 10 min investigating the three chambers with no mouse (dummy mouse) on the left side (D) and an unknown real mouse (R) on the right side. The mouse with HT1 clearly spends more time in the chamber without a real mouse. d An example of a 10 min trail from a WT-NTBC mouse is shown from a social novelty experiment. The WT-NTBC mouse spends more time investigating the novel mouse (N) compared to the familiar mouse (F)
In the social novelty test, both the WT-water and the WT-NTBC mice spent more time in the company of the novel mouse (Fig. 3b). WT-water mice spent 223.4 ± 16.58 s (n = 8) with the novel mouse and 157 ± 16.34 s (n = 8) with the familiar mouse (P = 0.0147). The WT-NTBC mice spent 259 ± 20.58 s (n = 9) with the novel mouse and 153.7 ± 19.34 s (n = 9) with the familiar mouse (P = 0.0018). The FAHmut-NTBC mice did not discriminate between novel and familiar mice (Fig. 3b) and spent equal amounts of time with each mouse, specifically 204 ± 12.51 s (n = 10) with the novel mouse and 195.3 ± 19.55 s (n = 10) with the familiar mouse (P = 0.7126).
The WT-NTBC are the control group for any effects of HT1 on behavior so we compared the two treated groups of mice. In the social experiment (Fig. 3a) FAHmut-NTBC mice spent about 50% more time in the zone without a mouse 283.1 ± 24.75 s (n = 10) than the WT-NTBC mouse 186.7 ± 22.46 s (n = 9, P = 0.0108). The WT-NTBC mice (Fig. 3a) spend about 70% more time in the zone with the real mouse 250.7 ± 35.51 s (n = 9) than the FAHmut-NTBC group of mice 148.1 ± 23.25 s (n = 10, P = 0.0245).
In the social novelty experiment (Fig. 3b) the WT-NTBC group of mice spent more time in the zone with the novel mouse 259 ± 20.58 s (n = 9) compared to the FAHmut-NTBC mice 204 ± 12.51 s (n = 10, P = 0.0319). There was no difference in mean times spent by either group of drug treated mice in the zone with the familiar mouse (Fig. 3b). WT-NTBC mice spent 153.7 ± 19.34 s (n = 9) and FAHmut-NTBC mice spent 195.3 ± 19.55 s (n = 10, P = 0.1500) with the familiar mouse.
An example of the trail taken by an FAHmut-NTBC mouse is shown (Fig. 3c) as it spends 10 min investigating the three chambers with the dummy mouse on the left side (D) and an unknown real mouse (R) on the right side. The mouse with HT1 clearly avoids contact with the real mouse and spends more time in the chamber without a mouse. For the social novelty experiment a WT-NTBC mouse trail is shown (Fig. 3d). The WT-NTBC mouse spends more time investigating the novel mouse (N) than the familiar mouse (F). Examples of mouse trails taken by the WT-water and WT-NTBC mice in the social behavior experiment (Fig. 3a) can be found in the Supplemental materials (Supplemental Fig. 1). Examples of mouse trails taken by the WT-water and FAHmut-NTBC mice in the social novelty experiment (Fig. 3b) can be found in the Supplemental materials (Supplemental Fig. 2).
These data are better explained using an avoidance/preference score (Fig. 4a). In the social behavior test (Fig. 4a, left side), the WT-water mice and the WT-NTBC avoided the no mouse side (dummy mouse) and preferred the real mouse side with a score of −20.94 ± 29.99 s (n = 8) and −64.07 ± 53.93 s (n = 9), respectively. The WT groups of mice drinking water or water containing 7.5 mg/L NTBC showed similar behavior and were not different from each other (P = 0.5103). However, the FAHmut-NTBC mice spent 135.1 ± 43.69 s (n = 10) more time in the no mouse side (dummy mouse) than the real mice side and this is different from both the WT-water mice (P = 0.0131) and the WT-NTBC mice (P = 0.0101) groups.
Mice with tyrosinemia type I avoid other mice but show normal exploratory behavior. a The three groups of mice were compared in an avoidance/preference score plot. The WT-water and the WT-NTBC mice both avoided the dummy mouse and preferred the real mouse and were not different from each other (P = 0.5103). However, the FAHmut-NTBC mice spend 135.1 ± 43.69 s (n = 10) more time with the dummy mouse than the real mouse and this is different from both the WT-water mice (P = 0.0131) and the WT-NTBC mice (P = 0.0101). In the social novelty test the WT-water and WT-NTBC mice behaved very similar to one another (P = 0.7599) and spent more time with the novel mouse. The FAHmut-NTBC mice did not discriminate between the novel and familiar mouse which was different from both the WT-water (P = 0.0375) and WT-NTBC (P = 0.0188) mice, respectively. b There was no difference in the basic exploratory behavior (distance travelled) between the three groups of mice during the 5 min habituation to the apparatus where the mouse only had access to the center zone (F (2, 24) = 1.073, P = 0.3578). There was also no difference in the distance travelled between the three groups of mice in the 10 min social behavior and social novelty experiments, both within a group (P = 0.7510 for WT-water, P = 0.4073 for WT-NTBC and P = 0.8279 for FAHmut mice) and between all three groups of mice in either of the two social behavior trials (F (5, 48) = 1.269, P = 0.2927). The data shown is for the habituation experiment (Hab), the dummy-real experiment (D/R) and the novel-familiar experiment (N/F). c There was no difference in zone entries between any of the three experimental mouse groups (WT-water, WT-NTBC and FAHmut) in any of the four social experiments (F (11, 96) = 0.8485, P = 0.5927)
In the social novelty test (Fig. 4a, right side) the WT-water and WT-NTBC mice behaved very similar (P = 0.7599) and spent 90.7 ± 32.9 s (n = 8) and 105.3 ± 33.2 s (n = 9) more time with the novel mouse. While the FAHmut-NTBC mice did not discriminate between the novel and familiar mouse spending only 8.7 ± 18.9 s (n = 10) more with the novel mouse, which is different from both WT-water (P = 0.0375) and WT-NTBC (P = 0.0188) mice respectively.
These data could be explained by confounding factors such as altered motor behavior and exploratory interest. We measured the total distance travelled (Fig. 4b) and number of zone entries (Fig. 4c) in the three groups of mice. There was no difference in the basic exploratory behavior of the three groups of mice during the 5 min habituation to the apparatus where the mouse only had access to the center zone (F (2, 24) = 1.073, P = 0.3578). The WT-water mice travelled 1568 ± 168 cm (n = 8), the WT-NTBC mice travelled 1308 ± 89 cm (n = 9) and the FAHmut-NTBC mice travelled 1535 ± 143 cm (n = 10). There was no difference between the distance travelled within a group of mice in the social behavior and the social novelty experiments (Fig. 4b), with P = 0.7510 for WT-water, P = 0.4073 for WT-NTBC and P = 0.8279 for FAHmut-NTBC mice. There was also no difference in the total distance traveled between the three groups of mice in either of the two social behavior trials (F (5, 48) = 1.269, P = 0.2927).
There was no difference in zone entries between any of the three experimental mouse groups WT-water, WT-NTBC and FAHmut-NTBC (Fig. 4c) in any of the 4 social experiments (F (11, 96) = 0.8485, P = 0.5927). Taking FAHmut-NTBC as an example, there was no difference in the number of zone entries between Dummy (22.1 ± 4.1), Real (16.9 ± 5.5), Novel (18.5 ± 2.7) or Familiar (18.5 ± 3.5) zones in the mice social behavior studies (F (3, 36) = 0.2889, P = 0.8331, n = 10).
In summary, mice with HT1 show abnormal social behavior compared to their WT littermates. In a three-chambered social choice experiment, the mice with HT1 spent more time in the zone without a mouse and did not discriminate between a novel mouse and a familiar mouse. This behavior correlates with the FAH mutation and not treatment with NTBC, as WT-NTBC mice behaved identically to WT mice drinking water in every aspect. There was no difference in the total distance travelled or the number of zone entries between the three mouse groups suggesting that the variable contributing to the differences is the amount of time the mouse spends in its preferred zone.
Mice with tyrosinemia type I show normal exploratory activity in an open field
The three groups of mice were tested for motor and exploratory activity in a 50 cm × 50 cm grey Perspex open field (Fig. 5). There was no difference in the distance traveled in 10 min (Fig. 5a) by the three groups of mice in the open field (F (2, 23) = 0.7031, P = 0.5054). WT-water mice travelled 4508 ± 171 cm (n = 10), WT-NTBC mice travelled 4954 ± 332 cm (n = 8) and FAHmut-NTBC mice travelled 4894 ± 395 (n = 8). There was also no difference between the three groups of mice in the percentage of time spent in the center zone (F (2, 23) = 0.9332, P = 0.4077). WT-water mice spent 13.5 ± 1.3% (n = 10), WT-NTBC mice spent 10.8 ± 1.2% (n = 8) and FAHmut spent 11.4 ± 2.0% (n = 8) of the time in the center zone (Fig. 5c). An example of the trail a WT-water and a FAHmut-NTBC mouse takes is shown in Fig. 5b and Fig. 5d respectively.
Tyrosinemia type I mice travel the same distance and show similar exploration in an open field. a There was no difference in the distance traveled in 10 min between the three groups of mice in the open field (F (2, 23) = 0.7031, P = 0.5054). c There was also no difference between the three groups of mice in the percentage of time spent in the center zone (F (2, 23) = 0.9332, P = 0.4077). An example of the trail that a WT-water mouse and an FAHmut-NTBC mouse takes is shown in b and d respectively. Only 5 min of trace is shown for clarity. e There was no difference in the number of entries into the center zone between the three groups of mice F (2, 23) = 0.828, P = 0.4495. f The mean velocity of the three groups of mice was also similar F (2, 23) = 2.061, P = 0.1502
Mice with tyrosinemia type I have equivalent exploratory activity, velocity and gross olfactory ability
There was no difference in the number of entries into the center zone between the three groups of mice F (2, 23) = 0.828, P = 0.4495 (Fig. 5e) with WT-water crossing 50.9 ± 3.9 times, WT-NTBC 49.1 ± 2.8 times and FAHmut-NTBC crossing 56.1 ± 4.5 times. The mean velocity of the three groups of mice was also similar (Fig. 5f) with WT-water travelling at 7.5 ± 0.3 cm·s−1, WT-NTBC moving at 8.2 ± 0.6 cm·s−1 and FAHmut-NTBC mice trotting at 8.7 ± 0.4 cm·s−1 (F (2, 23) = 2.061, P = 0.1502). Mice olfaction is one critical aspect of behavior, and is responsible for social bonding and recognition of kin and predators (Yang and Crawley 2009). In order to determine that any social deficit was not a result of olfactory impairments, mice were evaluated through the buried food test (Supplemental Material).
Mice with tyrosinemia type I have increased myelin staining and increased expression of myelin related genes in the brain
The mice brains were divided into 3–4 mm sections and the region from around bregma −1 was further trimmed into 20 μm slices and stained with Luxol Fast Blue to look for gross anatomical changes in the distribution of myelin. All three groups of mice were stained concurrently in the same slide cassette to control for minor differences in procedures or stain efficacy. It was immediately apparent that more intense myelin staining was observed in the FAHmut-NTBC mice compared to WT-NTBC mice (Fig. 6a). The diffuse increase in Luxol Fast Blue staining can be seen more easily in a 10× magnification of the cerebral cortex (Fig. 6a, lower panel). To quantify Luxol Fast Blue staining, mean pixel intensity was calculated for a 250 μm square taken close to the lateral boundary of the cerebral cortex (Fig. 6b). Pixel color intensity was measured as 47.63 ± 8.35 (n = 3) for WT-water, 28.46 ± 5.93 (n = 3) for WT-NTBC and was about four times more intense in FAHmut-NTBC mice (117.90 ± 11.11, n = 4, F (2, 7) = 26.31, P = 0.0006). There was no difference between both the wild-type mouse groups (P = 0.4137), but FAHmut-NTBC was different from WT-water mice (P = 0.0028) and WT-NTBC mice (P = 0.0007).
Mice with tyrosinemia type I show increased myelin expression in the cerebral cortex. a The upper panel shows Luxol Fast Blue stained 20 μm coronal brain slices from a WT-NTBC mouse (left) and an FAHmut-NTBC mouse (right). The brain slices from the FAHmut mice stained more intensely for myelin. The slices are from close to bregma −1 and the scale bar is 1 mm. The bottom panels shows 10× magnified regions taken from the cerebral cortex close to the tissue edge and the scale bar indicates 100 μm. Increased Luxol Fast Blue myelin staining was observed in the FAHmut-NTBC mouse brain compared to WT-NTBC. b Pixel color intensity was measured in a 250 μm square from the cerebral cortex of a WT-water 47.6 ± 8.4 (n = 3), a WT-NTBC 28.5 ± 5.9 (n = 3) and a FAHmut-NTBC 117.9 ± 11.1 (n = 4) mouse. c The levels of two myelin related protein gene transcripts were measured in the cerebral cortex relative to GAPDH. MBP levels were about 3-fold higher in the FAHmut-NTBC mice over the WT-NTBC mice 18.43 ± 2.11 (n = 4) vs 54.16 ± 10.54 (n = 4, P = 0.0159). PLP1 expression levels in FAHmut-NTBC mice were also increased over WT-NTBC mice 9.96 ± 1.85, (n = 4) vs 21.32 ± 4.51 (n = 4). The P value was P = 0.0586 but in this case we consider this different
We also performed RT-PCR on cDNA isolated from the cerebral cortex to quantify the message for two myelin associated proteins, myelin basic protein (MBP) and proteolipid protein 1 (PLP1), using GAPDH as a reference (Fig. 6c). MBP levels were about 3-fold higher in the FAHmut-NTBC mice over the WT-NTBC mice 18.43 ± 2.11 (n = 4) vs 54.16 ± 10.54 (n = 4, P = 0.0159). PLP1 expression levels in FAHmut-NTBC mice were also increased over WT-NTBC mice 9.96 ± 1.85, (n = 4) vs 21.32 ± 4.51 (n = 4, P = 0.0586).
In summary, mice with HT1 show abnormal social behavior in that they avoid the presence of other mice and do not show a social novelty preference with an unknown mouse. Their basic motor and exploratory behavior and olfactory sense is unchanged from wild-type mice drinking water and mice treated with NTBC. Brain histochemistry in HT1 mice (FAHmut-NTBC) showed a two to threefold more intense staining for myelin which agreed with quantitative RT-PCR measurements for myelin related protein genes. Hence, the absence of the FAH enzyme in these mice with HT1 is causing an alteration in brain myelin composition with associated changes in social behavior that is independent of treatment with NTBC.
Discussion
The exact cause of the cognitive decline and impaired learning, memory and social cognition observed in children with HT1 remains undetermined (Bendadi et al. 2014; Masurel-Paulet et al. 2008; Thimm et al. 2012; van Ginkel et al. 2016). Our previous study on long term memory and learning in mice suggests that these slower learning issues were caused by the pathophysiology of the disease itself and not by treatment with NTBC (Hillgartner et al. 2016). In order to evaluate the mouse as a model for social behavior differences in children with HT1, we studied social behavior in mice with HT1 using Crawley’s three-chambered sociability chamber (Moy et al. 2004).
Our key observations were that mice with HT1 (FAHmut) displayed abnormal social behavior by spending twice as much time in a zone without a mouse, compared to a zone containing a mouse. The FAHmut mice also showed no preference for a novel mouse over a familiar mouse. Upon gross histological examination of the brain, mice with HT1 showed increased myelin staining of the cerebral cortex compared to WT-NTBC mice. Although NTBC causes hypertyrosinemia, increased tyrosine transport into the brain and altered neurotransmitter levels, wild-type mice on long-term NTBC treatment display no alterations in brain myelin levels or changes in sociability compared to wild-type mice drinking only water. These findings provide evidence that these social deficits in the FAHmut mice result from the absence of functional FAH protein expression and not from NTBC treatment.
We finished our previous study by proposing that the altered learning in the Barnes maze could be explained by inhibition of myelin synthesis by buildup of toxic tyrosine breakdown products, specifically succinylacetone in the brain. This would result in a hypomyelination of the brain and altered neuronal connectivity explaining slower learning (Hillgartner et al. 2016). However, coronal brain slices stained for myelin showed more intense staining with Luxol Fast Blue, a quantitative stain for myelin (Scholtz 1977). The brain slice staining was performed concurrently with RT-PCR on frontal lobe cortex for the myelin related genes MBP and PLP1. Both assays were in agreement and showed a two to threefold increase in expression of myelin and of myelin protein related genes in the cerebral cortex of mice with HT1. We did not perform electron microscopy to determine the location of the myelin, so we cannot use the term “increased myelination” to describe our data although we will assume that the increased myelin expression in the cerebral cortex correlates to increased layers of myelin wrapped around axons. However, multiple genetic modifications in mice that target the myelin synthesis pathway have been found to alter myelin expression, and cause hypomyelination or hypermyelination, and change the layers of myelin wrapped around neurons (Flores et al. 2008; Fraser et al. 2008; Kwon et al. 2006; Yang et al. 2016). Hence, the process of myelination appears to be a robust basic biological process and our assumption of hypermyelination is not unreasonable.
In a recent RNA-Seq analysis of mouse brain cell types, it was found that the message for the enzyme HPD, the target of NTBC, was not detected in the brain (Zhang et al. 2014). Our previous concerns as to the efficiency of NTBC transport into the brain are now irrelevant, as even if NTBC crosses the blood brain barrier, there is no target to inhibit the tyrosine breakdown (Hillgartner et al. 2016). In fact, several of the early enzymes in tyrosine catabolism have undetectable expression in many brain cells, including astrocytes, neurons and oligodendrocytes (Zhang et al. 2014). This makes sense as the brain has evolved for a higher purpose and has left the toxic job of tyrosine breakdown to the liver. This begs us to ask the question of what the role of FAH is in the brain, if it is not functionally present to participate in tyrosine breakdown? In the rat brain, FAH has been shown at the protein level to be predominantly expressed in myelinating oligodendrocytes (Labelle et al. 1993) and we propose that FAH has an as of yet unrecognized role in the metabolism of myelin in mouse oligodendrocytes (Fig. 7).
A Schematic representation of our theory of altered social behavior in mice with tyrosinemia type I. The brain has evolved for higher purposes and does not participate in the catabolism of tyrosine (center pathway). However, the brain does synthesize dopamine and other neurotransmitters from tyrosine. The target of NTBC, HPD (4-hydroxyphenylpyruvate dioxygenase), is not expressed in the brain, hence NTBC will have no direct effect on brain function and does not inhibit tyrosine breakdown in the brain (center pathway, bottom). However, FAH is expressed in the brain and this raises the possibility that FAH in the brain has different substrates and a different role in brain biochemistry (center pathway, bottom). Due to its higher expression in oligodendrocytes, we propose that FAH has a role myelin synthesis, breakdown or turnover. The absence of functional FAH seen in HT1 could prevent the breakdown of an unknown metabolite, either of which could be the cause of increased myelin synthesis or decreased myelin breakdown (right panel). The HT1 mice have increased cerebral cortex myelin and by an as of yet undiscovered mechanism have altered social behavior
There is very little known about the effect of hypermyelination on behavior and brain function, but the few observations that have been published show alterations in mouse social behavior. Germ free mice without intestinal microbiota also show socially abnormal behavior including social avoidance and lack of preference for a novel mouse (Desbonnet et al. 2014). A follow up study from the same group showed that these mice had increased myelination of neurons in the prefrontal cortex (Hoban et al. 2016). The germ free mice showed a coordinated upregulation of gene expression in the PFC for pathways involved in myelin synthesis including MBP and PLP1 expression. The authors hypothesized that this could be due to increased neuronal activity or increased myelinogenesis caused by the absence of bacterial metabolites. Interestingly, in young mice socially isolated for 3–4 weeks the thickness of the myelin wrap on PFC neurons was reduced. The number of oligodendrocytes was not altered, but they showed altered morphology with fewer and shorter processes with a lower expression of myelin related proteins MBP and MAG (Makinodan et al. 2012). These mice showed decreased social exploration of other mice and deficits in working memory. Adult mice also deprived of social contact show hypomyelination and display altered social behavior, including social avoidance and lack of preference for social novelty (Liu et al. 2012). The regulation of myelination clearly remains dynamic throughout life (Bercury and Macklin 2015; Snaidero and Simons 2014) and is critical for normal cognition and social behavior. Neuronal circuits must develop properly and maintain the appropriate thickness of myelin sheaths to ensure accurate and efficient propagation of electrical signals (Seidl 2014).
How can decreasing or increasing neuronal myelin thickness produce the exact same changes in social behavior? The simplest hypothesis would be that axons and oligodendrocytes have a close relationship and send signals to each other to regulate the level of myelin to be at the optimum thickness either for efficient nerve conduction or for the optimum velocity for the processes that are dependent on it (Seidl 2014). This is thought to occur via signal molecule release from the axons that target receptors on the oligodendrocytes or from the actual electrical activity of the axons that may cause induction of myelination in the central nervous system (Demerens et al. 1996; Macklin 2010; Wake et al. 2011). The ratio of axon to myelin is termed the g-ratio, and is represented by axon diameter (d)/fiber diameter (D). G-ratio has evolved to be the most efficient at a value of 0.6 to 0.7 (Chomiak and Hu 2009). If the myelin is too thin, excessive current leakage may slow the action potential. In the situation of hypermyelination and increased thickness of the myelin layer, the other components of the system must then alter their volume to compensate for this compact closed system (Chklovskii et al. 2002). Hence the reduction in axonal volume and width will increase electrical resistance and also decrease the velocity of the action potential. It then follows that if the g ratio is greater or less than the optimum value of 0.6–0.7, then nerve conduction will be slower. This can explain the apparent paradoxical observation that both hypomyelination (an increase of g ratio) and hypermyelination (a decrease of g ratio) of nerves in the cerebral cortex can potentially contribute to abnormal social behavior in mice. (Chomiak and Hu 2009; Rushton 1951; Smith and Koles 1970). Myelin water fraction imaging with magnetic resonance imaging has been shown to correlate well with g ratios in mouse models of hypo- and hypermyelination (West et al. 2017). Myelin water fraction measurements also correlate well with Luxol fast blue staining optical densitometry analysis of brains from MS patient cadavers (Laule et al. 2006).
Worse neurocognitive outcomes have been observed in children with HT1 that start treatment with NTBC earlier (García et al. 2017) and lower IQ is negatively associated with a longer duration of NTBC treatment (van Ginkel et al. 2016). This could suggest an effect of NTBC on these outcomes. The high plasma tyrosine levels produced by NTBC treatment could also be contributing to neurocognitive outcomes by competing for transport of other amino acids into the brain, causing alterations in brain neurotransmitter levels or other brain biochemical processes. Our data show that increased tyrosine levels and NTBC treatment do not in themselves alter behavior in a healthy mouse, however increased plasma tyrosine levels may contribute to or magnify the behavioral differences that we observe in the mice with HT1. Our mice were fed a normal protein diet and the behavior of HT1 mice may be different on a zero tyrosine/low phenylalanine diet, implicating increased tyrosine levels in these behavioral changes. Although the mechanism causing neurocognitive and behavioral changes in HT1 remains unknown, future experiments will have to separate the effects of NTBC treatment, high plasma tyrosine levels and the mutated FAH enzyme causing HT1.
Conclusion
Our findings from the Crawley three-chambered social behavior experiments indicate that mice with HT1 mice show impaired social activity and that this effect is due to HT1 and not due to treatment with NTBC. The altered social behavior observed in mice with HT1 is associated with increased myelin expression in the cerebral cortex. The increased expression of myelin could be the basis behind the observed social behavior impairments due to altered axon conduction velocity and development. This in turn could lead to altered neuronal circuits and information processing that could explain the neurological issues seen in HT1. Social behavior ultimately comes down to patterns of neuronal activity and neurotransmitter release, resulting in altered connectivity between the PFC and other brain regions, producing the social experience in the mouse as measured by changes in social need and social novelty behavior.
Abbreviations
- HT1:
-
Hereditary Tyrosinemia Type I
- FAH:
-
Fumarylacetoacetate hydrolase
- NTBC:
-
2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1-3-dione
- HPD:
-
4-hydroxyphenylpyruvate dioxygenase
- MBP:
-
Myelin basic protein
- PLP1:
-
Proteolipid protein 1
References
Aponte JL, Sega GA, Hauser LJ, Dhar MS, Withrow CM, Carpenter DA, Rinchik EM, Culiat CT, Johnson DK (2001) Point mutations in the murine fumarylacetoacetate hydrolase gene: animal models for the human genetic disorder hereditary tyrosinemia type 1. Proc Natl Acad Sci U S A 98:641–645
Bendadi F, de Koning TJ, Visser G, Prinsen HC, de Sain MG, Verhoeven-Duif N, Sinnema G, van Spronsen FJ, van Hasselt PM (2014) Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J Pediatr 164:398–401
Bercury KK, Macklin WB (2015) Dynamics and mechanisms of CNS myelination. Dev Cell 32:447–458
Bicks LK, Koike H, Akbarian S, Morishita H (2015) Prefrontal cortex and social cognition in mouse and man. Front Psychol 6:1805
Brodkin ES, Hagemann A, Nemetski SM, Silver LM (2004) Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res 1002:151–157
Broek JA, Guest PC, Rahmoune H, Bahn S (2014) Proteomic analysis of post mortem brain tissue from autism patients: evidence for opposite changes in prefrontal cortex and cerebellum in synaptic connectivity-related proteins. Mol Autism 5:41
Chklovskii DB, Schikorski T, Stevens CF (2002) Wiring optimization in cortical circuits. Neuron 34:341–347
Chomiak T, Hu B (2009) What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One 4(11):e7754
Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C (1996) Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A 93:9887–9892
Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF (2014) Microbiota is essential for social development in the mouse. Mol Psychiatry 19:146–148
Fields RD (2005) Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist 11:528–531
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370
Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116:2093–2104
Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB (2008) Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci 28:7174–7183
Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ (2008) Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience 151:476–488
García MI, de la Parra A, Arias C, Arredondo M, Cabello JF (2017) Long-term cognitive functioning in individuals with tyrosinemia type 1 treated with nitisinone and protein-restricted diet. Mol Genet Metab Rep 11:12–16
van Ginkel WG, Jahja R, Huijbregts SC, Daly A, MacDonald A, De Laet C, Cassiman D, Eyskens F, Körver-Keularts IM, Goyens PJ, McKiernan PJ, van Spronsen FJ (2016) Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J Rare Dis 11:87
Hillgartner MA, Coker SB, Koenig AE, Moore ME, Barnby E, MacGregor GG (2016) Tyrosinemia type I and not treatment with NTBC causes slower learning and altered behavior in mice. J Inherit Metab Dis 39:673–682
Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF (2016) Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6:e774
Jahn O, Tenzer S, Werner HB (2009) Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol 40:55–72
Kluver H, Barrera E (1953) A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol 12:400–403
Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF (2006) Pten regulates neuronal arborization and social interaction in mice. Neuron 50:377–388
Labelle Y, Puymirat J, Tanguay RM (1993) Localization of cells in the rat brain expressing fumarylacetoacetate hydrolase, the deficient enzyme in hereditary tyrosinemia type 1. Biochim Biophys Acta 1180:250–256
de Laet C, Munoz VT, Jaeken J, François B, Carton D, Sokal EM, Dan B, Goyens PJ (2011) Neuropsychological outcome of NTBC-treated patients with tyrosinaemia type 1. Dev Med Child Neurol 53:962–964
de Laet C, Dionisi-Vici C, Leonard JV, McKiernan P, Mitchell G, Monti L, de Baulny HO, Pintos-Morell G, Spiekerkötter U (2013) Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis 8:8
Laule C, Leung E, Lis DK, Traboulsee AL, Paty DW, MacKay AL, Moore GR (2006) Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler 12:747–753
Lindblad B, Lindstedt S, Steen G (1977) On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A 74:4641–4645
Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B (1992) Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340:813–817
Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15:1621–1623
Macklin WB (2010) The myelin brake: when enough is enough. Sci Signal 3(140):pe32
Makinodan M, Rosen KM, Ito S, Corfas G (2012) A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337:1357–1360
Masurel-Paulet A, Poggi-Bach J, Rolland MO, Bernard O, Guffon N, Dobbelaere D, Sarles J, de Baulny HO, Touati G (2008) NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis 31:81–87
Mitchell G, Larochelle J, Lambert M, Michaud J, Grenier A, Ogier H, Gauthier M, Lacroix J, Vanasse M, Larbrisseau A, Paradis K, Weber A, Lefevre Y, Melançon S, Dallaire L (1990) Neurologic crises in hereditary tyrosinemia. N Engl J Med 322:432–437
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3:287–302
Nave KA (2010) Myelination and support of axonal integrity by glia. Nature 468:244–252
Nave KA, Werner HB (2014) Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol 30:503–533
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Rushton WA (1951) A theory of the effects of fibre size in medullated nerve. J Physiol 115:101–122
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Scholtz CL (1977) Quantitative histochemistry of myelin using Luxol fast blue MBS. Histochem J 9:759–765
Seidl AH (2014) Regulation of conduction time along axons. Neuroscience 276:126–134
Smith RS, Koles ZJ (1970) Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Phys 219:1256–1258
Snaidero N, Simons M (2014) Myelination at a glance. J Cell Sci 127:2999–3004
Thimm E, Richter-Werkle R, Kamp G, Molke B, Herebian D, Klee D, Mayatepek E, Spiekerkoetter U (2012) Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J Inherit Metab Dis 35:263–268
Ueda S, Niwa M, Hioki H, Sohn J, Kaneko T, Sawa A, Sakurai T (2015) Sequence of molecular events during the maturation of the developing mouse prefrontal cortex. Mol Neuropsychiatry 1:94–104
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333:1647–1651
West KL, Kelm ND, Carson RP, Gochberg DF, Ess KC, Does MD (2017) Myelin volume fraction imaging with MRI. NeuroImage. doi:10.1016/j.neuroimage.2016.12.067
Yang M, Crawley JN (2009) Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosc 48:8.24:8.24.1–8.248.24.12
Yang HJ, Vainshtein A, Maik-Rachline G, Peles E (2016) G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat Commun 7:10884
Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947
Acknowledgments
The authors are extremely grateful to Bruce Stallsmith for the use of his microscopes to document the mouse brain histology. We are grateful to the UAH Research or Creative Experience for Undergraduates (RCEU) program for student summer support to Megan Hillgartner (RCEU 2014), Ashton Koenig (RCEU 2015) and Marissa Moore (RCEU 2016).
Author information
Authors and Affiliations
Contributions
MM, AK, MH, CO, EB and GM designed research; MM, AK, MH, CO and GM performed research; GM analyzed data; MM and GM wrote the paper.
Gordon G. MacGregor accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Corresponding author
Ethics declarations
Conflict of interest
Marissa Moore, Megan Hillgartner, Ashton Koenig, Christopher Otap, Elizabeth Barnby and Gordon MacGregor declare that they have no conflict of interest.
Animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed. The care, housing, breeding and maze experiments of animals were approved by the UAH IACUC committee.
Funding
This study was funded internally by UAH. We are grateful to the UAH Research or Creative Experience for Undergraduates (RCEU) program for student summer support to Megan Hillgartner (RCEU 2014), Ashton Koenig (RCEU 2015) and Marissa Moore (RCEU 2016).
Electronic supplementary material
ESM 1
(DOCX 940 kb)
Rights and permissions
About this article
Cite this article
Moore, M.E., Koenig, A.E., Hillgartner, M.A. et al. Abnormal social behavior in mice with tyrosinemia type I is associated with an increase of myelin in the cerebral cortex. Metab Brain Dis 32, 1829–1841 (2017). https://doi.org/10.1007/s11011-017-0071-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0071-8