Abstract
Autism (MIM 209850) is a heterogeneous neurodevelopmental disease that manifests within the first 3 years of life. Numerous articles reported that dysfunctional folate-methionine pathway enzymes may play an important role in the pathophysiology of autism. Methylenetetrahydrofolate reductase (MTHFR) is a critical enzyme of this pathway and MTHFR C677T polymorphism reported as risk factor for autism in several case control studies. However, controversial reports were also published. Hence the present meta-analysis was designed to investigate the relationship of the MTHFR C677T polymorphism with the risk of autism. Electronic databases were searched for case control studies with following search terms - ‘MTHFR’, ‘C677T’, in combination with ‘Autism’. Pooled OR with its corresponding 95 % CI was calculated and used as association measure to investigate the association between MTHFR C677T polymorphism and risk of autism. Total of thirteen studies were found suitable for the inclusion in the present meta-analysis, which comprises 1978 cases and 7257 controls. Meta-analysis using all four genetic models showed significant association between C677T polymorphism and autism (ORTvs.C = 1.48; 95 % CI: 1.18–1.86; P = 0.0007; ORTT + CT vs. CC = 1.70, 95 % CI = 0.96–2.9, p = 0.05; ORTT vs. CC = 1.84, 95 % CI = 1.12–3.02, p = 0.02; ORCT vs.CC = 1.60, 95 % CI = 1.2–2.1, p = 0.003; ORTT vs.CT+CC = 1.5, 95 % CI = 1.02–2.2, p = 0.03). In total 13 studies, 9 studies were from Caucasian population and 4 studies were from Asian population. The association between C677T polymorphism and autism was significant in Caucasian (ORTvs.C = 1.43; 95 % CI = 1.1–1.87; p = 0.009) and Asian population (ORTvs.C = 1.68; 95 % CI = 1.02–2.77; p = 0.04) using allele contrast model. In conclusion, present meta-analysis strongly suggested a significant association of the MTHFR C677T polymorphism with autism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism is a complex neurodevelopmental disorder marked by deficits in social communication and interaction and by restricted, repetitive behaviors, interests, or activities (Schendel et al. 2016). Centers for Disease Control and Prevention (CDC 2012) reported the prevalence rate of autism spectrum disorder (ASD) as 1 in 68 children (1 in 42 boys and 1 in 189 girls). A number of factors such as genetic, environmental, autoimmune function, oxidative stress, and inflammatory biomarkers have been implicated in the etiology of ASD (Ali et al. 2011). Family and twin studies have conclusively described autism as a highly heritable neuropsychiatry disorder (Bailey et al. 1995).
Folate plays an important role in neurological development because it act as a methyl group transporter (Meguid et al. 2015). Folate-methionine pathway is crucial for DNA methylation and synthesis and also for redox balance in cell. Several studies reported that folate-methionine pathway was defective in autism patients (Selhub and Rosenberg 1996; Barbato et al. 2007; Main et al. 2010). Folate facilitates methionine synthesis from homocysteine by acting as a cofactor for methylenetetrahydrofolate reductase (MTHFR) enzyme, which converts 5,10-methylenetetrahydrofolate (CH2THF) to 5-methyltetrahydrofolate (CH3THF). Sufficient folate and vitamin B reduce levels of homocysteine, while deficiency of B vitamins can cause hyperhomocysteinemia, (Flickera et al. 2004; Almeida et al. 2005). Homocysteine in the body is metabolized along two pathways, remethylation to methionine or transsulfuration to cysteine (Miller 2003; James et al. 2004).
MTHFR is the crucial enzyme in folate-methionine metabolism and acts at the crossroads between DNA methylation and DNA synthesis. Decreased activity of MTHFR enzyme due to polymorphism may affect DNA synthesis and methylation (Ryan and Weir 2001; James et al. 2004) and favored increase levels of plasma homocysteine (Hcy) (James et al. 2004; Pasca et al. 2009). High levels of plasma Hcy and increased oxidative stress have generally been associated in the pathophysiology of many neuropsychiatric disorders including Autism (Suh et al. 2008; Tu et al. 2010).
Several MTHFR gene polymorphisms have been identified. A common genetic variation in the MTHFR gene, in which cytosine is replaced by thymidine at base position 677, resulted in substitution of alanine amino acid by valine at position 222 in MTHFR protein (Frosst et al. 1995). C677T polymorphism is associated with reduced enzyme activity (60 %) and results in approximately 20 % rise in serum Hcy levels (Frosst et al. 1995; Christensen et al. 1997; Devlin et al. 2006). MTHFR functions in dimeric form and flavin adenine dinucleotide (FAD) works as a co-factor for this enzyme, but variant MTHFR enzyme dissociates into monomers and its enzymatic activity reduces. Mutant T allele frequency range approximately from 0.24 to 0.44 in European and Caucasian populations, 0.06 in an African population, and 0.35 to 0.41 in Asian populations (Pepe et al. 1998; Botto and Yang 2000; Rai et al. 2012). The frequency of homozygosity (TT) ranges from 1 % in US African-American populations to more than 20 % in US Latinos; 5 % to 30 % in White populations in Europe and North America; 32.2 % in Mexico; 5.8 % in White Canadians in Alberta to 14.3 % in those in Quebec, Canada; 0.0 % in Sub-Saharan Africa; 10.7 % in Oceania; 11.5 % in Japanese and 16 % in Chinese (Botto and Yang 2000).
Several independent case control studies have investigated the role of the MTHFR C677T polymorphism in autism risk (Guo et al. 2012; Park et al. 2014), but the results are inconclusive. Hence, to estimate the overall risk of the C677T polymorphism for autism, a meta-analysis was performed on all published case-control studies.
Method
Meta-analysis was carried out according to MOOSE guidelines (Stroup et al. 2000).
Search strategy and identification of studies
Eligible studies were identified by searching PubMed, Google Scholar, Elsevier and Springer link databases. Following search terms were used: “MTHFR”, “methylenetetrahydrofolate reductase”, “C677T”, and “polymorphism” in combination with “Autism” up to January 15, 2016.
Inclusion/exclusion criteria
The inclusion criteria were as follows: studies should: 1) be original, 2) used case control approach, 3) be reported genotype frequency of cases and controls, and if not, the text provided data enabling such calculations, 4) used standard diagnostic criteria for autism patient diagnosis (Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) or the International Classification of Diseases (ICD)).
Studies were excluded if: 1) their sample was not independent from that investigated in another study, 2) incomplete raw data/information and not providing complete information for number of genotype and/or allele number calculation, 3) studies based on pedigree and 4) review, letter to editors and book chapters. If more than one study was published using the same dataset, the most recent study, or the study with the larger sample size, was selected.
Data extraction
The following information was extracted from the each identified studies: the first author family name, year of publication, sample size, country name, ethnicity, genotyping method, the numbers of patients and controls, and MTHFR C677T genotypes information and frequencies of alleles in all study. If important information was not given in the article, the relevant information was obtained by contacting authors.
Statistical analysis
The strength of association between the C677T polymorphism of MTHFR and autism risk was estimated by the odds ratio (OR) along with its 95 % confidence interval (CI). Heterogeneity among studies was examined with the χ2 test-based Q statistics (Lau et al. 1997) and P < 0.05 was considered significant. To quantify heterogeneity ‘I2’ index was used, which calculated as the percentage of the total variability in a set of effect sizes due to true heterogeneity (Higgins and Thompson 2002) and ‘I2’ > 50 % is considered high heterogenity (Whitehead 2002). Fixed and random-effects summary ORs were calculated using the Mantel-Haenszel and DerSimonian and Laird methods (Mantel and Haenszel 1959; DerSimonian and Laird 1986), random-effect summary ORs was used, when there was higher heterogeneity. The pooled ORs were performed for the allele contrasts/additive model (T versus C), homozygote model (TT versus CC), recessive model (TT versus CT+ CC), dominant model (TT + CT versus CC), and co-dominant/heterozygote model (CT versus CC). Control population of each study was tested for Hardy-Weinberg Equilibrium (HWE) using the χ2 test.
Sensitivity analysis was performed to evaluate the stability of the results by removing the studies not in Hardy–Weinberg equilibrium (HWE), and studies with small sample size. Cumulative meta-analysis was performed to see the effect of subsequent addition of each study. The publication bias was evaluated by a Begg’s test and Egger’s linear regression test (Begg and Mazumdar 1994; Egger et al. 1997). All statistical analysis was undertaken using MIX program version 1.7 (Bax et al. 2006). P values were two-tailed with a significance level of 0.05.
Results
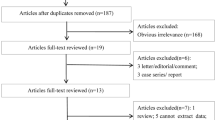
The literature search and detailed study selection procedures were presented in Fig. 1. The search strategy retrieved 28 studies from PubMed, Google Scholar, Elsevier and Springer Link databases. However, most of them were excluded after reviewing titles and abstracts, leaving 19 for full text review. Finally, 13 studies (Boris et al. 2004; James et al. 2006; Mohammad et al. 2009; Pasca et al. 2009; dos Santos et al. 2010; Liu et al. 2011; Schmidt et al. 2011; Guo et al. 2012; Divyakolu et al. 2013; Park et al. 2014; Sener et al. 2014; Shawky et al. 2014; Meguid et al. 2015) were included in present meta-analysis. The studies were published between 2004 and 2015. All thirteen studies were performed in different countries like- America (Boris et al. 2004; James et al. 2006; Schmidt et al. 2011), Brazil (dos Santos et al. 2010), Canada (Liu et al. 2011), China (Guo et al. 2012), India (Mohammad et al. 2009; Divyakolu et al. 2013), Romania (Pasca et al. 2009; Park et al. 2014), Turkey (Sener et al. 2014) and Egypt (Shawky et al. 2014; Meguid et al. 2015).
Characteristic of included studies
The number of cases varied from 20 (Shawky et al. 2014) to 356 (James et al. 2006), and the number of controls varied from 22 (Shawky et al. 2014) to 5389 (Boris et al. 2004) (Table 1). ORs for more than one were reported in ten studies and two studies did not show significant any association (Schmidt et al. 2011; Park et al. 2014). In all thirteen studies, total cases were 1978 with CC (788), CT (925) and TT (265), and controls were 7257 with CC (3466), CT (2975), and TT (816) genotypes. In controls genotypes, percentage of CC, CT and TT were 47.76 %, 40.99 %, and 11.24 % respectively. In total cases, genotype percentage of CC, CT, and TT was 39.83 %, 46.76 % and 13.39 % respectively. Frequencies of CC genotype and C allele were highest in both cases and controls (Table 1). Except one study (Boris et al. 2004), control populations of all studies were in Hardy-Weinberg equilibrium.
Meta-analysis
Meta-analysis with allele contrast showed significant association with both fixed effect (ORTvs.C = 1.37; 95 % CI: 1.25–1.50; P < 0.0001) and random effect (ORTvs.C = 1.48; 95 % CI: 1.18–1.86; P = 0.0007) models (Table 2; Fig. 2). Higher heterogeneity was found so random effect model was applied. C677T polymorphism had a significant association with susceptibility to autism in other four genetic models also (for TT + CT vs. CC (dominant model): OR =1.7, 95 % CI = 0.96–2.9, p = 0.05; for TT vs. CC (homozygote model): OR =1.84, 95 % CI = 1.12–3.02, p = 0.01(Figure3); for CT vs. CC (co-dominant/heterozygote model): OR = 1.6, 95 % CI = 1.2–2.1, p = 0.003; for TT vs. CT + CC (recessive model): OR = 1.5, 95 % CI = 1.0–2.2, p = 0.03).
Subgroup analysis
The association between the C677T polymorphism and autism was further stratified by ethnicity. As shown in Table 2 and Fig. 3, in Asian populations C677T polymorphism significantly increased the risk of autism only in allele contrast and co-dominant models (T vs. C: OR =1.68,95%CI = 1.02–2.77, P = 0.04; for TT vs. CC: OR = 1.68, 95%CI = 0.69–4.08,P = 0.24; for TT + CT vs. CC: OR =2.78, 95%CI = 0.53–14.56, p = 0.22; for CT vs. CC: OR =1.66, 95%CI = 1.00–2.76, P = 0.04; for TT vs. CT + CC: OR =1.56, 95%CI = 0.61–3.95, P = 0.35). Results of the meta-analysis of nine Caucasian studies showed significant association between C677T polymorphism and autism was found in allele contrast and co-dominant genetic models (T vs. C: OR =1.43,95%CI = 1.1–1.87, P = 0.009; for CT vs. CC: OR =1.48, 95%CI = 1.03–2.10, P = 0.03) (Table 2; Fig. 4).
Heterogeneity and sensitivity analysis
A true heterogeneity existed between studies for allele contrast model (Pheterogeneity = <0.0001, Q = 58.97, df = 11, I2 = 81.3 %, t2 = 0.11), dominant model (Pheterogeneity = <0.0001, Q = 147.34, df = 11, I2 = 92.9 %, t2 = 0.638). The ‘I2’ value of more than 50 % for between studies comparison in both allele and genotype analysis shows high level of true heterogeneity. In allele contrast meta-analysis, high heterogeneity was observed in Asian (Pheterogeneity = 0.0004, I2 = 83.57 %) and Caucasian (Pheterogeneity = <0.0001, I2 = 84.32 %) population studies.
In overall allele contrast meta-analysis, sensitivity analysis performed by exclusion of the studies in which control population was not in Hardy Weinberg equilibrium and studies with small sample size. Control population of one study (Boris et al. 2004) were not in HW equilibrium and sample size in three studies was less than 50 (Pasca et al. 2009, n = 39; Shawky et al. 2014, n = 20; Meguid et al. 2015, n = 24). Exclusion of these four studies decreased heterogeneity and (p = 0.0001, I2 = 76.08 %) also decreased odds ratio (OR =1.3;95%CI = 1.0–1.67;P = 0.02).
Publication bias
Publication bias was not observed in additive/allele contrast, co-dominant, homozygote and recessive models (Begg’s p = 0.195, Egger’s p = 0.34 for T vs. C; Begg’s p = 0.458, Egger’s p = 0.5 for TT vs. CC; Begg’s p = 0.026, Egger’s p = 0.6 for CT vs. CC; and Begg’s p = 0.987, Egger’s p = 0.97 for TT vs. CC + CT) of overall meta-analysis (Table 2). Publication bias was not observed in subgroup analysis based on ethnicity as Asian and Caucasian populations (Tables 2) by using of Begg’s and Egger’s test. Funnel plots were showed in Fig. 5.
Discussion
MTHFR enzyme is important enzyme of folate-methionine pathway and is involved in to two important processes -DNA methylation and DNA synthesis. It converts 5,10-methylenetetrahydro folate (5,10-methylene THF) in to 5-methyltetrahydrofolate (5-methyl THF), which donates methyl group for the remethylation of Hcy in to methionine. Methionine is converted in to S-adenosylmethionine (SAM) which is the main methyl group donor in the cellular methylation reaction of DNA, RNA, proteins and lipid. Under the condition of folate deficiency and/or hypofunctional MTHFR may result in less conversion of 5, 10-methylene THF to less 5-methyl THF, and less conversion of Hcy to methionine and consequently increased the concentration of plasma Hcy (James et al. 2006; Melnyk et al. 2011) which may contribute independently to the cause of autism.
Normal activity of MTHFR is required for normal genome methylation and imprinting. Genetic, epigenetic and environment factors play role in autism rate and symptom severity (Schaevitz and Berger-Sweeney 2012). The DNA methylation or epigenetic programming is essential for gene imprinting and cell differentiation during embryogenesis (Li 2002). Most critical period of epigenetic programming are prenatal and early post natal, when DNA methylation is essential for development of normal brain and neuron networks (Schaevitz and Berger-Sweeney 2012). The main neuroanatomical abnormalities in autistic children at birth are mainly in hippocampal and prefrontal cortex regions and following abnormalities were reported like (i) overgrowth of some specific regions of the brain (Anagnostou and Taylor 2011; Courchesne et al. 2007), (ii) network of long distance connections were underdeveloped (Wass 2011) and (iii) networks of short range were over developed (Wass 2011).
The epigenetic mechanism controls several processes during neurodevelopment which occurs prenatally and early post-natal up to 2 years of age like (i) establishment of neuron networks, (ii) selected cell death, (iii) synaptogenesis and (iv) pruning of inappropriate dendritic arbors and synapses etc. High concentration of Hcy and its metabolites inhibit activity of methyl transferases like Catechol-O-methyl transferase (COMT) (Schatz et al. 1981) and experiments on animal models have showed that COMT activity is high during early embryogenesis at the time of development of sympathetic nervous system (Ignarro and Shideman 1968). COMT degrades dopamine neurotransmitter by transferring methyl group from SAM to dopamine. Excess dopamine inhibits expression of brain derived neurotrophic factor (BDNF) (Fumagalli et al. 2003), which is essential for normal brain development (Schmidt et al. 2011). Abnormal methylation due to variant MTHFR enzyme reduced the activity of COMT and increased the concentration of dopamine, which consequently inhibit the synthesis of BDNF and abnormal neurodevelopment is resulted.
Meta-analysis is a statistical procedure for combining the results of several individuals studies to produce a pooled estimate for summarizing inconsistent results from different studies (Munafo and Flint 2004). Several meta-analyses were published accessing MTHFR as risk factor to various diseases/disorders like- neural tube defects (Yadav et al. 2015), down syndrome (Rai et al. 2014), cleft lip and palate (Zhao et al. 2014), cardiovascular disease (Gao et al. 2014), anxiety (Peerbooms et al. 2011), schizophrenia (Hu et al. 2014), depression (Rai 2014a), bipolar disorder (Rai 2011; Hu et al. 2014), Alzheimer’s disease (Zhang et al. 2010) and cancer (Rai 2014b).
Author identified two meta-analyses (Frustaci et al. 2012; Pu et al. 2013) concerning similar topic during the literature search. Frustaci et al. (2012) conducted a meta-analysis on MTHFR C677T and autism risk based on six publications (Boris et al. 2004; dos Santos et al. 2010; James et al. 2006; Liu et al. 2011; Mohammad et al. 2009; Pasca et al. 2009) and reported significant association. Pu et al. (2013) included only eight studies in their meta-analysis with 1672 case and 6762 control subjects. They reported random effect (OR = 1.42; 95 % CI = 1.09–1.85) models of meta-analysis showed significantly increased autism risk in the presence of mutant T allele. There are several published studies which were not included in these previous meta-analyses. Hence comprehensive meta-analysis with the largest number of studies and number of samples was conducted to investigate the possible relationship between MTHFR C677T polymorphism and the risk of Autism. The quality of meta-analysis is compromised by presence of publication bias, sampling method and variations in genetic background of the subjects due to different ethnicity. However to minimize these limitation, author tried to use appropriate inclusion and exclusion criteria, performed sensitivity analysis and subgroup analysis to reduce selection bias and to lower heterogeneity, (Zhang et al. 2014) but failed to minimize the heterogeneity.
There were few limitations in present meta-analysis: (i) used crude ORs in the pooled analysis without adjustment, (ii) some studies with small sample sizes were also included in the meta-analysis, (iii) did not done subgroup analysis by diagnostic subgroups because of the lack of information, (iv) in the subgroup analyses, the number of studies in Asian subgroup was relatively small, which could lead to a lack of sufficient statistical power to explore the true association, (v) analyses stratified by other related susceptible factors, such as maternal infection, smoking, drink abuse status during pregnancy have not been conducted in the present study due to unavailability of sufficient data, and (vi) due to lack of data, gene–gene and gene–environment interactions could not be included. Along with limitations, present meta-analysis had some strength also like substantial number of cases and controls were pooled from different studies, which significantly increased the statistical power of the analysis.
Results of present meta-analysis suggested that C677T polymorphism of MTHFR gene was a risk factor for autism susceptibility in overall population as well as, Asian and Caucasian populations. Further autism is a multifactorial disease; therefore, single susceptibility gene polymorphisms might have modest effects. More case control studies stratified by environmental exposure, or other risk factors, should be performed in the future to better understand the role of the MTHFR C677T polymorphism in the pathogenesis of autism.
References
Ali A, Waly MI, Al-Farsi YM, Essa MM, Al-Sharbati MM, Deth RC (2011) Hyperhomocysteinemia among Omani autistic children: A case-control study. Acta Biochim Pol 58:547–551
Almeida OP, Flicker L, Lautenschlager NT, Leedman P, Vasikaran S, Van Bock-Xmeer FM (2005) Contribution of the MTHFR gene to the causal pathway for depression, anxiety and cognitive impairment in later life. Neurobiol Aging 26:251–257
Anagnostou E, Taylor MJ (2011) Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Mol Autism 2:4–13
Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25:63–77
Barbato JC, Catanescu O, Murray K, DiBello PM, Jacobsen DW (2007) Targeting of metallothioneine by L-homocysteine: a novel mechanism for disruption of zinc and redox homeostasis. Arterioscler Thromb Vasc Biol 27:49–54
Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG (2006) Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol 6:50
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Boris M, Goldblatt A, Galanko J, James J (2004) Association of MTHFR gene variants with autism. J Am Phys Surg 9:106–108
Botto LD, Yang Q (2000) 5, 10-methylenetetrahydrofolate reductase variants and congenital anomalies: A huge review. Am J Epidemiol 151:862–877
Centers for Disease Control and Prevention (2012) Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ 61:1–19
Christensen B, Frosst P, Lussier-Cacan S, Selhub J, Goyette P, Rosenblatt DS, Gravel RA, Forbes P, Rozen R (1997) Correlation of a common mutation in the methylenetetrahydrofolate reductase gene with plasma homocysteine in patients with premature coronary artery disease. Arterioscler Thromb Vasc Biol 17:569–573
Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J (2007) Mapping early brain development in autism. Neuron 56:399–413
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Devlin AM, Clarke R, Birks J, Evans JG, Halsted CH (2006) Interactions among polymorphisms in folate-metabolizing genes and serum total homocysteine concentrations in a healthy elderly population. Am J Clin Nutr 83:708–713
Divyakolu S, Tejaswini Y, Thomas W, Thumoju S, Sreekanth VR, Vasavi M, OmSai VR, Nagaratna V, Hasan Q, Ahuja YR (2013) Evaluation of C677T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) gene in various neurological disorders. Neurol Disord 2:142–146
dos Santos PA, Longo D, Brandalize AP, Schüler-Faccini L (2010) MTHFR C677T is not a risk factor for autism spectrum disorders in South Brazil. Psychiatr Genet 20:187–189
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. British Med J 315:629–634
Flickera L, Martins RN, Thomas J, Acres J, Taddei K, Norman P, Jamrozik K, Almeida OP (2004) Homocysteine, Alzheimer genes and proteins, and measures of cognition and depression in older men. J Alzheimer’s Dis 6:329–336
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Mathews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, van den Heuvel LP, Rozen R (1995) A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113
Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Bernardina BD, et al. (2012) Oxidative stress-related biomarkers in autism: systematic review and meta-analysis. Free Rad Med 52(10):2128–2141
Fumagalli F, Racagni G, Colombo E, Riva MA (2003) BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol Psychiatr 8(11):898–899
Gao XH, Zhang GY, Wang Y, Zhang HY (2014) Correlations of MTHFR 677C.T polymorphism with cardiovascular disease in patients with end-stage renal disease: a meta-analysis. PLoS one 9(7):e102323
Guo T, Chen H, Liu B, Ji W, Yang C (2012) Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet Test Mole Biomark 16:968–973
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, Yang HY, Sun YH (2014) Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm 122(2):307–320
Ignarro LJ, Shideman FE (1968) Catechol-O-methyl transferase and monoamine oxidase activities in the heart and liver of the embryonic and developing chick. J Pharmacol Exp Ther 159(1):29–37
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80:1611–1617
James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW (2006) Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med genet. part B. Neuropsychiat Genet 141B:947–956
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127:820–826
Li E (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673
Liu X, Solehdin F, Cohen IL, Gonzalez MG, Jenkins EC, Lewis MES, Holden JA (2011) Population- and family-based studies associate the MTHFR gene with idiopathic autism in simplex families. J Autism Develop Dis 41:938–944
Main PAE, Angley MT, Thomas P, O’Doherty CE, Fenech M (2010) Folate and methionine metabolism in autism: a systematic review. Am J Clin Nutr 91:1598–1620
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Meguid N, Khalil R, Gebril O, El-Fishawy P (2015) Evaluation of MTHFR genetic polymorphism as a risk factor in Egyptian Autistic Children and Mothers. J Psychiatry 18:1. doi:10.4172/Psychiatry.1000179
Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. (2011) Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord 42:367–377
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8:7–19
Mohammad NS, Jain JM, Chintakindi KP, Singh RP, Naik U, Akella RR (2009) Aberrations in folate metabolic pathway and altered susceptibility to autism. Psychiat Genet 19:171–176
Munafo MR, Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20:439–444
Park JW, Ro MJ, Pyun JA, Kwack KB, Nam M, Bang HJ, Yang JW, Choi KS, Kim SK, Chung JH (2014) MTHFR 1298 A > C is a risk factor for autism spectrum disorder in the Korean population. Psychiat Res 215:258–259
Pasca SP, Dronca E, Kaucsar T, Craciun EC, Endreffy E, Ferencz BK, Iftene F, Benga I, Cornean R, Banerjee R, Dronca M (2009) One carbon metabolism disturbances and the C677T MTHFR gene polymorphism in children with autism spectrum disorders. J Cell Mole Med 13:4229–4238
Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L (2011) Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun 25(8):1530–1543
Pepe G, Venegas OC, Giusti B, Brunelli T, Marcucci R, Attanasio M, Rickards O, De Stefano GF, Prisco D, Gensini GF, Abbate R (1998) Heterogeneity in world distribution of thermolabile C677T mutation in 5,10-methylenetetrahydrofolate reductase. Am J Hum Genet 63:917–920
Pu D, Shen Y, Wu J (2013) Association between MTHFR gene polymorphisms and the risk of autism spectrum disorders: a meta-analysis. Autism Res 6(5):384–392
Rai V (2011) Evaluation of methylenetetrahydrofolate reductase gene variant (C677T) as risk factor for bipolar disorder. Cell Mol Biol 57:OL1558–OL1566
Rai V (2014a) Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) gene and susceptibility to depression in Asian population: a systematic meta-analysis. Cell Mol Biol 60(3):29–36
Rai V (2014b) The methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Asian populations. Asian Pac J Cancer Prev 15:5853–5860
Rai V, Yadav U, Kumar P (2012) Prevalence of methylenetetrahydrofolate reductase C677T polymorphism in Eastern Uttar Pradesh. Ind J Hum Genet 18:43–46
Rai V, Yadav U, Kumar P, Yadav SK, Mishra OP (2014) Maternal methylenetetrahydrofolate reductase C677T polymorphism and down syndrome risk: a meta-analysis from 34 studies. PLoS one 9:e108552
Ryan BM, Weir DG (2001) Relevance of folate metabolism in the pathogenesis of colorectal cancer. J Lab Clin Med 138:164–176
Schaevitz LR, Berger-Sweeney JE (2012) Gene–environment interactions and epigenetic pathways in autism: the importance of one-carbon metabolism. ILAR J 53(3–4):322–340
Schatz RA, Wilens TE, Sellinger OZ (1981) Decreased transmethylation of biogenic amines after in vivo elevation of brain S-adenosyl-l-homocysteine. J Neurochem 36(5):1739–1748
Schendel DE, Overgaard M, Christensen J, Hjort L, Jørgensen M, Vestergaard M, Parner ET (2016) Association of psychiatric and neurologic comorbidity with mortality among persons with autism Spectrum disorder in a Danish population. JAMA Pediatr. doi:10.1001/jamapediatrics.2015.3935
Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F, Hertz-Picciotto I (2011) Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiol 22:476–485
Selhub J, Rosenberg IH (1996) Folic acid. In: Ziegler EE, filer LJ Jr, present knowledge in nutrition, 7th edn. International Life Sciences Institute Press, Washington, DC, pp. 206–219
Sener EF, Oztop DB, Usuf Ozkul Y (2014) MTHFR gene C677T polymorphism in Autism Spectrum Disorders. Genet Res Int 2014:Article ID 698574
Shawky RM, El-baz F, Kamal TM, Elhossiny RM, Ahmed MA, El Nady GH (2014) Study of genotype–phenotype correlation of methylene tetrahydrofolate reductase (MTHFR) gene polymorphisms in a sample of Egyptian autistic children. Egypt J Med Hum Gene 15:335–341
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15):2008–2012
Suh JH, Walsh WJ, McGinnis WR, Lewis A, Ames BN (2008) Altered sulfur amino acid metabolism in immune cells of children diagnosed with autism. Am J Biochem Biotech 4:105–113
Tu MC, Huang CW, Chen NC, Chang WN, Lui CC, Chen CF, et al. (2010) Hyperhomocysteinemia in Alzheimer dementia patients and cognitive decline after 6 months follow-up period. Acta Neurol Taiwan 19:168–177
Wass S (2011) Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn 75(1):18–28
Whitehead A (2002) Meta-Analysis of controlled clinical trials. Chichester, West Sussex, England, John Wiley & Sons Ltd
Yadav U, Kumar P, Yadav SK, Mishra OP, Rai V (2015) Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: an updated meta-analysis. Meta Brain Dis 30:7–24
Zhang MY, Miaoa L, Li YS, Hub GY (2010) Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to Alzheimer’s disease. Neurosci Res 68:142–150
Zhang P, Gao X, Zhang Y, Hu Y, Ma H, Wang W, et al. (2014) Association between MTHFR C677T polymorphism and venous thromboembolism risk in the Chinese population: A meta-analysis of 24 case-control studies. Angiology 66(5):422–432
Zhao M, Ren Y, Shen L, Zhang Y, Zhou B (2014) Association between MTHFR C677T and A1298C polymorphisms and NSCL/P risk in Asians: a meta- analysis. PLoS one 9:e88242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, V. Association of methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism with autism: evidence of genetic susceptibility. Metab Brain Dis 31, 727–735 (2016). https://doi.org/10.1007/s11011-016-9815-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9815-0