Abstract
The ketogenic diet (KD) has been shown to be effective as an antiepileptic therapy in adults, but it has not been extensively tested for its efficacy in neonatal seizure-induced brain damage. We have previously shown altered expression of zinc/lipid metabolism-related genes in hippocampus following penicillin-induced developmental model of epilepsy. In this study, we further investigated the effect of KD on the neurobehavioral and cognitive deficits, as well as if KD has any influence in the activity of zinc/lipid transporters such as zinc transporter 3 (ZnT-3), MT-3, ApoE, ApoJ (clusterin), and ACAT-1 activities in neonatal rats submitted to flurothyl-induced recurrent seizures. Postnatal day 9 (P9), 48 Sprague-Dawley rats were randomly assigned to two groups: flurothyl-induced recurrent seizure group (EXP) and control group (CONT). On P28, they were further randomly divided into the seizure group without ketogenic diet (EXP1), seizure plus ketogenic diet (EXP2), the control group without ketogenic diet (CONT1), and the control plus ketogenic diet (CONT2). Neurological behavioral parameters of brain damage (plane righting reflex, cliff avoidance reflex, and open field test) were observed from P35 to P49. Morris water maze test was performed during P51–P57. Then hippocampal mossy fiber sprouting and the protein levels of ZnT3, MT3, ApoE, CLU, and ACAT-1 were detected by Timm staining and Western blot analysis, respectively. Flurothyl-induced neurobehavioral toxicology and aberrant mossy fiber sprouting were blocked by KD. In parallel with these behavioral changes, rats treated with KD (EXP2) showed a significant down-regulated expression of ZnT-3, MT-3, ApoE, clusterin, and ACAT-1 in hippocampus when compared with the non-KD-treated EXP1 group. Our findings provide support for zinc/lipid transporter signals being potential targets for the treatment of neonatal seizure-induced brain damage by KD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is a typical metal ion that contributes to the epileptogenesis under pathologic conditions such as seizure attack, ischemia, traumatic brain injury, autism spectrum disorders, etc. [1–4]. Hippocampal mossy fibers (MFs), which contains abundant zinc and is important in keeping the balance between excitatory and inhibitory system, has been well established to be involved in the pathological process of epileptogenesis following an initial brain-damaging insult [5], which is represented by the aberrant MF regenerative sprouting revealed by Timm staining. Previous studies have demonstrated that up-regulated expression changes of zinc transporter 3 (ZnT-3) in the rat hippocampus may be responsible for this pathological hallmark using adult or developmental seizure models [6, 7]. However, few studies have investigated the post-seizure modulation of ZnT-3 and its association with neurobehavioral and cognitive changes.
In this study, we investigated the effect of a high-fat low-carbohydrate ketogenic diet (KD) on neonatal seizure-induced neurobehavioral deficits, as well as if KD has any influence in the activity of ZnT-3 in hippocampus. KD has been used for drug-resistant refractory epilepsy since the 1920s. Considering the fact that 20–25 % patients develop therapeutic failure with conventional antiepileptic drugs (AEDs) [8] and the serious physiological and psychological harm to patients of AEDs, this traditional diet therapy has attracted intensive attention in recent clinical practice.
Hypothesized anticonvulsant mechanisms of KD focus on decreased excitability and/or increased inhibition through energy metabolism mechanism, especially the partitioning of ketone bodies toward GABA [9, 10]. Yet the key metabolic pathways in vivo remains poorly understood with neuronal effects. Despite transient ketosis, the classic high-fat ketogenic diet could also induce marked changes in fatty acid metabolism, which may contribute to the seizure protection by KD as well [11]. We have recently found up-regulated expressions of lipid metabolism-related genes in hippocampus following developmental seizures, such as apolipoprotein J (clusterin), apolipoprotein E, and ACAT-1, which could be down-regulated by pretreatment with autophagy inhibitors before the acute seizure attacks [12]. Coincidently, Lee JY et al. reported that the level of histochemically reactive zinc (principally synaptic zinc) was significantly reduced in the ApoE-deficient brain compared to wild type, suggesting that ApoE may affect the cerebral free zinc pool that contributes to Alzheimer’s disease pathology [13]. This prompts us to predict that the interaction of Zn2+ with lipid metabolism signals may exist in experimental neonatal seizure model.
In this study, we investigated the effect of KD on neurobehavioral deficits, as well as if KD has any influence in the activity of zinc/lipid signal-related genes, such as ZnT-3, ApoE, ApoJ, and ACAT-1 in Sprague-Dawley rats submitted to recurrent neonatal seizures.
Materials and Methods
Animal Preparation
Sprague-Dawley rats (number = 48) at postnatal day 8 (P8, weighing between 12.59 and 15.30 g) were obtained from the Chinese academy of sciences, Shanghai Experimental Animal Center, China. Rats were kept in an environment-controlled room which was away from bright light and noise under a 12 h/12 h light/dark cycle. The animals were adapted for 1 day before the study and had free access to standard laboratory food and water ad libitum. The animals were treated in accordance with the guidelines set by the National Institutes of Health for the humane treatment of animals. Attempts were made to reduce animal suffering and the number of animals used. Forty-eight Sprague-Dawley rats were randomly assigned to two groups: flurothyl (bis-2,2,2-triflurothyl ether; Sigma-Aldrich Chemical, WI, USA)-induced recurrent seizure group (EXP, n = 24) and control group (CONT, n = 24). Seizures were induced in EXP rats with volatile flurothyl (bis-2,2,2-triflurothyl ether, Aldrich-Sigma Chemical, WI, USA), a potent and rapidly acting central nervous system stimulant that produces seizures within minutes of exposure once a day for eight consecutive days, from P9 to P16. The procedure of seizure induction had been described in detail previously [14]. CONT rats were placed into the container for an equal amount of time to their counterpart without exposing to flurothyl.
At weaning day P21, rats were further randomly divided into four groups: the seizure group without KD (EXP1, n = 12), the seizure plus KD (EXP2, n = 12), the control group without KD (CONT1, n = 12), and the control plus KD (CONT2, n = 12). At P28, rats in EXP2 and CONT2 groups received KD, while rats in EXP1 and CONT1 groups received normal diet. The formula of KD was reported in detail previously [15]. KD (70 % fat, 20 % protein, and no carbohydrate) and normal diet (50 % fat, 20 % protein, and 4.5 % carbohydrate) were obtained from Chinese Academy of Sciences, Shanghai Experimental Animal Center, China. All rats were given food and water ad libitum for 4 weeks.
Neurobehavioral Tests
Neurological behavioral parameters of brain damage (plane righting reflex, cliff avoidance reflex, and open field test) were observed on P35, P42, and P49 according to the procedure previously described [16, 17].
Morris Water Maze Test
During the five consecutive days (P51–P55), rats (n = 12 each group) were tested in the Morris water maze to evaluate visual-spatial learning and memory ability. The procedure has been described previously [18, 19]. In brief, for the place navigation test, each rat was allowed to find the hidden platform for a maximum of 60 s. If an animal did not find the platform in the special time, it would be placed onto the platform for 10 s to identify spatial cues before being returned to the cage. The escape latency (the duration for finding the platform) was automatically recorded by a video/compute system. For the spatial probe test, the platform was removed from the pool on alternate days after the navigation test (P57). Then each rat was placed in the water for 60 s and the frequency (number of times) of passing through the platform quadrant was recorded to reflect the spatial memory ability.
Timm Staining
On P58, a subset of rats was sacrificed by decapitation for Timm staining (n = 6/each group). The pyramidal/infra-pyramidal CA3 region and the inner molecular layer of the dentate gyrus of hippocampus were assessed on each section. Mossy fiber sprouting was analyzed using a semiquantitative scale for terminal sprouting in the CA3 and the dentate gyrus [20].
Western Blot Analysis
Protein levels were detected by Western blot method [19] on P58 from the rest part of rats after water maze test (n = 6/each group). Polyvinylidene fluoride membrane blots after blocking solution TBST were incubated with one of the following antibodies: a rabbit anti-ACAT-1 polyclonal antibody (1:200, Cayman Chemical), a goat anti-ApoE polyclonal antibody (1:200, Santa Cruz), a goat anti-clusterin polyclonal antibody (1:500, Santa Cruz), a goat anti-ZnT-3 (1:200, Santa Cruz) or MT3 (1:200, Santa Cruz) polyclonal antibody in Tris-buffered saline containing 0.2 % Tween-20 (TBST), and 5 % nonfat dry milk overnight at 4 °C. The blot was then incubated with the secondary antibody for about 2 h at ambient temperature. Antibody reactions were exposed with Kodak X-ray film using the ECL detection system Amersham. The relative changes of the intensity of each immunoreactive band were evaluated with Sigma Scan Pro 5 and were normalized to a loading control GAPDH.
Statistical Analysis
The behavioral measures, escape latency of place navigation test, the frequency of passing through the platform quadrant of spatial probe test in the water maze, Timm staining, and the protein levels were analyzed with post hoc comparisons using a Bonferroni test after ANOVA by SAS 8.0 statistical software. Data were presented as the mean ± SD, and statistical significance was considered as a P < 0.05.
Results
Neurological Behavior
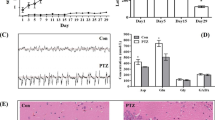
The effects of neonatal seizure and chronic treatment by KD on neurological development may be presented by different neurological reflexes and open field test. As a result, the EXP1 group showed a significant delay or reduction of plane righting reflex and cliff avoidance reflex, as well as poor performance in open field test (Fig. 1a–d). On the other hand, these deleterious changes were reversed by KD post-seizure treatment in the EXP2 group compared with those in the EXP1 group (Fig. 1a–d).
At P35, P42, and P49, times of each group in plane righting test and cliff avoidance test (a, b). In open filed test, the immobility time and the frequency of locomotor activity were recorded at P42 and P49 (c, d). And in water maze test, during the 5 days of training, mean escape latency for each group is plotted (e). The frequencies of crossing the platform were recorded at P57 (f).*P < 0.05, compared with CONT1; # P < 0.05, compared with EXP1. (n = 12/group)
Morris Water Maze Test
It could be seen from Fig. 1e that the escape latencies from the water maze were significantly longer in rats of the EXP1 group from P51 to P55 than in control rats; on the other hand, however, the latency was significantly decreased in rats of the KD-treated EXP2 group than that of the EXP1 group at P54 and P55. As far as spatial probe test was concerned, the frequency of passing through the platform quadrant was significantly lower in the EXP1 group than in the control. This decrease was reversed by KD as compared between the EXP1 and EXP2 groups in the probe tests (Fig. 1f).
Timm Staining
The Timm staining pattern in rats subjected to recurrent seizures differed from the control rats. In rats of EXP1 (Fig. 2c) and EXP2 (Fig. 2d) groups, there was an increased distribution of Timm granules in the region of stratum pyramidale of CA3 subfield and supragranular of dentate gyrus, while remaining barely visible in CONT1 (Fig. 2a) and CONT2 (Fig. 2b). In addition, the Timm score decreased remarkable in EXP2 group compared with that in EXP1 group (Fig. 2c).
Example of mossy fiber sprouting by Timm staining in CONT1 [a (1)–(2)], CONT2 [b (1)–(2)], EXP1 [c (1)–(2)], EXP2 [d (1)–(2)]. a (1)–d (1) represent dentate gyrus subfield and a (2)–d (2) represent CA3 subfield from CONT1 to EXP2, respectively. Note the excessive amount of Timm staining in the inner molecular layer of the granule cells [c (1)] and the stratum pyramidale of CA3 subfield [c (2)] in EXP1 group (arrows). Calibration bars = 30 μm
Western Blot Analysis
Western blot was employed to evaluate the relative protein levels of ZnT3, MT3, ApoE, CLU, and ACAT-1 in hippocampus after Morris water maze analysis. As shown in Fig. 3, EXP1 rats had a higher amount of ZnT3, MT3, ApoE, CLU, and ACAT-1 in hippocampus when compared with control rats. In addition, there were long-term decrease of ZnT3, MT3, ApoE, CLU, and ACAT-1 of KD-treated EXP2 rats compared with that in EXP1 group.
Discussion
Recent clinical and experimental studies have shown that KD could decrease peripheral vitamin and mineral levels, including zinc [21–23]. To our knowledge, alteration of zinc metabolism-related gene expressions in the brain after KD treatment, however, has not been investigated so far. In the current study, we found increased expression of ZnT-3 and MT-3 in hippocampus following neonatal seizures which was inhibited by chronic KD treatment. It has been documented that the physiological activity of free zinc in the normal brain might largely depend on the pool of synaptic vesicle zinc that is determined by ZnT-3, as well as MT-3 [24, 25]. Thus, the increased level of ZnT-3 and MT-3 in this study would, in principle, imply a raised hippocampal synaptic vesicle zinc levels [26]. It is reported that ZnT-3 is regulated by age, hormones, fatty acids, zinc chelation, and glucose [27]. Lee JY et al. showed that estrogen decreased ZnT-3 expression and synaptic vesicle zinc levels in mouse brain through changing AP-3 delta expression [28]. Here, we provided additional evidence that KD could modulate not only the expression of hippocampal ZnT-3 and MT-3 but also lipid metabolism-related ApoE, ApoJ, and ACAT-1 following neonatal seizure-induced brain damage, which may provide new insights into the mechanism of ZnT-3 regulation in the brain.
As long as zinc-induced lipid peroxidation is concerned, clinical and experimental studies have demonstrated that proper levels of zinc and antioxidant enzyme activity are crucial for the brain in maintaining normal neurological functions. Dietary zinc deficiency can cause increased lipid peroxidation while zinc supplementation inhibited the increased oxidative stress by activating the antioxidant system [29–31]. In addition, acute zinc neurotoxicity involves lipid peroxidation-dependent rises in intracellular levels of reactive oxygen species (ROS) [32–34]. Furthermore, disturbance of trace element level (including Fe, Cr, Se, and Zn) combined with declined antioxidant activity plays a significant role in responsible for the etiology of cerebral ischemia [35]. Whether this zinc-induced lipid peroxidation and oxidative stress is also a cause of developmental neurodegenerative processes such as hippocampal mossy fiber sprouting and neurobehavioral damage following developmental seizures, on the other hand, has not been examined. Interestingly, recent studies on Alzheimer’s disease and epilepsy highly suggest that this may quite be the case. For example, metal ions (copper, iron, and zinc), ApoE, clusterin, and cholesterol affect parallel molecular and biochemical pathways involved in the progression of AD pathology [36–39]. Coincidently, elevated expression of ApoE, clusterin, and acyl-coenzyme A:cholesterol acyltransferase (ACAT1) in rat hippocampus, the enzyme involved in cholesteryl ester biosynthesis has been found after kainic acid excitotoxicity [40, 41], which could be prevented by KD [42]. In addition, ApoE ablation decreases synaptic vesicular zinc in the brain [13]. In this study, we found a long-term increase in the expression of ZnT-3, MT-3, ApoE, clusterin, and ACAT-1 in the hippocampus of rats following flurothyl-induced neonatal seizures. These results are in line with hypothesized changes in the aberrant sprouting of regenerative mossy fibers and behavioral deficits. More importantly, chronic treatment with a ketogenic diet (KD) reversed the adverse pathological, neurobehavioral, and neurochemical changes. Thus, this report extends the observation in experimental epilepsy models suggesting that adaptive energy metabolism changes in gene expression are involved in KD’s anticonvulsant effects [43, 44].
In conclusion, this study supports the notion that zinc/lipid transporter pathways are involved in the neurodegeneration following developmental seizures, especially the long-term aberrant mossy fiber sprouting in hippocampus. The results may provide important clues towards zinc/lipid transporter signals being potential targets for the treatment of neonatal seizure-induced brain damage by KD.
References
Kim JH, Jang BG, Choi BY et al (2012) Zinc chelation reduces hippocampal neurogenesis after pilocarpine-induced seizure. PLoS One 7:e48543
Choi BY, Kim JH, Kim HJ et al (2014) Zinc chelation reduces traumatic brain injury-induced neurogenesis in the subgranular zone of the hippocampal dentate gyrus. J Trace Elem Med Biol 28:474–481
Suh SW, Won SJ, Hamby AM et al (2009) Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J Cereb Blood Flow Metab 29:1579–1588
Grabrucker S, Jannetti L, Eckert M et al (2014) Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain 137:137–152
Sanchez RM, Ribak CE, Shapiro LA (2012) Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy. Epilepsia 53:98–108
Linkous DH, Flinn JM, Koh JY et al (2008) Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J Histochem Cytochem 56:3–6
Cole TB, Wenzel HJ, Kafer KE et al (1999) Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A 96:1716–1721
Anovadiya AP, Sanmukhani JJ, Tripathi CB (2012) Epilepsy: novel therapeutic targets. J Pharmacol Pharmacother 3:112–117
Zhang Y, Zhang S, Marin-Valencia I et al (2015) Decreased carbon shunting from glucose toward oxidative metabolism in diet-induced ketotic rat brain. J Neurochem 132:301–312
Bough K (2008) Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia 49:91–93
Taha AY, Ryan MA, Cunnane SC (2005) Despite transient ketosis, the classic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabolism 54:1127–1132
Ni H, Ren SY, Zhang LL et al (2013) Expression profiles of hippocampal regenerative sprouting-related genes and their regulation by E-64d in a developmental rat model of penicillin-induced recurrent epilepticus. Toxicol Lett 217:162–169
Lee JY, Cho E, Kim TY et al (2010) Apolipoprotein E ablation decreases synaptic vesicular zinc in the brain. Biometals 23:1085–1095
Ni H, Jiang YW, Bo T et al (2005) c-Fos, N-methyl-d-aspartate receptor 2C, GABA-A-alpha1 immonoreactivity, seizure latency and neuronal injury following single or recurrent neonatal seizures in hippocampus of Wistar rat. Neurosci Lett 380:149–154
Ziegler DR, Araújo E, Rotta LN et al (2002) A ketogenic diet increases protein phosphorylation in brain slices of rats. J Nutr 132:483–487
Rothstein S, Simkins T, Nuñez JL (2008) Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience 152:959–969
de Araújo DP, De Sousa CN, Araújo PV et al (2013) Behavioral and neurochemical effects of alpha-lipoic acid in the model of Parkinson’s disease induced by unilateral stereotaxic injection of 6-ohda in rat. Evid Based Complement Alternat Med 2013:571378
Mychasiuk R, Hehar H, van Waes L et al (2014) Diet, age, and prior injury status differentially alter behavioral outcomes following concussion in rats. Neurobiol Dis 73C:1–11
Ni H, Yan JZ, Zhang LL et al (2012) Long-term effects of recurrent neonatal seizures on neurobehavioral function and related gene expression and its intervention by inhibitor of cathepsin B. Neurochem Res 37:31–39
Sliwa A, Plucinska G, Bednarczyk J et al (2012) Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett 509:105–109
Christodoulides SS, Neal EG, Fitzsimmons G et al (2012) The effect of the classical and medium chain triglyceride ketogenic diet on vitamin and mineral levels. J Hum Nutr Diet 25:16–26
Hayashi A, Kumada T, Nozaki F et al (2013) Changes in serum levels of selenium, zinc and copper in patients on a ketogenic diet using Ketonformula. No To Hattatsu 45:288–293
Frommelt L, Bielohuby M, Stoehr BJ et al (2014) Effects of low-carbohydrate, high-fat diets on apparent digestibility of minerals and trace elements in rats. Nutrition 30:869–875
Lee JY, Kim JS, Byun HR et al (2011) Dependence of the histofluorescently reactive zinc pool on zinc transporter-3 in the normal brain. Brain Res 1418:12–22
Lee JY, Kim JH, Palmiter RD et al (2003) Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol 184:337–347
Linkous DH, Flinn JM, Koh JY et al (2008) Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J Histochem Cytochem 56:3–6
Smidt K, Rungby J (2012) ZnT3: a zinc transporter active in several organs. Biometals 25:1–8
Lee JY, Kim JH, Hong SH et al (2004) Estrogen decreases zinc transporter 3 expression and synaptic vesicle zinc levels in mouse brain. J Biol Chem 279:8602–8607
Baltaci AK, Mogulkoc R, Ayyildiz M et al (2014) Lipid peroxidation in kidney and testis tissues in experimental hypothyroidism: the role of zinc. Bratisl Lek Listy 115:498–501
Ozturk A, Baltaci AK, Mogulkoc R et al (2003) Effects of zinc deficiency and supplementation on malondialdehyde and glutathione levels in blood and tissues of rats performing swimming exercise. Biol Trace Elem Res 94:157–166
Shaheen AA, el-Fattah AA (1995) Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int J Biochem Cell Biol 27:89–95
Kim YH, Kim EY, Gwag BJ et al (1999) Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience 89:175–182
Noh KM, Koh JY (2000) Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci 20:RC111
Kim EY, Koh JY, Kim YH et al (1999) Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. Eur J Neurosci 11:327–334
Fang KM, Cheng FC, Huang YL et al (2013) Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol Trace Elem Res 152:66–74
Wong BX, Hung YH, Bush AI et al (2014) Metals and cholesterol: two sides of the same coin in Alzheimer’s disease pathology. Front Aging Neurosci 6:91
Xu H, Finkelstein DI, Adlard PA (2014) Interactions of metals and apolipoprotein E in Alzheimer’s disease. Front Aging Neurosci 6:121
Hughes TM, Lopez OL, Evans RW et al (2014) Markers of cholesterol transport are associated with amyloid deposition in the brain. Neurobiol Aging 35:802–807
Nuutinen T, Suuronen T, Kauppinen A et al (2010) Valproic acid stimulates clusterin expression in human astrocytes: implications for Alzheimer’s disease. Neurosci Lett 475:64–68
Kim JH, Ee SM, Jittiwat J et al (2011) Increased expression of acyl-coenzyme A: cholesterol acyltransferase-1 and elevated cholesteryl esters in the hippocampus after excitotoxic injury. Neuroscience 185:125–134
Zhang XM, Mao XJ, Zhang HL et al (2012) Overexpression of apolipoprotein E4 increases kainic-acid-induced hippocampal neurodegeneration. Exp Neurol 233:323–332
Noh HS, Kim DW, Kang SS et al (2005) Ketogenic diet prevents clusterin accumulation induced by kainic acid in the hippocampus of male ICR mice. Brain Res 1042:114–118
Danial NN, Hartman AL, Stafstrom CE et al (2013) How does the ketogenic diet work? Four potential mechanisms. J Child Neurol 28:1027–1033
Bough K (2008) Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia 49:91–93
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81271458, 81471337), Jiangsu Province’s Key Provincial Talents Program (RC2011113), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, T., Ni, H. & Sun, Bl. Neurobehavioral Deficits in a Rat Model of Recurrent Neonatal Seizures Are Prevented by a Ketogenic Diet and Correlate with Hippocampal Zinc/Lipid Transporter Signals. Biol Trace Elem Res 167, 251–258 (2015). https://doi.org/10.1007/s12011-015-0285-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0285-8