Abstract
The presence and severity of a systemic inflammatory response is a major predictor of brain edema and encephalopathy in acute liver failure (ALF) and polymorphisms of the gene coding for the proinflammatory cytokine TNF-alpha are known to influence the clinical outcome in ALF. Recent reports provide robust evidence for a role of neuroinflammation(inflammation of the brain per se) in ALF with the cardinal features of neuroinflammation including activation of microglial cells and increased production in situ of pro-inflammatory cytokines such as TNF-alpha and interleukins IL-1beta and IL-6. Multiple liver-brain signalling pathways have been proposed to explain the phenomenon of neuroinflammation in liver failure and these include direct effects of systemically-derived cytokines, recruitment of monocytes relating to microglial activation as well as effects of liver failure-derived toxins and altered permeability of the blood-brain barrier. Synergistic mechanisms involving ammonia and cytokines have been proposed. Currently-available strategies aimed at lowering of blood ammonia such as lactulose, probiotics and rifaximin have the potential to dampen systemic inflammation as does the anti-oxidant N-acetyl cysteine, mild hypothermia and albumin dialysis. Experimental studies demonstrate that deletion of genes coding for TNF-alpha or IL-1 leads to attenuation of the CNS consequences of ALF and administration of the TNF-alpha receptor antagonist etanercept has comparable beneficial effects in experimental ALF. Together, these findings confirm a major role for central neuroinflammatory mechanisms in general and mechanisms involving TNF-alpha in particular in the pathogenesis of the cerebral consequences of ALF and open the door to novel therapeutic interventions in this often fatal disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) and brain edema leading to intracranial hypertension are severe central nervous system complications of acute liver failure (ALF). HE in ALF is characterized by disturbances of both cognitive and motor function starting with personality changes progressing rapidly through more severe cognitive symptoms to stupor and coma. Progression to coma may occur in a matter of days. Since the appearance of neurological symptoms in ALF heralds a poor prognosis potentially having a significant impact on liver transplant priority and outcome as well as on patient survival, effective therapies are urgently required.
The mechanisms responsible for HE and brain edema in ALF are still not completely understood. A great deal of attention continues to be focused on ammonia toxicity in the pathogenesis of these CNS complications. In support of this concept, several studies have shown that arterial ammonia concentrations are useful predictors of brain herniation (Clemmesen et al. 1999) and independent predictors of survival in patients with ALF (Bernal et al. 1998) However some ammonia-lowering agents have so far been found to be of limited value in prevention of the central nervous system (CNS) complications of ALF (Acharya et al. 2009). Recent research in ALF patients and studies in experimental animal models confirm that inflammation, acting alone or in concert with ammonia, plays an important role in the pathogenesis of the neurological complications of ALF.
Neuroglial pathology in acute liver failure
Studies in material from patients with ALF reveal primarily swelling of the astrocytes (Kato et al. 1992) leading to brain edema that is primarily cytotoxic in nature, the cell-type expressing the greatest degree of swelling being the astrocyte.
More recently, alterations of a second cell type of the glial linage namely microglial cells have been reported in ALF. Microglial cells are the immunomodulator cells of the brain being bone marrow-derived myeloid lineage cells. In the absence of an inflammatory stimulus, microglial cells remain quiescent, being involved principally in surveillance (the so-called “resting” phenotype). However in the presence of an inflammatory stimulus, they become reactive (the “activated” phenotype) with the task of prevention and control of CNS damage due to altered homeostasis associated with a wide range of insults and/or cell death. Activation of microglia is indicative of neuroinflammation and is observed in a wide range of disorders including Multiple Sclerosis and the AIDS-Dementia Complex and also in disorders such as stroke and Alzheimer’s disease consistent with the presence of a significant neuroinflammatory component in the pathogenesis of the neurological symptoms of these disorders also. Activation of microglia was first reported in 2005 in brain sections from experimental animals with ALF resulting from liver ischemia (Jiang et al. 2005). These finding were subsequently confirmed (Jiang et al. 2009a, b) and the findings of microglial activation were extended to animals with ALF resulting from toxic liver injury (Bemeur et al. 2010a) and to a patient with ALF associated with viral hepatitis (Butterworth 2011).
Systemic inflammation in acute liver failure
Patients with ALF are susceptible to infection as a result of their compromised immune status and significant numbers of patients listed for liver transplantation die as a result of brain edema and its complications (intacranial hypertension, brain herniation) or sepsis with multiple organ failure before a donor liver becomes available (Stravitz and Kramer 2009). A systemic inflammatory response syndrome (SIRS) is commonly encountered in patients with ALF and the presence of SIRS is associated with a more critical illness, worsening of HE and increased mortality. A significant correlation exists between the presence of SIRS and the severity of CNS complication of ALF (Rolando et al. 2000). In a study of 227 ALF patients aimed at the identification of predictive factors for worsening HE, Vaquero et al. (2003) observed that the acquisition of infection and/or SIRS during mild stages of HE was a major predictor of worsening encephalopathy grade particularly in patients with ALF due to acetaminophen overdose. Moreover, HE in these patients is influenced by macrophage-derived cytokines such as the interleukins IL-1beta, IL-6 and tumour necrosis factor alpha TNF-alpha (Rolando et al. 2000).Circulating levels of TNF-alpha and other pro-inflammatory cytokines are invariably increased in ALF patients and TNF gene polymorphisms have been reported to influence the clinical outcome in these patients (Bernal et al. 1998). Moreover decreases in TNF-alpha production have been shown to be protective against the development of severe HE in patients with ALF resulting from acetaminophen ingestion (Bernal et al. 1998).Plasma cytokine profiles in experimental ALF resulting from toxic liver injury invariably manifest increases of TNF alpha but additionally, may show both similarities and differencesIn other cytokines depending upon the nature of the toxin (Bemeur and Butterworth 2013).
Neuroinflammation in acute liver failure
Although systemic inflammation is well established in ALF, evidence of neuroinflammation in liver failure was not provided until the publication of a report of a study suggesting increased production of pro-inflammatory cytokines in brain in ALF patients (Wright et al. 2007).Unequivocal evidence of neuroinflammation was subsequently provided by studies in both human (Butterworth 2011) and experimental animal models of ALF. For example, in a report by Jiang et al. (2009a) microglial activation was demonstrated together with concomitantly increased expression of genes coding for pro-inflammatory cytokines in ALF resulting from ischemic liver injury in the rat (Fig. 1).
Neuroinflammation in ALF. Panel A: Microglial activation indicated by increased OX-42 immunostaining in frontal cortex of a rat with acute liver failure resulting from hepatic devascularisation at coma/edema stage of encephalopathy (ALF-coma) compared to a sham-operated control (Sham). Original magnification: X200. Panel B: Increased expression of genes coding for the pro-inflammatory cytokines interleukin-1-beta (IL-1b) and tumor necrosis factor alpha (TNFa) in samples of frontal cortex from rats with acute liver failure at coma/edema stage of encephalopathy (ALF-coma) compared to sham-operated controls (Sham). Histograms represent mean+/− SE values from n = 6 animals per group. Values that are significantly different from Sham indicated by *p < 0.02 **p < 0.01 by Student t test
In this study, increases in expression of the major histo-compatibility complex class 11 antigen marker CD11b/c (OX-42) was observed, a feature that is characteristic of microglial activation. Microglial activation occurred early in the progression of ALF and increased significantly as HE worsened and brain edema became evident.
Liver-brain proinflammatory signalling in acute liver failure
The precise nature of the signalling between the failing liver and the brain in ALF leading to neuroinflammation remains unknown. Possibilities (D’Mello et al. 2009) include (i) direct transfer of cytokines, (ii) interaction with receptors on circumventricular organs lacking a blood-brain barrier or (iii) activation of afferent neurons of the vagus nerve. In addition systemic inflammatory signals have the potential to result in increased permeability of the blood-brain barrier in ALF (Fig. 2).
Trace amounts of lipopolysaccharide (LPS) lead to breakdown of the blood-brain barrier in ALF. Left panel: Increased extravasation of Immunoglobulin G (IgG) in brain of rats with ALF due to hepatic devascularisation followed by administration of trace amounts of LPS. CTRL: control; LPS: lipopolysaccharide alone; ALF-8 h: Acute liver failure 8 h post-op/precoma stage; ALF-Coma: Acute liver failure at coma stage; ALF + LPS: Acute liver failure plus LPS administration. Right panel; Data from n = 10 animals per group; columns indicate mean values +/− SD. * indicates significant difference from all other groups with p < 0.01 by ANOVA. Data from Chastre et al. 2014 with permission
In addition to these pro-inflammatory signals, there is evidence to suggest that neuroactive and/or neurotoxic substances generated by the failing liver play a role in the pathogenesis of neuroinflammation in ALF. A wide range of molecules with the potential to threaten the functional integrity of the brain have the capacity to trigger the transformation of the microglia from the resting to the active state. Such molecules include ammonia, lactate, glutamate as well as certain neurosteroids, all of which have been reported to be increased in concentration in the brain in ALF. Brain ammonia and lactate concentrations have consistently been reported to be increased in an animal model of ALF resulting from hepatic devascularisation (Swain et al. 1992; Chatauret et al. 2003) and in humans with ALF (Tofteng et al. 2002) using the technique of in vivo microdialysis. Moreover, EEG changes characteristic of HE in ALF animals have been shown to be significantly associated with increased brain lactate concentrations (Deutz et al. 1988) and increased brain lactate is associated with the occurrence of intercranial hypertension and poor outcome following ALF in dogs (Nyberg et al. 1998). Finally using the technique of 13C-NMR spectroscopy, brain lactate was shown to be predictive of the presence and severity of HE and brain edema in rats with ALF due to hepatic devascularisation (Chatauret et al. 2003). Increased brain lactate in liver failure has been attributed to an inhibitory effect of ammonia on brain glucose oxidation. Increased brain lactate synthesis is significantly correlated with severity of encephalopathy and brain edema, with microglial activation and with brain cytokine production in experimental ALF (Jiang et al. 2009a; Chatauret et al. 2003). Of particular interest to signalling mechanisms leading to neuroinflammation, it has been shown that exposure of cultured microglial cells to concentrations of lactate equivalent to those reported in brain in ALF leads to several-fold increases in release of the pro-inflammatory cytokines TNF-alpha and IL-6 (Andersson et al. 2005).
Synergistic effects of ammonia and pro-inflammatory cytokines in acute liver failure
Glutamate is the principal excitatory neurotransmitter of mammalian brain and one important function of astrocytes is the rapid removal of neuronally-released glutamate. This mechanism represents the major inactivation step in the regulation of the glutamatergic neurotransmitter system. For this purpose, astrocytes express high affinity high capacity glutamate transporters. Studies in cultured astrocytes reveal that exposure to either ammonia (Norenberg et al. 1991) or pro-inflammatory cytokines (Hu et al. 2000) causes loss of expression of glutamate transporters and a consequent reduction in the capacity for high affinity glutamate uptake. Expression of the most abundant glutamate transporter, EAAT-2, is decreased in experimental ALF (Knecht et al. 1997). Moreover administration of the endotoxin component LPS to rats with ALF results in further increases in loss of expression of EAAT-2 in brain leading to a more rapid progression of encephalopathy and brain edema (Jiang et al. 2009b). These findings provide a probable explanation for the clinical observation of the precipitation of severe encephalopathy in ALF patients by inflammation and suggest a role for the glutamatergic neurotransmitter system in ammonia/cytokine synergism.
More recent studies provide direct evidence for additional ammonia/pro-inflammatory cytokine synergism at the cellular/molecular level (Chastre et al. 2010). Exposure of primary cultures of cortical rat astrocytes to recombinant IL-1beta and ammonia resulted in significant increases in expression of genes coding for both hemoxygenase-1 (HO-1) and inducible nitric oxide synthase (iNOS). The effects were additive suggestive of synergism.
Implications for novel therapeutics
The consistent findings of induction of central neuro-inflammatory processes in ALF have the potential to impact significantly on diagnostic, management and treatment options for the future.
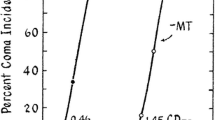
Therapies directly targeting neuro-inflammatory processes include those aimed at inhibition of microglial activation or inhibition of the actions of pro-inflammatory cytokines. One such example is mild hypothermia. Just two degrees of hypothermia in ALF has been shown to delay the onset of HE, prevent brain edema and impair both microglial activation and the increased expression of genes coding for pro-inflammatory cytokines (Jiang et al. 2009a). A more recent study showed that deletion (knockdown) of the gene coding for TNF-alpha or IL-1beta likewise delays HE onset and attenuates brain edema in mice with ALF resulting from toxic liver injury (Bemeur et al. 2010b). Studies demonstrate that treatment with the TNF-alpha receptor antagonist etanercept led to slowing in progression of HE (Fig. 3) and prevention of brain edema in experimental ALF (Chastre et al. 2012).
Slowing of progression of HE in ALF following treatment with etanercept (ETA) 30 min, prior to, 3 h following and 6 h following liver ischemia. Columns represent mean values +/− SD of n = 6 animals with ALF resulting from liver ischemia *Indicates significant lengthening of time-to-coma in hours with p < 0.01 by ANOVA
Some existing therapies used in the management of HE in ALF that were presumed to act by lowering levels of circulating ammonia may also act by reducing levels of pro-inflammatory cytokines. For example, the use of the albumen dialysis (MARS) system in patients with ALF resulted in removal of TNF-alpha and clinical improvement with better outcome in ALF (Guo et al. 2003). Another example of an existing therapy that appears to act, at least in part, by reduction of inflammation is mild hypothermia which is increasingly being used in the management of CNS complications of ALF (Jalan et al. 1999). Mechanisms implicated in the mediation of the beneficial effects of hypothermia in ALF involve anti-inflammatory mechanisms at both the hepatic and cerebral levels (Vaquero and Butterworth 2007).
An interesting new dimension in the search for novel anti-inflammatory agents for potential application in the treatment of the CNS complications of liver failure was recently provided by the report that minocycline, an agent with well-established potent inhibitory properties on microglial activation that are independent of its antimicrobial properties inhibits pro-inflammatory cytokine production in brain, delays progression of HE and attenuates brain edema in experimental ALF (Jiang et al. 2009a).
These findings confirm a major role for central neuroinflammatory mechanisms involving TNF-alpha in particular in the pathogenesis of the cerebral consequences of ALF and provide a rationale for the use of novel central anti-inflammatory agents in the treatment of the CNS complications of this often fatal disorder.
References
Acharya SK, Bhatia V, Sreenivas V, et al. (2009) Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind randomized, placebo-controlled trial. Gastroenterology 136:2159–2168
Andersson AK, Ronnback L, Hansson E (2005) Lactate induces tumor necrosis factor-alpha, interleukin-6 and interleukin-1beta release in microglial and astroglial-enriched primary cultures. J Neurochem 93:1327–1333
Bemeur C, Butterworth RF (2013) Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis 28:145–150
Bemeur C, Vaquero J, Desjardins P, Butterworth RF (2010a) N-Acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis 25:241–249
Bemeur C, Qu H, Desjardins P, Butterworth RF (2010b) IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int 56:213–216
Bernal W, Donaldson P, Underhill J, et al. (1998) Tumor necrosis factor genomic polymorphism and outcome of acetaminophen(paracetamol)-induced acute liver failure. J Hepatol 29:53–59
Butterworth RF (2011) Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 53:1372–1376
Chastre A, Jiang W, Desjardins P, Butterworth RF (2010) Ammonia and proinflammatory cytokines modify expression of genes coding for astrocytic proteins implicated in brain edema in acute liver failure. Metab Brain Dis 25:17–21
Chastre A, Belanger M, Beauchesne E, et al. (2012) Inflammatory cascades driven by tumor necrosis factor alpha play a major role in the progression of acute liver failure and its neurological complications. PLoS One 7:e49670
Chastre A, Belanger M, Nguyen BN, Butterworth RF (2013) Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int 34(3):353–361 doi:10.1111/liv.12252
Chatauret N, Zwingmann C, Rose C, et al. (2003) Effects of hypothermia on brain glucose metabolism in acute liver failure : a H/C nuclear magnetic resonance study. Gastroenterology 125:815–824
Clemmesen JO, Larsen FS, Kondrup J, et al. (1999) Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentrations. Hepatology 29:648–653
D’Mello C, Le T, Swain MG (2009) Cerebral microglia recruit monocytes into the brain in response to tumour necrosis factor alpha signalling during peripheral organ inflammation. J Neurosci 29:2089–2102
Deutz NEP, DeGraaf AA, DeHaan JG et al., In Vivo brain 1 H-NMR spectroscopy (1 H-MRS) during acute hepatic encephalopathy (HE), In Soeters PD et al., Advances in ammonia metabolism and hepatic encephalopathy, Amsterdam: Excerpta Medica, 1988, 439–446
Guo LM, Liu JY, Xu DZ, et al. (2003) Application of molecular absorbents re-circulating system to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int 23(Suppl 3):16–20
Hu S, Sheng W, Ehrlich L, et al. (2000) Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7:153–159
Jalan R, Olde Daminck SW, Deutz NE, et al. (1999) Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet 1:1164–1168
Jiang W, Qu H, Desjardins P, Butterworth RF (2005) Unequivocal evidence for cytokine accumulation in brain in experimental acute liver failure. Hepatology 44(Suppl. 1):366A
Jiang W, Desjardins P, Butterworth RF (2009a) Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem 109:485–493
Jiang W, Desjardins P, Butterworth RF (2009b) Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J Cereb Blood Flow Metab 29:944–952
Kato M, Hughes RD, Keays RT, Williams R (1992) Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 15:160–1066
Knecht K, Michalak A, Rose C, Butterworth RF (1997) Decreased glutamate transporter (GLT-1) expression in frontal cortex of rats with acute liver failure. Neurosci Lett 229:201–203
Norenberg MD, Baker I, Lo N, et al. (1991) Ammonia-induced astrocyte swelling in primary culture. Neurochem Res 16:833–836
Nyberg SL, Cerra FB, Gruetter R, et al. (1998) Brain lactate by magnetic resonance spectroscopy during fulminant hepatic failure in the dog. Liver Transpl Surg 4:158–165
Rolando N, Wade J, Davalos M, et al. (2000) The systemic inflammatory response syndrome in acute liver failure. Hepatology 32:734–739
Stravitz RT, Kramer DJ (2009) Management of acute liver failure. Nat Rev Gastroenterol Hepatol 6:542–553
Swain M, Butterworth RF, Blei AT (1992) Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 15:449–453
Tofteng F, Jorgensen L, Hansen BA, et al. (2002) Cerebral microdialysis in patients with fulmionant hepatic failure. Hepatology 36:1333–1340
Vaquero J, Butterworth RF (2007) Mild hypothermia for the treatment of acute liver failure: what are we waiting for? Nat Clin Pract Gastroenterol Hepatol 10:528–529
Vaquero J, Chung C, Cahill ME, et al. (2003) Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis 23:259–269
Wright G, Shawcross D, Olde Daminck SW, Jalan R (2007) Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis 22:375–388
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butterworth, R.F. The concept of “the inflamed brain” in acute liver failure: mechanisms and new therapeutic opportunities. Metab Brain Dis 31, 1283–1287 (2016). https://doi.org/10.1007/s11011-015-9747-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9747-0