Abstract
It remains unclear whether prolonged febrile seizures (pFS) in childhood facilitate mesial temporal lobe epilepsy (MTLE) in adulthood. Interleukin (IL)-1β is associated with seizures in children and immature animal models. Here, we use a rat model of pFS to study the effects of IL-1β on adult epileptogenesis, hippocampal damage, and cognition. We produced prolonged hyperthermia-induced seizures on postnatal days (P) 10–11 and administered IL-1β or saline intranasally immediately after the seizures. Motor and cognitive functions were assessed at P85 using rotarod and passive avoidance tests. Electroencephalogram recordings were conducted at P90 and P120. Hippocampal CA1 and CA3 neurons and gliosis were quantified at the end of the experiment. Spontaneous seizure incidence was significantly greater in rats that had received IL-1β than in those that had received saline or those without hyperthermia-induced seizures (p < 0.05). Seizure frequency did not differ significantly between the three groups and no motor deficits were observed. Passive avoidance learning was impaired in rats that received IL-1β compared with controls (p < 0.05), but was not different from that in rats that received saline. Hippocampal cell numbers and gliosis did not differ between the three groups. These results indicate that neuronal loss and gliosis are not prerequisites for the epileptogenic process that follows pFS. Our results suggest that infantile pFS combined with IL-1β overproduction can enhance adulthood epileptogenesis, and might contribute to the development of MTLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some patients with mesial temporal lobe epilepsy (MTLE) have a history of prolonged febrile seizures during childhood, and a few prospective studies have reported the development of MTLE in children with febrile status epilepticus (Tanabe et al. 2011; Lewis et al. 2014). In experimental studies, prolonged hyperthermia-induced seizures (pHS) in developing rats are associated with elevated electroclinical seizure susceptibility in adulthood, with or without hippocampal cell injury (Dubé et al. 2000, 2006; Fukuda et al. 2014).
Interleukin (IL)-1β has acute and long-lasting effects on neuronal excitability. The −511C/T polymorphism in the IL1B gene is associated with sporadic development of simple febrile seizures (Kira et al. 2005). IL-1β receptor-deficient immature mice are resistant to hyperthermia-induced seizures (Dubé et al. 2005), and administration of IL-1β increases hyperthermic seizure susceptibility in developing rats (Fukuda et al. 2009). Furthermore, prenatal stress enhances infantile drug-induced febrile seizures as a result of enhanced IL-1β release (Qulu et al. 2012). With respect to long-lasting effects, it was reported that upregulation of the IL-1β–microRNA-146a axis, a mediator of inflammation, is associated with seizures in children and immature animal models and might facilitate later epileptogenesis (Omran et al. 2012). Pre-exposure to lipopolysaccharide on postnatal day (P) 14 enhances seizure susceptibility in a number of rat models, which is reversed by IL-1 receptor antagonism, and produces a long-lasting, tumor necrosis factor α-dependent increase in in vitro hippocampal excitability (Auvin et al. 2010; Galic et al. 2008). Furthermore, repetitive postnatal pHS combined with IL-1β administration in rats enhances adulthood kainic acid-induced seizures with mild hippocampal neuronal injury, implicating IL-1β in the development of MTLE in humans (Fukuda et al. 2014).
Here, we test the hypothesis that elevation of proinflammatory cytokines after pHS in infantile rats enhances spontaneous seizures, neuronal cell injury, and disturbance of higher brain functions in adulthood.
Materials and methods
Animals and experimental grouping
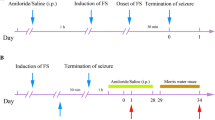
Lewis rats were born and housed with their mothers in a quiet, temperature-controlled room on a 12 h light–dark cycle, with food and water freely available. The pups were assigned to three groups (Fig. 1a): pHS-IL1b (n = 14); pHS-saline (n = 16); and control (n = 15). All experimental procedures conformed to the guidelines from the Ministry of Education of Japan and were approved by the animal experimental committee of our University (No. TE-17-2).
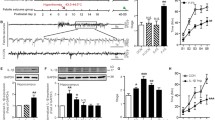
Experimental design and EEG findings. (a) Schematic showing the timescale of pHS induction, developmental IL-1β administration, and behavioral and histological tests in adulthood. (b) Representative EEG showing sporadic interictal events in the hippocampus, and spontaneous electrographic seizures recorded from hippocampal electrodes, consisting of polyspikes or sharp-wave trains that spread to the cortex
Seizure induction
We induced seizures in the pHS-IL1b and pHS-saline groups on P10–11 using a stream of heated air as described previously (Fukuda et al. 2014). Core temperature was controlled at 40.5–41.8 °C. Seizures were not induced in control rats. On P21, all pups were weaned and housed three per cage.
Administration of cytokine
Recombinant human IL-1β (500 ng; PeproTech, Inc., Rocky Hill, NJ, USA) was dissolved in 0.9 % (w/v) saline and administered intranasally (20 μL) immediately after pHS, and again the following day, to simulate the long-lasting elevation of cytokines observed after pHS (Dubé et al. 2010; Fukuda et al. 2009; Lawrence 2002).
Assessment of motor and cognitive function
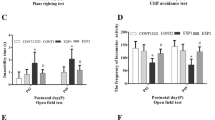
The rotarod test was performed on P85–87 to evaluate motor coordination. Animals were initially trained to stay on the rotarod (rotating at 4 rpm) for three consecutive sessions of 2 min, separated by 3 h intervals during which the animals were returned to their home cages. For the test, the rod accelerated from 4 to 20 rpm; motor integrity and coordination were assessed over four trials as the mean latency from the beginning of the trial until the rat fell from the rotarod.
The passive avoidance paradigm began the next day to evaluate learning and memory. Each rat was placed individually in the illuminated chamber, and upon entry into the dark chamber received electric shocks (100 V, 1.0 A, 2 s) twice to the feet through the floor grid. Rats were immediately returned to the home cage. The following day, the rats were placed into the same illuminated chamber and the interval between placement into the apparatus and entry into the dark compartment (step-through latency) was recorded, with a cut-off time of 300 s.
Assessment of seizure activity
After the passive avoidance test, rats were anesthetized with pentobarbital (25 mg/kg i.p.) and silver electroencephalogram (EEG) electrodes were implanted in the left hippocampus (AP −4.0, L 3.5, V 3.5 mm relative to bregma) and cortex (over the left occipital cortex). After 3–4 days’ recovery, long-term EEG recordings and visual observation were carried out over two separate 4 h sessions (6–10 p.m.) at P90–95 and P120–125 (Fig. 1a). Electrographic seizures were defined as events consisting of polyspikes or sharp waves (amplitude > twofold background) that lasted more than 6 s (Dubé et al. 2010).
Hippocampal neuron quantification and evaluation of gliosis
After EEG monitoring, rats were deeply anesthetized with pentobarbital and killed by cervical dislocation. Brains were removed, perfused with 4 % (w/v) paraformaldehyde, and embedded in paraffin for sectioning. Using a microtome, 10 μm coronal sections were cut from the septal area where the two blades of the dentate are equal in size and form a V shape (2.8 mm posterior to bregma), to a point 3.3 mm posterior to bregma. Every 10th section was stained with cresyl violet, and cell counts were performed in the pyramidal cell layers of CA1 and CA3 (defined as described previously; Fukuda et al. 2014) of the right hippocampus by two investigators who were blind to the experimental grouping. Neurons were included when the nucleus and nucleolus were clearly identifiable.
Every 20th section was used for glial fibrillary acidic protein (GFAP) staining. Sections were deparaffinized, rehydrated and washed; endogenous peroxidase was quenched with 3 % (v/v) hydrogen peroxide in water. Sections were incubated overnight at 4 °C with goat anti-GFAP polyclonal primary antibody (Chemicon, Temecula, CA, USA). The following day, the sections were incubated with the secondary antibody, HRP-conjugated rabbit anti-goat IgG (Dako Japan, Co., Ltd. Kyoto, Japan) for 3 h at room temperature. Staining was visualized using diaminobenzidine (Dako). The area of GFAP staining was measured in CA1 and CA3 using Adobe Photoshop CS4 Extended (Adobe Systems, San Jose, CA) and ImageJ 1.47 (Wayne Rasband, National Institute of Health, Bethesda, ML, USA).
Statistical analysis
Data were analyzed using a one-way ANOVA followed by Scheffe’s multiple comparisons test for parametric data; the Kruskal–Wallis test followed by Dunn’s test for nonparametric data; or χ 2 followed by Bonferroni’s test (SPSS version 20; SPSS Japan, Tokyo, Japan). Values are expressed as the mean ± S.E. and p < 0.05 was considered significant.
Results
Prolonged hyperthermia-induced seizures in developing rats
Hyperthermia was maintained for 30 min after seizure onset (40.2 ± 0.2 °C), and total seizure duration was 20–24 min. The maximum core temperature during hyperthermia was 41.9 ± 0.3 °C. All rats showed stereotyped seizures, consisting of facial automatisms and repetitive sudden arrests of hyperkinesia during hyperthermia. These movements were occasionally followed by body flexion and loss of posture control, and sometimes clonic convulsions.
Assessment of adulthood seizure activity
Electrographic seizures recorded from hippocampal electrodes consisted of polyspikes or sharp-wave trains (Fig. 1b). Behavioral correlates of these seizures were sudden cessation of activity with or without facial automatisms. The incidence of spontaneous seizures was significantly greater in pHS-IL1b rats (35 %) than in pHS-saline rats (6 %) and control rats (0 %) (p < 0.05) (Fig. 2a). There was no significant difference between the two pHS groups in the number of seizures per hour (pHS-IL1b, 0.4 ± 1.0; pHS-saline, 0.02 ± 0.1; Fig. 2b) or their duration (pHS-IL1b, 12 ± 3 s; pHS-saline, 9 s; Fig. 2c).
Incidence, number and duration of EEG seizures. (a) Epilepsy incidence was higher in rats in the pHS-IL1b group than in the pHS-saline and control groups. (b) Number of seizures per hour was not significantly different between the pHS-IL1b and pHS-saline groups. (c) Seizure durations were not different between the pHS-IL1b and pHS-saline groups. *p < 0.05
Assessment of brain function and pathology
In the passive avoidance test, step-through latency in the pHS-IL1b group (median, 220 s; range 5–360 s) was significantly shorter than in the control group (360 s; p < 0.05), but was not different from that in the pHS group (median, 300 s; range, 72–360 s; Fig. 3b). The rotarod test did not reveal any significant differences in performance between the three groups (pHS-IL1b, 44 ± 4 s; pHS-saline, 56 ± 4 s; control, 51 ± 4 s; Fig. 3a). In the hippocampus, no differences were observed between the three groups in the number of neurons in the CA1 (pHS-IL1b, 49 ± 1; pHS-saline, 45 ± 1; control, 44 ± 2) or CA3 (pHS-IL1b, 42 ± 2; pHS-saline, 39 ± 2; control, 39 ± 2; Fig. 4a–c). Similarly, the GFAP-immunopositive area was not significantly different between the three groups in the CA1 (pHS-IL1b, 1.4 ± 0.2 × 100 μm2; pHS-saline, 1.2 ± 0.2 × 100 μm2; control, 1.5 ± 0.1 × 100 μm2) or CA3 (pHS-IL1b, 1.7 ± 0.2 × 100 μm2; pHS, 1.3 ± 0.2 × 100 μm2; control, 1.5 ± 0.1 × 100 μm2).
Cognitive and motor evaluation. (b) Step-through latency in the passive avoidance task was significantly shorter in the pHS-IL1b group than in the control group, but not the pHS group. (a) Latency to fall from the rotarod was not different between the three groups. *p < 0.05. Data are median and range (b) or mean ± SE (a)
Neuronal counts and astrogliosis. (a) 250,000 μm2 areas delineated for pathological analysis of the CA1 and CA3 pyramidal cell layers. (b, c) Neuronal cell counts were not different between groups. (d, e) GFAP-immunopositive staining was not different between groups. Data are presented as means ± S.E
Discussion
Our study is the first to use the infantile pHS model to study the effects of IL-1β on adult epileptogenesis. We have shown that IL-1β administration after postnatal pHS enhances epileptogenesis and impairs cognitive function in adulthood.
Reports using rodents have identified a relationship between seizure susceptibility in adulthood and pretreatment with lipopolysaccharide or IL-1β (Fukuda et al. 2009; Lee et al. 2012), but few studies have examined the influence of proinflammatory cytokines in the infantile brain on adulthood epileptogenesis after prolonged neonatal febrile seizures, either in animals or in humans. Intranasal administration of 500 ng recombinant human IL-1β is sufficient to significantly elevate IL-1β protein concentrations in the frontal lobes of immature rats to 288 pg/mg (range, 222–534) (Fukuda et al. 2009), hence a 500 ng dose of IL-1β was considered appropriate for the present study.
It was previously reported that pHS in developing rats induced long-lasting endogenous IL-1β production in the hippocampus, and that de novo synthesis of IL-1β was induced for at least 24 h before declining to control levels (Dubé et al. 2010). However, in a study of children with prolonged febrile seizures or acute encephalitis/encephalopathy, IL-1β concentrations in cerebrospinal fluid were elevated only in the patients with encephalitis/encephalopathy (from <4.0 to 45 pg/mL) and not in those with febrile seizures (Ichiyama et al. 1998). A genetic predisposition to IL-1β hyperproduction in patients who experience prolonged febrile seizures, however, may be important for subsequent epileptogenesis in adulthood. Indeed, the −511C/T polymorphism in the IL1B gene, which is related to a hyperproduction of IL-1β, is associated with development of MTLE (Kanemoto et al. 2003).
The mechanisms of action of IL-1β are numerous, including increasing blood–brain barrier permeability, inhibiting γ-aminobutyric acid (GABA)-mediated Cl− flux, promoting angiogenesis, and inhibiting or delaying the release of IL-1RA (an endogenous IL-1 antagonist) (Ravizza et al. 2011; Vezzani and Granata 2005; Vezzani et al. 2008). Early-life elevation of IL-1β via maternal or neonatal infection results in complex, long lasting changes in inflammatory cytokines in the neocortex, hippocampus, and serum (Arrode-Bruses and Bruses 2012; Garay et al. 2012), which may underlie increases in adulthood seizure susceptibility (Galic et al. 2009). Furthermore, IL-1β is thought to affect the function of N-methyl-D-aspartate (NMDA) receptor agonists, and NMDA receptor function is important in the pathogenesis of both febrile seizures and MTLE in humans (Viviani et al. 2003). IL-1β enhances NMDA receptor-mediated Ca2+ currents in rat hippocampal pyramidal neurons (Viviani et al. 2003) and is involved in kindling progression (Ravizza et al. 2008). Kindling epileptogenesis is associated with activation of the IL-1β system in astrocytes, likely triggered by the repetitive epileptogenic stimulation of the hippocampus (Ravizza et al. 2008). Another study indicated that an NMDA receptor blocker suppresses hyperthermia-induced seizures and their kindling in developing rats (Morimoto et al. 1995). Together, this evidence indicates that the enhanced adulthood epileptogenesis observed in the pHS-IL1b group in our study was a result of enhanced kindling induced by the action of IL-1β on NMDA receptors, and that endogenous IL-1β might exert its epileptogenic effects in children with febrile seizures via NMDA receptor activation. Future experiments will examine the expression patterns and function of NMDA receptors in adult rats with developmental IL-1β exposure.
In the passive-avoidance test, step-through latency was shorter in the pHS-IL1b group than in the control group, but was not significantly different from that in the pHS-saline group. It is possible that seizure activity before the passive avoidance test might bias the test in the learning paradigm and this may have contributed to the high variance in the pHS-IL1b group. Seizure monitoring should therefore be performed before cognitive testing.
Although memory impairment in MTLE is reported to correlate with the degree of hippocampal sclerosis (Cendes et al. 2012), and gliosis is a recognized characteristic of epileptic tissue (Pitkanen and Sutula 2002; Sharma et al. 2007), we found no abnormalities in hippocampal cell number or gliosis, consistent with previous findings in rats (Fukuda et al. 2014; Lee et al. 2012). However, hippocampal specimens obtained from surgically treated MTLE patients revealed five distinct neuropathological subgroups, one of which shows no significant pathological changes (Blümcke et al. 2007). Therefore, the pHS-IL1b group in our study may represent that specific MTLE subtype, indicating that significant hippocampal pathology is not a prerequisite for the epileptogenic process that follows febrile status epilepticus.
A limitation of our study is the lack of a fourth group, intranasal IL-1β without prior pHS. However, this condition was examined in a previous study, in which it was found that IL-1β alone has no significant influence on adulthood susceptibility to drug-induced seizures (Fukuda et al. 2014). This indicates that endogenous IL-1β hyperproduction in the developing rat is not in itself enough to influence epileptogenesis in adulthood.
In the present study, the incidence of epilepsy in the pHS-saline group was 6 %. This figure is remarkably consistent with data obtained cohort studies investigating the incidence of subsequent epilepsy in children with febrile seizures (Shorvon and Goodridge 2013). This similarity highlights the validity of the pHS model as a tool for the study of infantile febrile seizures.
In conclusion, we have shown that postnatal pHS and an excess of IL-1β enhances epileptogenesis in adulthood. Our results indicate that prolonged febrile seizures in childhood, coupled with IL-1β overproduction, may contribute to the development of MTLE.
References
Arrode-Bruses G, Bruses JL (2012) Maternal immune activation by poly(I:C) induces expression of cytokines IL-1beta and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J Neuroinflammation 9:83
Auvin S, Shin D, Mazarati A, Sankar R (2010) Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia 51(Suppl 3):34–38
Blümcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstöck J, Stefan H, Hildebrandt M (2007) A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 113:235–244
Cendes F, Kahane P, Brodie M, Andermann F (2012) The mesio-temporal lobe epilepsy syndrome. In: Bureau M, Genton P, Dravet C, Delgado-Escueta AV, Tassinari CA, Thomas P, Wolf P (eds) Epileptic syndrome in infancy, childhood, and adolescence, 5th edn. John Libbery Eurotext Ltd, Paris, pp 383–399
Dubé C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ (2000) Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol 47:336–344
Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ (2005) Interleukin-1β contributes to the generation of experimental febrile seizures. Ann Neurol 57:152–155
Dubé C, Richichi C, Bender RA, Chung G, Littt B, Baram TZ (2006) Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain 129:911–922
Dubé C, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30:7484–7494
Fukuda M, Suzuki Y, Ishizaki Y, Kira R, Kikuchi C, Watanabe S, Hino H, Morimoto T, Hara T, Ishii E (2009) Interleukin-1β enhances susceptibility to hyperthermia-induced seizures in developing rats. Seizure 18:211–214
Fukuda M, Hino H, Suzuki Y, Takahashi H, Morimoto T, Ishii E (2014) Postnatal interleukin-1β enhances adulthood seizure susceptibility and neuronal cell death after prolonged experimental febrile seizures in infantile rats. Acta Neurol Belg 114:179–185
Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ (2008) Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci 28:6904–6913
Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ (2009) Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis 36:343–351
Garay PA, Hsiao EY, Patterson PH, McAllister AK (2012) Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68
Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S (1998) Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures: comparison with acute encephalitis/encephalopathy. Neurology 50:407–411
Kanemoto K, Kawasaki J, Yuasa S, Kumaki T, Tomohiro O, Kaji R, Nishimura M (2003) Increased frequency of interleukin-1β-511 T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia 44:796–799
Kira K, Torisu H, Takemoto M, Nomura A, Sakai Y, Sanefuji M, Sakamoto K, Matsumoto S, Gondo K, Hara T (2005) Genetic susceptibility to simple febrile seizures: interleukin-1β promoter polymorphisms are associated with sporadic cases. Neurosci Lett 384:239–244
Lawrence D (2002) Intranasal delivery could be used to administer drugs directly to the brain. Lancet 359:1674
Lee SH, Kim BJ, Kim YB, Chung PW, Moon HS, Suh BC, Yoon WT, Jin DK, Park YS, Lee YT, Park KY (2012) IL-1β induction and IL-6 suppression are associated with aggravated neuronal damage in a lipopolysaccharide-pretreated kainic acid-induced rat pup seizure model. Neuroimmunomodulation 19:319–325
Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, Xu Y, MacFall J, Gomes WA, Moshé SL, Mathern GW, Pellock JM, Nordli DR Jr, Frank LM, Provenzale J, Shinnar RC, Epstein LG, Masur D, Litherland C, Sun S, FEBSTAT Study Team (2014) Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann Neurol 75:178–185
Morimoto T, Kida K, Nagao H, Yoshida K, Fukuda M, Takahashi S (1995) The pathogenic role of the NMDA receptor in hyperthermia-induced seizures in developing rats. Dev Brain Res 84:204–207
Omran A, Peng J, Zhang C, Xiang QL, XueJ GN, Kong H, Yin F (2012) Interleukin-1β and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia 53:1215–1224
Pitkanen A, Sutula TP (2002) Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol 1:173–181
Qulu L, Daniels WM, Mabandia MV (2012) Exposure to prenatal stress enhances the development of seizures in young rats. Metab Brain Dis 27:399–404
Ravizza T, Noé F, Zardoni D, Vaghi V, Sifringer M, Vezzani A (2008) Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol Dis 31:327–333
Ravizza T, Balosso S, Vezzani A (2011) Inflammation and prevention of epileptogenesis. Neurosci Lett 497:223–230
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW et al (2007) Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol 35:984–999
Shorvon SD, Goodridge DM (2013) Longitudinal cohort studies of the prognosis of epilepsy: contribution of the National General Practice Study of Epilepsy and other studies. Brain 136:3497–3510
Tanabe T, Hara K, Shimakawa S, Fukui M, Tamai H (2011) Hippocampal damage after prolonged febrile seizure: one case in a consecutive prospective series. Epilepsia 52:837–840
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743
Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22:797–803
Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartifai T, Binaglia M, Corsini E, Luca MD, Galli CL, Marinovich M (2003) Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 24:8692–8700
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology (No. 22591132) and The Setsuro Fujii Memorial Osaka Foundation for Promotion of Fundamental Medical Research, and a Research Grant of the Japan Epilepsy Research Foundation. We are grateful to the staff of the Animal Center of INCS of Ehime University for their care of our animals, and Takeshi Kiyoi for help with histological staining.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures conformed to the guidelines from the Ministry of Education of Japan and were approved by the animal experimental committee of our University. This article does not contain any studies with animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, M., Ito, M., Yano, Y. et al. Postnatal interleukin-1β administration after experimental prolonged febrile seizures enhances epileptogenesis in adulthood. Metab Brain Dis 30, 813–819 (2015). https://doi.org/10.1007/s11011-014-9648-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-014-9648-7