Abstract
D-galactose (GAL) causes aging-related changes and oxidative stress in the organism. We investigated the effect of whole fresh blueberry (BB) (Vaccinium corymbosum L.) treatment on oxidative stress in age-related brain damage model. Rats received GAL (300 mg/kg; s.c.; 5 days per week) alone or together with 5 % (BB1) and 10 % (BB2) BB containing chow for two months. Malondialdehyde (MDA),protein carbonyl (PC) and glutathione (GSH) levels, and Cu Zn-superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and glutathione transferase (GST) activities as well as acetylcholinesterase (AChE) activities were determined. Expressions of B cell lymphoma-2 (Bcl-2), Bax and caspase-3 were also evaluated in the brain by immunohistochemistry. MDA and PC levels and AChE activity increased, but GSH levels, SOD and GSH-Px activities decreased together with histopathological structural damage in the brain of GAL-treated rats. BB treatments, especially BB2 reduced MDA and PC levels and AChE activity and elevated GSH levels and GSH-Px activity. BB1 and BB2 treatments diminished apoptosis and ameliorated histopathological findings in the brain of GAL-treated rats. These results indicate that BB partially prevented the shift towards an imbalanced prooxidative status and apoptosis together with histopathological amelioration by acting as an antioxidant (radical scavenger) itself in GAL-treated rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
D-galactose (GAL) overload was reported to induce changes that resemble the normal aging process in tissues of rodents (Hsieh et al. 2009; Hsia et al. 2012; Banji et al. 2013). High doses of GAL results in accumulation of galactitol, leading to osmotic stress and generation of reactive oxygen species (ROS) (Hsieh et al. 2009; Anand et al. 2012; Hsia et al. 2012; Tsai and Yin 2012; Banji et al. 2013). It has been reported that high dose GAL-treated animals show increased production of ROS and lowered activities of antioxidant enzymes in the brain (Hsieh et al. 2009; Hsia et al. 2012; Lan et al. 2012; Yang et al. 2012; Prakash and Kumar 2013; Wu et al. 2014). GAL also reacts readily with the free amines of amino acids in proteins to form advanced glycation end products (AGEs). AGEs can also cause the accumulation of ROS, especially superoxide radicals and hydrogen peroxide (Tsai and Yin 2012; Zhang et al. 2013). Since oxidative stress is suggested as one of the main mechanisms of naturally aging (Harman 2001; Parıldar-Karpuzoğlu et al. 2008; Aydın et al. 2010), this GAL-induced aging model has been frequently used for brain aging and antiaging pharmacology studies.
Blueberries (BB; Vacciniumcorymbosum L.) are among the fruits with high antioxidant power and contain several polyphenolic compounds such as flavonoids, nonflavonoids and phenolic acids. A subset of the flavonoids known as anthocyanins is particularly abundant in BB and they may play an important role in protective effect of BB. These compounds have antioxidant, anti-inflammatory and metal chelating activities (Neto 2007; Zafra-Stone et al. 2007; Tsao 2010; Schaffer and Halliwell 2012). The use of BB was suggested to be useful in inflammation, cancer, diabetes mellitus, hepatic, cardiovascular and neuronal disorders (Neto 2007; Zafra-Stone et al. 2007). It has also been proposed that BB may have antiaging and neuroprotective effects (Giacalone et al. 2011; Shukitt-Hale 2012). BB extract was reported to prolong the main lifespan and slow down the aging-related declines in C.elegans (Wilson et al. 2006) and fruit flies (Peng et al. 2012). It has also been suggested that BB can retard physiological and functional deficits in aged humans (Joseph et al. 2005; Krikorian et al. 2010) and rats (Goyarzu et al. 2004; Andres-Lacueva et al. 2005; Malin et al. 2011).
As it is known, the ability of polyphenolic compounds to cross blood–brain barrier (BBB) may influence their neuroprotective effects (Milbury and Kalt 2010; Shukitt-Hale 2012). The berry anthocyanins were reported to be able to cross the BBB and reach brain to directly exert their effects. The overall effects provided by whole BB were found to be much stronger than each of these compounds, since different polyphenols may show their effects via different mechanisms (Shukitt-Hale 2012).
To our knowledge, there is no study investigating the effect of BB on prooxidant and antioxidant balance in brain tissue in aging. Considering the role of oxidative stress in GAL-induced aging model and antioxidant effects of whole BB, we aimed to investigate the effect of BB treatment on prooxidant and antioxidant status in brain tissues in GAL-treated rats. For this reason, malondialdehyde (MDA) and protein carbonyl (PC) and glutathione (GSH) levels, and CuZn-superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and glutathione transferase (GST) activities as well as acetylcholinesterase (AChE) activities were determined in brain tissue of GAL-treated rats. Histopathological changes and expressions of B cell lymphoma-2 (Bcl-2), Bax and caspase-3 were also evaluated in the brain tissue.
Materials and methods
Chemicals
GAL and other chemicals were supplied from Sigma-Aldrich (St Louis, Missouri, USA).
The preparation of BB containing diets
Northern highbush “Patriot” BB (Vacciniumcorymbosum L.) were donated by Gedik Flora (Kartal-Istanbul). They were homogenized using a blender and BB homogenates were mixed with powdered rat chow by using a mixer for 15 min. Then, this mixture were dried and prepared as a pellet chow containing 5 and 10 % BB (w/w) by Barbaros Denizeri (Gebze-Kocaeli). The BB containing diets were made by replacing 5 and 10 % sucrose in the control diet with 5 and 10 % BB. Diets were stored at 4 °C.
Total phenolic and total flavonoid assay in BB
Total phenolic compounds were determined with the Folin-Ciocalteu reagent and expressed as mg gallic acid equivalent per 100 g BB (Liu et al. 2010). The total flavonoid levels were measured with aluminum chloride colorimetric method. The results are presented as mg quercetin equivalents per 100 g fresh BB (Yin et al. 2008). Total phenolic compounds and total flavonoid levels were detected as 260 mg gallic acid equivalents and 105 mg catechin equivalents per 100 g BB, respectively.
Animals and experimental design
Male Wistar rats aged 3–4 months weighing 200–220 g were used in the study. They were obtained from the Experimental Medicine Research Institute of Istanbul University. Rats were housed in a light- and temperature-controlled room on a 12/12-hr light/dark cycle. The animals allowed free access to food and water and were kept in wire-bottomed stainless steel cages. The experimental procedure used in this study met the guidelines of the Animal Care and Use Committee of the Istanbul University.
Rats were divided into six groups; a) Control group (n = 8): Rats were fed with commercial rat chow containing 11 % moisture, 10 % crude ash, 15 % protein, 3.5 % crude fat; 47 % carbohydrate, 7.5 % cellulose, 3.5 % salt mixture and 2 % vitamin mixture (AIN 76); b) GAL group (n = 8): Rats were treated with GAL (300 mg/kg; s.c.; 5 days for week) c) GAL + BB1 group (n = 8): They were fed with 5 % BB containing diet and were treated with GAL (300 mg/kg; s.c; 5 days per week). d) GAL + BB2 group (n = 8): They were fed with 10 % BB containing diet and treated with GAL (300 mg/kg; s.c; 5 days per week).e) BB1 group (n = 6): Rats were fed with 5 % BB (w/w) containing diet. f) BB2 group (n = 6): Rats were fed with 10 % BB (w/w) containing diet. Control, BB1 and BB2 groups were treated by 0.9 % NaCl/day (s.c.) as vehicle.
At the end of experimental period for two months, all rats were sacrificed by taking blood via cardiac puncture under sodium thiopental anesthesia (50 mg/kg, i.p.). Whole brains except cerebellum were quickly removed and washed in 0.9 % NaCl and tissue samples were frozen at −80 °C for later uses. Brain tissue was homogenized in ice-cold 0.15 M KCl (10 %; w/v) and postmitochondrial fraction of the brain tissue was obtained for SOD, GSH-Px, GST and AChE activities. In brief, brain homogenates were centrifuged at 600 × g for 10 min at 4 °C to remove crude fractions. Then, supernatants were centrifuged at 10,000 × g for 20 min.
Determination of MDA levels
Lipid peroxidation was assessed by measuring the levels of MDA by thiobarbituric acid (TBA) test (Ohkawa et al. 1979). For this reason, 0.2 ml tissue homogenate, 0.2 ml 8.1 % sodium dodecyl sulfate, 1.5 ml 20 % acetic acid (adjusted to pH 3.5), 1.5 ml 0.9 % TBA and 0.6 ml distilled water were vortex mixed and this mixture was placed in a water bath at 95 °C for 1 hour. After cooling to room temperature, 1.0 ml distilled water and 5.0 ml butanol:pyridine mixture (15:1; v/v) were added and vortex mixed. After centrifugation at 3,000 rpm for 10 minutes, absorbances were read at 532 nm spectrophotometrically. The breakdown product of 1, 1, 3, 3-tetraethoxypropane was used as a standard.
Determination of PC levels
The oxidative protein damage was measured by the quantification of carbonyl groups based on spectrophotometric detection of the reaction between 2, 4-dinitrophenylhydrazine (DNPH) and PC to form protein hydrazones (Reznick and Packer 1994). In brief, brain tissues (approximately 150–200 mg) were homogenized in 3 ml 50 mM phophate buffer pH 7.4 containing 1 mM EDTA and a coctail of protease inhibitors. 1 ml of homogenates were incubated with DNPH (4.0 ml;10 mM in 2.5 M HCl) in glass test tubes, allowed to stand for 1 h in the dark and stirred every 15th min. Then, 5 ml 20 % trichloracetic acid was added to the reaction mixture. Tubes were left in ice bucket for 10 min and centrifuged for 5 min to collect the protein precipitates. The precipitates were washed 3 times with 4 ml of an ethanol– ethylacetate (1:1; v/v) mixture to remove the free DNPH and other concomitants. The final precipitates were dissolved in 2.0 ml of 6 M guanidine hydrochloride solution and left 10 min at 37 °C. The absorbance was measured at 360 nm. PC was determined using a molar extinction coefficient of DNPH (22,000 M−1 cm−1) and expressed as nmoles of carbonyls/mg protein.
Determination of GSH levels
GSH levels in brain homogenates were measured by using 5,5’-dithiobis-(2-nitrobenzoate) (DTNB) at 412 nm spectrophotometrically (Beutler et al. 1963). In brief, 1 ml homogenate (10 %; w/v) and 1 ml 0.15 M KCl were mixed and deproteinized by addition of 3 ml of metaphosphoric acid solution (30 g NaCl, 1.67 g metaphosphoric acid, and 0.2 g EDTA in 100 ml distilled water). After centrifugation at 3,000 rpm for 20 min, 0.5 ml supernatant was added to 2 ml 0.3 M Na2HPO4.2H2O and 0.5 ml DTNB (0.4 ml/ml in 1 % sodium citrate) solution. Absorbances at 412 nm were measured immediately after mixing. GSH levels were calculated using extinction coefficient (13,600 M−1 cm−1).
Determination of SOD activity
SOD activity was assayed by its ability to increase the riboflavin-sensitized photooxidation of o-dianisidine (Mylorie et al. 1986). Cuvettes containing 2.7 ml 50 mM potassium phoshate buffer (pH 7.8) with 0.1 mM EDTA, 0.05 ml distilled water, 0.1 ml 0.39 mM riboflavin in 10 mM potassium phosphate (pH 7.5), 0.1 ml of 6 mM 0-dianisidine-2HCl in distilled water and 0.05 ml postmitochondrial fraction were illuminated with 20 W fluorescence lambs at 37 °C. Absorbance readings were taken before and after 8 min of illumination at 460 nm. SOD activity was calculated according to the difference in absorbances. A standard curve was prepared by using bovine SOD and results were expressed as U/mg protein.
Determination of GSH-Px activity
GSH-Px activities were measured using cumene hydroperoxide as substrate (Lawrence and Burk 1976). The assay mixture contained 50 mM potassium phoshate buffer (pH:7.0), 1 mM EDTA, 1 mM sodium azide, 0.2 mM NADPH, 1 mM GSH, 0.5 IU/ml glutathione reductase, 1.2 mM cumene hydroperoxide and 0.1 ml diluted postmitochondrial fraction in a total volume of 1 ml. Reaction was followed spectrophotometrically (340 nm) at 37 °C after the addition of cumene hydroperoxide. Results were calculated using extinction coefficient of NADPH (6.22 × 103 M−1 cm−1) and expressed as nmol/min/mg protein.
Determination of GST activity
GST activity was measured using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate (Habig and Jacoby 1981). The assay mixture contained 100 mM potassium phoshate buffer (pH 7.0), 1 mM CDNB, 1 mM GSH and 0.2 ml diluted postmitochondrial fraction in a total volume of 3 ml. Reaction was started with the addition of CDNB and followed spectophotometrically (340 nm) at 25 °C. Results were calculated using the extinction coefficient (9,600 M−1 cm−1) of the product formed by the conjugation of GSH and CDNB. Values were expressed as nmol/min/mg protein.
Determination of AChE activity
AchE activity is a marker of loss of cholinergic neurons in the brain. The AChE activity was assessed by Ellman method (Ellman et al. 1961). Briefly, 100 μL post mitochondrial fraction was added to 2.65 ml 100 mM potassium phosphate buffer (pH:7.4). The reaction was started with the addition of 100 μL 24 mM acetylcholine iodide and change in absorbance (412 nm) was noted every 30 second for 150 seconds at 25 °C. Results were calculated using the extinction coefficient (13,600 M−1 cm−1) of the break-down product of acetylcholine iodide and expressed as nmol acetylcholine iodide hydrolysed /min/mg protein.
Determination of protein levels
Protein levels were determined using bicinchoninic acid (Smith et al. 1985). Briefly, 10 μl of diluted postmitochondrial fraction was added to 200 μL bicinchoninic acid containing 0.08 % CuSO4, incubated for 30 min at 37 °C and absorbance was read at 562 nm.
Histopathological analysis
The brain tissues were fixed in 10 % formalin, for 24 h. After routine automated tissue processing (Thermo Scientific Excelsior Tissue Processor), the tissues containing cortical areas of parietotemporal and frontal lobes were embedded in paraffin. 4 μm thick sections obtained from each paraffin block were stained with haematoxylen and eosin (H&E) for histopathological evaluation under digital light microscope Olympus BX51.
Additionally for detecting apoptotic activity Bax and Caspase 3 and for detecting anti-apoptotic activity Bcl-2 immunohistochemical studies were also performed.
Immunohistochemical evaluation
After routine tissue processing 3 μm thick 3 sections from each paraffine block for the immunohistochemistry were deparaffinized in xylene and dehydrated in graded ethyl alcohol. Following deparaffinization, the slides for Bcl-2, Bax and Caspase 3 were boiled for 20 minutes in 10 mM citrate buffer, pH 6.0; followed by cooling at room temperature for 20 minutes, and then rinsed with distilled water. The slides were immersed for 30 minutes in 0.3 % hydrogen peroxide in methanol for endogenous peroxide inactivation followed by three washes in phosphate buffer saline (PBS, pH 7.4) at room temperature. Subsequently, non-specific binding was blocked by PBS containing 1 % goat serum and 1 % bovine serum albumin which was applied for 30 minutes. Next, for each slide Bax (dilution 1:100, Santa Cruz, Europe), Bcl-2 (MS-123-R7, ready to use kit, Rat monoclonal antibody, Thermo, Neomarkers, Fremont, USA), Caspase 3 (dilution 1:10, Rabbit anti-active polyclonalantibody, Chemicon, Europe) were applied for 1 hour at room temperature. After washing in PBS, peroxidase activity was localized with chromogen 3, 3’-diaminobenzidine (DAB; DAKO Liquid DAB-Substrate-chromogen K-3466, CA, USA) and 0.03 % hydrogen peroxide. Sections were counter-stained with Haematoxylen, cleaned and mounted. Negative control studies were performed concurrently in the absence of the primary antibody. Positive control studies were also performed simultaneously in sections of human breast carcinoma sections for Bax and Bcl-2, human tonsil section for Caspase-3, as stated in data sheets. Brown staining in the cytoplasm of neuronal cells was considered as “positive” and no staining as “negative” for Bcl-2, Bax and Caspase-3 antibodies. Positive staining for all antibodies was graded as: 3 + for positive stained total neuronal cell number >50 %, 2 + for 25–49 %, 1 + for 10–24 % and 0 for <9 % positive staining.
Statistical analysis
The results were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post-hoc test was used for equal variances. Kruskal-Wallis test was performed for unequal variances.
Results
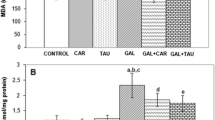
Significant increases were detected in brain MDA and PC levels in GAL-treated rats. BB1 and BB2 decreased MDA and PC levels in the brain of GAL-treated rats. However, decreases in MDA levels was observed not to be significant in GAL-treated rats due to BB1 treatment (Fig. 1).
Significant decreases in GSH levels and SOD and GSH-Px activities were observed in the brain of rats due to GAL treatment. However, GST activities remained unchanged. BB2 treatment increased the GSH levels and GSH-Px activities, but SOD and GST activities did not change. Only GSH-Px activity increased in GAL-treated rats due to BB1 treatment (Fig. 2). GAL treatment resulted in significant increases in AChE activities in the brain of rats. Both BB1 and BB2 diminished brain ACHE activities in GAL-treated rats (Fig. 3).
Effects of two doses of blueberry (BB1 and BB2) treatments on brain glutathione (GSH) levels and CuZn-superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and glutathione transferase (GST) activities in D-galactose (GAL)-treated rats (mean ± SD). a p < 0.05 as compared to controls; b p < 0.05 as compared to GAL group
Bax and Caspase-3 expression was increased in GAL-treated rats (2+) when compared to control (0), GAL + BB1 and GAL + BB2 (+1; 0) groups. Bcl-2 expression was slightly decreased in GAL-treated group when compared to control, GAL + BB1 and GAL + BB2 groups (Figs. 4, 5, 6).
Effects of two doses of blueberry (BB1 and BB2) (BB) treatments on brain Bax protein expression (Brown staining represents the Bax positive neuronal and glial cells) in D-galactose (GAL)-treated rats. Bax expression was increased in GAL- treated rats (2+) when compared to control (0), GAL + BB1 and GAL + BB2 (+1; 0) groups (Original magnification × 200) (a) Control (b) GAL (c) GAL + BB1 (d) GAL + BB2
Effects of two doses of blueberry (BB1 and BB2) blueberry (BB) treatments on brain B-cell lymphoma-2 (Bcl-2) protein expression (Brown nuclear staining represents the Bcl-2 positive neuronal and glial cells) in D-galactose (GAL)-treated rats. Bcl-2 expression was slightly decreased in GAL-treated group when compared to control, GAL + BB1 and GAL + BB2 groups (Original magnification × 200) (a) Control (b) GAL (c) GAL + BB1 (d) “GAL” + BB2
Effects of two doses of blueberry (BB1 and BB2) blueberry (BB) treatments on brain caspase-3 protein expression (Brown staining represents the Caspase positive neuronal and glial cells) in D-galactose (GAL)-treated rats. Caspase-3 expression was increased in GAL- treated rats (2+) when compared to control (0), GAL + BB1 and GAL + BB2 (+1; 0) groups (Original magnification × 200) (a) Control (b) GAL (c) GAL + BB1 (d) GAL + BB2
Normal brain structure was observed in control, BB1 and BB2 groups histopathologically
In the GAL- treated rats, the histopathological examination of brain revealed marked vacuolar changes, slight edema and mild inflammatory infiltration in cortical areas when compared to control group. (Those findings may found in brain ischemia or neurodegenerative processes). In GAL + BB1 and GAL + BB2 groups, the histopathological examination of brain revealed moderate decrease in vacuolar changes; moderate decrease in edema and marked decrease in inflammation, when compared to only GAL-treated rats (Fig. 7).
Effects of two doses of blueberry (BB1 and BB2) blueberry (BB) treatments on brain histopathology in D-galactose (GAL)-treated rats (H&E, original magnification × 200) (a) Control (b) GAL (areas of vacuolar degeneration could be seen in cortical area) (c) GAL (mild inflammation of glial tissue could be detected) (d) GAL + BB1 E) GAL + BB2
BB1 and BB2 treatments alone did not alter examined biochemical and apoptosis parameters, and histologic structure in brain tissue of normal rats.
Discussion
GAL treatment (100–500 mg /kg body weight; s.c.) for two months to rats is suitable to produce age-related disease model. Increased MDA and PC levels (Hsieh et al. 2009; Anand et al. 2012; Hsia et al. 2012; Banji et al. 2013; Prakash and Kumar 2013; Wu et al. 2014) and AGEs formation (Tsai and Yin 2012; Zhang et al. 2013), histopathological changes (Anand et al. 2012; Banji et al. 2013; Wu et al. 2014) and deterioration in learning and memory capacity (Lan et al. 2012; Yang et al. 2012; Banji et al. 2013; Prakash and Kumar 2013) were detected in GAL-treated rodents. In our study, rats were given GAL (300 mg/daily/s.c; 5 times per week) for two months. Brain MDA and PC levels increased, however, GSH levels and SOD and GSH-Px activities were found to decrease following GAL treatment. Lower levels of GSH, the substrate of GSH-Px, may cause the diminished GSH-Px activity. In addition, accumulation of H2O2 leads to decreased SOD activity. These findings show that brain oxidative stress was stimulated in GAL-treated rats.
Several investigators have reported that BB has neuroprotective and antiaging effects (Giacalone et al. 2011; Shukitt-Hale 2012). However, there is no study in the literature about the effect of BB on prooxidant-antioxidant balance and tissue damage in the brain of GAL-treated rats. Only, Çoban et al. (2014) recently reported that BB supplementation restored liver prooxidant status together with histopathological amelioration in age-related liver injury model due to GAL treatment. BB contains high amounts of polyphenols and flavonoids and has powerful antioxidant actions which may be due to its free radical scavenger properties (Neto 2007; Zafra-Stone et al. 2007). In the current study, BB concentration was chosen according to previous studies which describe that 5–10 % whole BB was an effective concentration (Kim et al. 2010; Çoban et al. 2013; Çoban et al. 2014). The daily consumption of BB in 5 % and 10 % BB containing diets is roughly equivalent to 0.75 and 1.5 g BB/ per rat, respectively. In our study, BB treatment, especially its high dose was observed to decrease brain MDA and PC levels and increase in GSH levels and GSH-Px activities in GAL-treated rats. These results obtained from brain tissue of GAL-treated rats may be related to free radical scavenger properties of polyphenols and flavonoids in BB. Indeed, in the study of Çoban et al. (2014), although an increase in activities of these enzymes in liver due to BB treatment was also observed, mRNA expressions of hepatic SOD and GSH-Px enzymes did not alter in GAL-treated rats.
On the other hand, chronic administration of GAL showed marked increase of the activity of AChE enzyme, one of the specific cholinergic markers (Prakash and Kumar 2013; Zhang et al. 2013; Ruan et al. 2014). Increases in AchE activity caused by GAL treatment may lead to a reduction of cholinergic neurotransmission due to a decrease in acetylcholine levels in synaptic cleft (Zhong et al. 2009; Xian et al. 2011; Zhang et al. 2011). Thus, modulation of cholinergic neurotransmission may be one of the mechanisms involved in the impairment of cognitive functions of GAL-treated rodents (Zhong et al. 2009; Xian et al. 2011; Zhang et al. 2011). In our study, both BB1 and BB2 treatments were observed to decrease high AChE activity in brain of GAL-treated rats. Decrease in AchE activity may improve cholinergic neurotransmission by restorating acetylcholine levels in synaptic cleft. Indeed, it has been reported that a polyphenol-rich wild BB extract resulted in decreases in AChE activity and an improvement in learning and memory in adult mice (Papandreou et al. 2009).
Mitochondrial apoptotic pathway plays an important role in brain aging in GAL-treated rodents (Mao et al. 2007; Tsai and Yin 2012; Wu et al. 2014; Prakash and Kumar 2013; Lan et al. 2012). In our study, increased proapoptotic Bax and decreased antiapoptotic Bcl-2 protein expressions together with increased caspase-3 protein expressions were detected in GAL-treated rats by immunohistochemical procedures. However, BB1 and BB2 treatments resulted in decreases in caspase-3 and Bax expressions and increases in Bcl-2 expressions in GAL-treated rats. BB1 and BB2 also ameliorated GAL-induced histopathological changes in the brain. These results indicate that BB may have antiapoptotic effects as previously reported (Wang et al. 2005; Bingül et al. 2013).
In conclusion, treatment with BB decreased prooxidant status, apoptosis and neurotoxicity by acting as an antioxidant (radical scavenger) itself in brain tissue of GAL-treated rats.
References
Anand KV, Jaabir MSM, Thomas PA, Geraldine P (2012) Protective role of chrysin against oxidative stress in D-galactose-induced aging in an experimental rat model. Geriatr Gerontol Int 12:741–750
Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA (2005) Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci 8:111–120
Aydın AF, Küçükgergin C, Ozdemirler-Erata G, Koçak-Toker N, Uysal M (2010) The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology 11:103–109
Banji D, Banji OJF, Dasaroju S, Kumar K (2013) Curcumin and piperine abrogate lipid and protein oxidation induced by galactose in rat brain. Brain Res 1515:1–11
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bingül I, Başaran-Küçükgergin C, Tekkeşin MS, Olgaç V, Doğru-Abbasoğlu S, Uysal M (2013) Effect of blueberry pretreatment on diethylnitrosamine- induced oxidative stres and liver injury in rats. Environ Toxicol Pharmacol 36:529–538
Çoban J, Betül-Kalaz E, Aydın AF, Doğan-Ekici I, Doğru-Abbasoğlu S, Uysal M (2014) Bluberry treatment attenuates D-galactose-induced oxidative stress and tissue damage in rat liver. Geriatr Gerontol Int 14:490–497
Çoban J, Evran B, Özkan F, Çevik A, Doğru-Abbasoğlu S, Uysal M (2013) The effect of blueberry feeding on lipids and oxidative stress in serum, liver and aorta of guinea pigs fed on high cholesterol diet. Biosci Biotechnol Biochem 77:389–391
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Giacalone M, DiSacco F, Traupe I, Topini R, Forfori F, Giunta F (2011) Antioxidant and neuroprotective properties of blueberry polyphenols: a critical review. Nutr Neurosci 14:119–125
Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, Moy E, Moy D, Lippold S, Shukitt-Hale B, Joseph JA (2004) Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci 7:75–83
Habig WH, Jacoby WB (1981) Assays for differentation of glutathione s-transferases. Methods Enzymol 77:398–405
Harman D (2001) Aging: overwiev. Ann N Y Acad Sci 928:1–21
Hsia CH, Wang CH, Kuo YW, Ho YJ, Chen HL (2012) Fructo-oligosaccharide systemically diminished D-galactose-induced oxidative molecule damages in BALB/cJ mice. Br J Nutr 107:1787–1792
Hsieh HM, Wu WM, Hu ML (2009) Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6 J mice treated with D-galactose. Food Chem Toxicol 47:625–632
Joseph JA, Shukitt-Hale B, Casadesus G (2005) Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr 81(1):313S–316S
Kim H, Bartley GE, Rimando AM, Yokoyama W (2010) Hepatic gene expressions related to lower plasma cholesterol in hamsters fed high-fat diets supplemented with blueberry peels and peel extract. J Agric Food Chem 58:3984–3891
Krikorian R, Shidler MD, Nash TA, Kalt W, Vinqvist-Tymchuk S-HB, Joseph JA (2010) Blueberry supplementation improves memory in older adults. J Agric Food Chem 958:3996–4000
Lan Z, Liu J, Chen L, Fu Q, Luo J, Qu R, Kong L, Ma S (2012) Danggui-Shaoyao-San ameliorates cognition deficits and attenuates oxidative stress-related neuronal apoptosis in d-galactose-induced senescent mice. J Ethnopharmacol 141:386–395
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium–deficient rat liver. Biochem Biophys Res Commun 71:952–958
Liu LC, Wang CJ, Lee CC, Su SC, Chen HL, Hsu JD, Lee HJ (2010) Aqueous extract of Hibiscus sabdariffa L. decelerates acetaminophen-induced acute liver damage by reducing cell death and oxidative stress in mouse experimental models. J Sci Food Agric 90:329–337
Malin DH, Lee DR, Goyarzu P, Chang YH, Ennis LJ, Beckett E, Shukitt-Hale B, Joseph JA (2011) Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition 27:338–342
Mao Z, Zheng YL, Zhang YQ, Han BP, Zhu XW, Chang Q, Hu XB (2007) The anti-apoptosis effects of daidzein in the brain of D-galactose treated mice. Molecules 12:1455–1470
Milbury PE, Kalt W (2010) Xenobiotic metabolism and berry flavonoid transport across the blood–brain barrier. J Agric Food Chem 58:3950–3956
Mylorie AA, Collins H, Umbles C, Kyle J (1986) Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicol Appl Pharmacol 82:512–520
Neto CC (2007) Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res 51:652–664
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN (2009) Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res 198:352–358
Parıldar-Karpuzoğlu H, Mehmetçik G, Özdemirler-Erata G, Doğru-Abbasoğlu S, Koçak-Toker N, Uysal M (2008) The effect of taurine treatment on prooxidant-antioxidant balance in livers and brains of old rats. Pharmacol Rep 60:673–678
Peng C, Zuo Y, Kwan KM, Liang Y, Ma KY, Chan HYE, Huang Y, Yu H, Chen ZY (2012) Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp Gerontol 47:170–178
Prakash A, Kumar A (2013) Pioglitazone alleviates the mitochondrial apoptotic pathway and mito-oxidative damage in the d-galactose-induced mouse model. Clin Exp Pharmacol Physiol 40:644–651
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Ruan Q, Hu X, Ao H, Ma H, Gao Z, Liu F, Kong D, Bao Z, Yu Z (2014) The neurovascular protective effects of huperzine A on D-galactose-induced inflammatory damage in the rat hippocampus. Gerontology (In press)
Schaffer S, Halliwell B (2012) Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr 7:99–109
Shukitt-Hale B (2012) Blueberries and neuronal aging. Gerontology 58:518–523
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Tsai SJ, Yin MC (2012) Anti-oxidative, anti-glycativeand anti-apoptotic effects of oleanolic acid in brain of mice treated by D-galactose. Eur J Pharmacol 689:81–88
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutrients 2:1231–1246
Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC (2005) Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp Neurol 193:75–84
Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA (2006) Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 5:59–68
Wu W, Wang X, Xiang Q, Meng X, Peng Y, Du N, Liu Z, Sun Q, Wang C, Liu X (2014) Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct 5:158–166
Xian YF, Lin ZX, Zhao M, Mao QQ, Ip SP, Che CT (2011) Uncaria rhynchophylla ameliorates cognitive deficits induced by D-galactose in mice. Planta Med 77:1977–1983
Yang YC, Lin HY, Su KY, Chen CH, Yu YL, Lin CC, Yu SL, Yan HY, Su KJ, Chen YL (2012) Rutin, a flavonoid that is a main component of saussurea involucrata, attenuates the senescence effect in D-galactose aging mouse model. Evid Based Complement Alternat Med 2012:980276
Yin J, Heo SI, Wang MH (2008) Antioxidant and antidiabetic activities of extracts from Circium japonicum roots. Nutr Res Pract 2:247–251
Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D (2007) Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res 51:675–683
Zhang WW, Sun QX, Liu YH, Gao W, Li YH, Lu K, Wang Z (2011) Chronic administration of Liu Wei Dihuang protects rat’s brain against D-galactose-induced impairment of cholinergic system. Acta Physiol Sin 63:245–255
Zhang X, Jin C, Li Y, Guan S, Han F, Zhang S (2013) Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem Toxicol 58:50–55
Zhong SZ, Ge QH, Qu R, Li Q, Ma SP (2009) Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci 277:58–64
Acknowledgments
The present work was supported by Research Fund of Istanbul University (Project No: 16727 and 31335).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çoban, J., Doğan-Ekici, I., Aydın, A.F. et al. Blueberry treatment decreased D-galactose-induced oxidative stress and brain damage in rats. Metab Brain Dis 30, 793–802 (2015). https://doi.org/10.1007/s11011-014-9643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-014-9643-z