Abstract
Ferroptosis is a type of cell death that is caused by the oxidation of lipids and is dependent on the presence of iron. It was first characterized by Brent R. Stockwell in 2012, and since then, research in the field of ferroptosis has rapidly expanded. The process of ferroptosis-induced cell death is genetically, biochemically, and morphologically distinct from other forms of cellular death, such as apoptosis, necroptosis, and non-programmed cell death. Extensive research has been devoted to comprehending the intricate process of ferroptosis and the various factors that contribute to it. While the majority of these studies have focused on examining the effects of lipid metabolism and mitochondria on ferroptosis, recent findings have highlighted the significant involvement of signaling pathways and associated proteins, including Nrf2, P53, and YAP/TAZ, in this process. This review provides a concise summary of the crucial signaling pathways associated with ferroptosis based on relevant studies. It also elaborates on the drugs that have been employed in recent years to treat ferroptosis-related diseases by targeting the relevant signaling pathways. The established and potential therapeutic targets for ferroptosis-related diseases, such as cancer and ischemic heart disease, are systematically addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research studies have indicated that the accumulation of lipid peroxidation in cells can trigger ferroptosis. Lipid peroxidation of cell and organelle membranes can lead to ferroptosis, with the endoplasmic reticulum membrane being the primary site of lipid peroxidation. Further studies have suggested that lipid peroxidation in membranes of the endoplasmic reticulum is an early event in the occurrence of ferroptosis, whereas lipid peroxidation of mitochondrial and other cell membranes takes place late in the process [1,2,3]. It is worth noting the vital contribution of mitochondria in ferroptosis. Iron ions, via the Fenton reaction, enhance the production of reactive oxygen species (ROS) by mitochondria, which in turn activate the enzyme 15-lipoxygenase. This enzyme is responsible for the oxidation of free polyunsaturated fatty acids esterified in phosphatidylcholine, leading to the damage of cellular membranes and ultimately resulting in ferroptosis [4].
It is important to note that the role of mitochondria in ferroptosis is dependent on specific background conditions. In cases where there is a deficiency of cysteine, it results in the hyperpolarization of the mitochondrial membrane potential and an accumulation of lipid peroxides, which triggers ferroptosis. However, when the mitochondrial tricarboxylic acid cycle loop or the electron transport chain is inhibited, the hyperpolarization of the mitochondrial membrane potential is reduced, and the ferroptosis caused by the lack of cysteine is effectively suppressed. This is due to the fact that mitochondrial metabolism significantly promotes the rapid depletion of glutathione(GSH), which leads to the generation of lipid ROS and ultimately ferroptosis. However, once glutathione peroxidase 4 (GPX4) is eliminated or pharmacologically inhibited in the cell, the cell can undergo ferroptosis independent of the mitochondria [5].
From a lipid metabolism perspective, ferroptosis is caused by the accumulation of harmful lipid peroxides. It disturbs cellular processes such as the balance of iron, redox, and antioxidant systems [6]. The buildup of toxic lipid ROS is caused by the deactivation of intracellular GSH-dependent antioxidant defenses [7]. GPX4 is a crucial enzyme that regulates ferroptosis by converting harmful lipid peroxides into harmless alcohols. It prevents the accumulation of these substances and ultimately prevents cell death. A decrease in GPX4 can lead to the accumulation of lipid peroxides, damaging the cell membrane and causing cell death [8].
The role of system Xc- is highly significant in the advancement of ferroptosis. system Xc- is a multifaceted protein responsible for regulating cystine uptake and glutamate excretion within cells. Its primary function is to facilitate the production of GSH by transporting cystine into the cell, where it undergoes reduction to form cysteine. In combination with GPX4, GSH reduces the effects of reactive oxygen and nitrogen species. However, obstruction of system Xc- impedes cystine uptake, reducing intracellular GSH synthesis. This depletion of GSH leads to a decrease in the activity of GPX4, which makes it unable to break down intracellular lipid peroxides (ROOH) into ROH and H2O2. As a result, there is a significant increase in ROS, which disrupts the cell's redox balance and lead to damage from cellular lipid peroxidation, ultimately resulting in the ferroptosis process [7, 9].
Iron plays a critical role in the manifestation of ferroptosis. However, excessive amounts of free reactive iron can result in damage to tissues. In the case of iron overload-related cardiomyopathy, iron chelation therapy is highly recommended as a treatment option [10]. The mitochondria play a critical role in regulating iron levels within cells, containing up to 50% of the total iron content in the body. An imbalance in iron levels can lead to the accumulation of iron within the mitochondria, which can significantly hinder their normal functioning [11]. Furthermore, iron can transfer electrons to oxygen and hydrogen peroxide, giving rise to ROS, which can be harmful to cells [7, 11, 12].
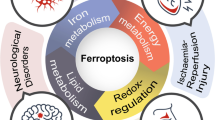
Ferroptosis plays an essential role in a variety of diseases, including neurological disorders (e.g., stroke and Alzheimer's disease), cancer, and cardiovascular disease [13,14,15]. Cellular metabolism relies heavily on iron, making the study of ferroptosis a critical aspect of maintaining adequate iron levels while also preventing cell death. A thorough comprehension of ferroptosis's mechanisms and regulatory networks is essential for devising novel strategies to address associated diseases [13, 16]. In recent years, there has been an increasing interest in studying the signaling pathways involved in ferroptosis, as well as its essential regulatory genes. A few critical signaling pathways and regulatory proteins, such as P53 and nuclear factor-erythroid 2-related factor 2 (Nrf2), have been identified, and their effects on ferroptosis should not be overlooked. Ferroptosis, due to its intricate relationship with various diseases, demands an in-depth comprehension of the underlying molecular mechanisms. Recent developments in the study of ferroptosis have led to the identification of novel targets and strategies that could potentially aid in the prevention and treatment of these ailments.
P53 in ferroptosis
P53 is a crucial regulatory protein that is commonly known as the "guardian of the cell." It plays a pivotal role in regulating cell growth, division, repair, and apoptosis. P53 also plays multiple roles in ferroptosis. In this context, we demonstrate how it contributes significantly to ferroptosis by targeting several important factors (Fig. 1).
An overview of the role of P53 on Ferroptosis. Abbreviations: ALOX12, Arachidonic 12-lipoxygenase; Cys, cystine; DPP4, Dipeptidyl peptidase 4; ROS, Reactive oxygen species; SAT1, Spermidine/spermine N1-acetyltransferase 1; SLC7A11, Solute carrier family 7 member 11; Ub, Ubiquitin; USP7, Ubiquitin-specific protease 7
Solute carrier family 7 member 11(SLC7A11)
Recent studies have revealed the crucial role of the P53 protein in regulating the expression of the SLC7A11 gene, which is responsible for modulating the ferroptosis process. Inhibition of SLC7A11 expression is a key marker of ferroptosis, and P53 also contributes to this process by suppressing the expression of SLC7A11 to some degree [17, 18]. SLC7A11 serves as a vital antioxidant defense mechanism in cells and plays a significant role in the absorption of cystine. A decline in the cellular levels of cystine can hinder the production of GSH, leading to an untimely removal of ROS. Inhibition of SLC7A11 by P53 results in a compromised ability of cells to effectively respond to ROS stress, rendering them more vulnerable to ferroptosis. Nevertheless, it is essential to note that the P53-mediated inhibition of SLC7A11 alone is insufficient to trigger ferroptosis, as additional cellular damage (e.g., ROS stress or erastin exposure) is required to induce ferroptosis [17, 19]. P53 increases cellular sensitivity to ferroptosis by inhibiting SLC7A11 gene expression, especially when cells are subjected to oxidative stress or other injuries. SLC7A11 is a promising target for future research into cancer treatment.
Spermidine/spermine N1-acetyltransferase 1 (SAT1)
Notably, despite the absence of conventional P53 functions(e.g., P53-mediated cell cycle arrest, apoptosis, and senescence), cells are still capable of exercising a tumor suppressor effect [19]. Additionally, it has been demonstrated that even when the P53 protein has lost its typical functions, such as cell cycle arrest and apoptosis, it is still able to promote ferroptosis, thereby achieving tumor suppression [18, 20]. The P53 protein serves as a transcription factor that can effectively regulate various target genes through transcriptional processes. Upon activation, P53 initiates the upregulation of SAT1 expression, which in turn increases cellular sensitivity to oxidative stress. SAT1 is a crucial enzyme in polyamine metabolism as it plays a rate-limiting role in the conversion of arginine to putrescine. SAT1 has been found to upregulate the expression of arachidonic 15-lipoxygenase (ALOX15), which is a lipoxygenase that plays a crucial role in the peroxidation of arachidonic acid. The increased expression of ALOX15 leads to further lipid peroxidation. However, the precise mechanism through which SAT1 modulates the expression of ALOX15 is not fully understood. The activation of SAT1 expression results in the induction of lipid peroxidation and an elevated cellular response to ROS-induced stress, ultimately contributing to ferroptosis [21].
Arachidonate 12-lipoxygenase (ALOX12)
The expression level of ALOX12 is significantly diminished in a variety of human cancers, including cervical squamous cell carcinoma, head and neck squamous cell carcinoma, esophageal squamous cell carcinoma, and acute myeloid leukemia. ALOX12 plays a crucial role in P53-induced ferroptosis. It is a lipoxygenase enzyme that facilitates the production of peroxides from polyunsaturated fatty acids [22]. Evidence suggests that ALOX12 mutations in human tumors impede its catalytic activity and its ability to oxidize polyunsaturated fatty acids. Knockdown of the ALOX12 gene has revealed a significant reduction in the inactivation of P53-mediated ferroptosis [23]. The significance of P53 in Ferroptosis among vascular endothelial cells has been established through research. Findings indicate a notable increase in the expression of P53 and cytochrome ALOX12 in angiotensin II-treated human umbilical vein endothelial cells. This suggests that the angiotensin II type 1 receptor and angiotensin II type 2 receptor trigger cellular ferroptosis through the P53-ALOX12 signaling axis. Additionally, P53 indirectly contributes to ALOX12's function by regulating the transcription of SLC7A11 [24, 25]. If cells have excess ROS, it could intensify P53 protein-mediated ferroptosis [26].
H2B
It is well known that H2B is a crucial component of histones. However, the relationship between H2B and ferroptosis has yet to be investigated. The phenomenon of ferroptosis is intricately tied to the extent of H2B ubiquitination modification (H2Bub1). The reduction of H2Bub1 contributes to an escalation in ferroptosis. The activation of solute carrier family member 11 is attained through epigenetic mechanisms instigated by H2Bub1. Recent studies have shown that P53 can negatively regulate the levels of H2Bub1 by promoting the nuclear translocation of the ubiquitin-specific protease 7 deubiquitinating enzyme. The function of P53 is independent of its role as a transcription factor and establishes a connection between P53 and ferroptosis through H2Bub1-mediated epigenetic pathways. These findings shed new light on the complex mechanisms underlying chromatin regulation and provide important insights into the role of P53 in cancer and other diseases [27].
Fe
The induction of cell death via an overabundance of iron is governed by the regulatory function of P53, which oversees the transcriptional output of transferrin and the intracellular concentration of free iron [28, 29]. The tumor suppressor protein P53 has been shown to regulate intracellular iron ion levels through the modulation of the H ferritin gene. As a critical intracellular iron storage protein, H ferritin plays a vital role in protecting cells against ROS by binding to free iron ions. Overexpression of H ferritin has been found to reduce hydrogen peroxide-induced cytotoxicity, thereby mitigating oxidative stress and promoting cellular survival. According to recent research, P53 has demonstrated the ability to stimulate the expression of ferritin. Interestingly, this mechanism of action occurs post-transcriptionally, rather than through transcriptional regulation. However, the exact mechanisms underlying this process remain unresolved and require further investigation [30]. A different research study found that P53 has the potential to efficiently interact with the H ferritin promoter, leading to suppression of ferritin expression [31]. Furthermore, overexpression of P53 can inhibit H ferritin gene transcription, which in turn reduces the intracellular expression [31]. P53 also regulates iron metabolism by inducing the expression of ferredoxin reductase, which in turn regulates iron metabolism [32]. However, the role of P53 in ferroptosis is complex, as it can either impede or facilitate the process [18]. It is noteworthy that ferritin has the ability to induce P53 expression under conditions of oxidative stress [29, 33].
P21
The P21 protein plays a crucial role in ferroptosis regulation. Understanding the regulatory mechanisms in this process can help to comprehensively understand ferroptosis and its therapeutic potential [34]. P53/P21 is a crucial signaling pathway. Studies have shown that inhibiting the P53/P21 pathway can prevent ferroptosis and slow the onset and progression of hypertensive nephropathy [35]. It is important to note that the P53-P21 signaling pathway is connected to GSH levels. This pathway can help cancer cells survive without serine by increasing GSH levels and maintaining a balanced redox state. Studies have shown that blocking P21 can cause lipid peroxidation, ROS production, and a series of ferroptosis events when exposed to radiation [36].
Dipeptidyl peptidase 4 (DPP4)
It has been discovered that the level of DPP4 expression in tumors is associated with the tumor's biological aggressiveness [37]. Additionally, DPP4 plays a crucial role in regulating ferroptosis, and its function is linked to reactions involving lipid peroxidation [38]. In colorectal cancer cells, P53 and DPP4 interact. This interaction affects the location and enzymatic activity of DPP4, which in turn plays a significant role in regulating the onset of ferroptosis. Specifically, P53 promotes the relocation of DPP4 to the nucleus and limits its enzymatic activity. This helps to reduce the likelihood of ferroptosis. However, if P53 has mutations or deletions, DPP4 accumulates on the cell membrane and promotes lipid peroxidation, increasing the likelihood of ferroptosis. Additionally, DPP4 binds to NADPH oxidase 1, which further promotes lipid peroxidation and enhances the likelihood of ferroptosis [16, 39].
The various functions of P53 in ferroptosis are intertwined with diverse pathways and have significant implications for comprehending the mechanisms controlling cell death and tumor growth. Nonetheless, it is crucial to conduct additional laboratory and clinical research to confirm these discoveries and gain a better understanding of the connection between P53 and ferroptosis.
Nrf2 in ferroptosis
Nrf2 is a significant transcription factor involved in regulating the intracellular antioxidant stress response and maintaining the stability of the intracellular environment. As a result, it plays a critical role in protecting cells against oxidative damage. In this section, we will focus on the vital role of Nrf2 in ferroptosis and its important associated targets (Fig. 2).
An overview of the role of Nrf2 on Ferroptosis. Abbreviations: FECH, Ferrochelatase; FPN, Ferroportin; FTH, Ferritin heavy chain; FTL, Ferritin light chain; GCLC, Glutamate-cysteine ligase catalytic subunit; GCLM, Glutamate-cysteine ligase modifier subunit; GPX4, Glutathione peroxidase 4; G6PD, Glucose-6-phosphate dehydrogenase; NQO1, NAD(P)H quinone oxidoreductase 1; Nrf2, Nuclear factor erythroid 2-related factor 2; ROS, Reactive oxygen species; SLC7A11, Solute carrier family 7 member 11
Ferroportin (FPN)
Nrf2 is a protein that plays a significant role in the regulation of iron metabolism within cells. FPN) is another essential protein that helps in the transport of iron and maintains its balance both inside and outside the cell. If a cell does not have enough iron, ferritin heavy chain 1(FTH1) and nuclear receptor co-activator protein 4 (NCOA4) combine to form a complex that releases iron through ferritin autophagy. Conversely, if there is an excess of iron inside the cell, FPN1 transports it outside the cell [40]. Nrf2 is responsible for controlling the expression of iron transport proteins and factors linked with ferroptosis at the transcriptional level. Brain microvascular endothelial cells express FPN1, and its expression is regulated by Nrf2. FPN1 plays a significant role in facilitating iron entry into the brain. A deficiency in Nrf2 results in reduced expression of FPN1 in microvascular endothelial cells, which hinders the entry of iron [41]. Research has revealed that Nrf2 and FPN are crucial for protecting macrophages from ferroptosis. When FPN or Nrf2 is suppressed, iron levels and lipid peroxidation within cells increase, making them more susceptible to ferroptosis triggered by RSL3, a GPX4 inhibitor. It was later discovered that FPN expression, triggered by RSL3, is dependent on Nrf2 [42]. Additionally, research has revealed that heme plays a crucial role in regulating the transcription of FPN1. This is accomplished through its interaction with BTB and CNC homology 1 (Bach1), Nrf2, and the MARE/ARE sequence situated at the -7007 position of the FPN1 promoter. As a result, heme can impact iron transport and metabolism [42, 43]. It is evident that the Nrf2-FPN signaling pathway does not have only a single regulatory effect in regulating ferroptosis but varies with changes in the tissue environment and other factors.
FTH/ ferritin light chain (FTL)
Iron can be stored in cells as ferritin, which consists of FTH and FTL. FTH acts as an iron reductase, and FTL stores large amounts of iron [44, 45]. FTH, which Nrf2 targets, is crucial for regulating iron metabolism and antioxidant systems in ferroptosis. Increasing FTH expression can help counteract ferroptosis and restore cellular balance. Studies have shown that inhibiting autophagy decreases FTH expression and activating Nrf2 increases it [46]. When the function of lysosomes is impeded, the degradation of FTH is also hindered, resulting in a decrease in the manifestation of ferroptosis symptoms, such as lipid peroxidation and iron accumulation [47].
After a traumatic brain injury, the brain has a natural defense mechanism, with Nrf2 playing a crucial role in this process. By regulating ferroptosis and controlling ferritin levels, Nrf2 helps safeguard the brain. However, in the absence of Nrf2, decreased FTH levels can lead to increased free iron levels, which may cause neurological abnormalities. Nrf2 is known to play a role in promoting the expression of FTL, a protein involved in iron metabolism. Studies have shown that deletion or mutation of FTL can disrupt iron balance in the brain, leading to ferroptosis in neuronal cells. This can cause early morphological signs of neurodegenerative lesions and impairments in motor coordination in mice [44]. Studies have shown that different FTH/FTL ratios have different functions, with higher FTH1/FTL ratios effectively inhibiting ferroptosis [48]. Therefore, it is essential to understand the role of Nrf2 in brain protection and to investigate potential therapies to enhance its function. [49, 50].
Ferrochelatase (FECH)
FECH is an important enzyme involved in heme synthesis. It is a vital enzyme that facilitates the integration of iron ions into protoporphyrin IX, which is necessary for the synthesis of heme [51]. This procedure efficiently restricts the accumulation of iron ions in the human body; this may lead to oxidative stress and lipid peroxidation. Inhibition of FECH can lead to increased oxidative stress and the onset of ferroptosis [52, 53]. Nrf2 can affect the process of heme biosynthesis by directly increasing the transcription of the FECH gene, thereby allowing the binding of iron to porphyrin to form heme [54, 55]. Notably, researchers have discovered that Nrf2 activates various genes related to heme regulation, including FECH. It has been proposed that both heme byproducts and heme itself can impact the immune system, and heme is essential for maintaining iron balance [56].
GPX4
GPX4 is a protein that belongs to the GPX family and is crucial in the process of ferroptosis [8, 57]. Until recently, the critical regulators of ferroptosis were unknown. However, a study was conducted that used targeted metabolomics analysis and chemical proteomics strategies to identify two classes of ferroptosis inducers. The first class led to the inactivation of GPX4 by depleting GSH, and the second class directly inhibited the activity of GPX4. The study also found that altering the expression of GPX4 could modulate the effects of ferroptosis-inducing agents on cells. Furthermore, two representative ferroptosis-inducing agents were found to be effective in preventing the growth of mouse xenograft tumors. In a sensitivity analysis of 177 cancer cell lines, GPX4-regulated ferroptosis was particularly effective against diffuse large B-cell lymphoma and renal cell carcinoma [8]. Therefore, GPX4 is a crucial factor in regulating ferroptosis and is essential in causing cancer cell death through ferroptosis.
In recent years, an increasing number of researchers have started investigating the correlation between GPX4 and Nrf2. Under conditions of high oxidative stress, Nrf2 is activated and promotes the transcription of a range of target genes, including GPX4, heme oxygenase-1 (HO-1), and SLC7A11. However, Nrf2 expression is inhibited with atorvastatin, which suppresses the expression of GPX4 and SLC7A11 [58]. Furthermore, when atorvastatin is administered, it can impair the expression and function of GPX4 in mitochondria and other organelles within the cell. This is due to the downregulation of Nrf2, which inhibits the expression of GPX4 and SLC7A11, ultimately contributing to the ferroptosis state of the cell [58]. In an oxygen–glucose deprivation and reoxygenation (OGD/R)-induced PC12 cell model, OGD/R stimulation led to the onset of ferroptosis and a significant reduction in the protein expression levels of GPX4, FTH1, and FPN. According to the study, the harmful effects of OGD/R can be reversed, and cellular ferroptosis can be prevented by the knockdown of TNF alpha-induced protein 1 (TNFAIP1). This knockdown helps in regulating the Nrf2/GPX4 signaling pathway, which inhibits ferroptosis and reduces OGD/R-induced neuronal cell injury. The results of the study suggest that the Nrf2/GPX4 signaling pathway plays a crucial role in regulating ferroptosis [59].
In addition to its role in neuronal cells, there is experimental evidence that GPX4 plays an essential protective role in autoimmune hepatitis and autoimmune hepatitis induced by liver-specific antigen S100. Interference with GPX4 expression by using AAV8-m-GPX4 showed that GPX4 knockdown resulted in a significant increase in the expression of ferroptosis markers, including cyclooxygenase-2, acyl-CoA synthetase long chain family member 4 (ACSL4), and FTH1, as well as led to increased liver injury and inflammatory cell infiltration [60]. According to recent studies, two crucial pathways relevant to HO-1 and sirtuin6 (SIRT6) have been discovered. The Nrf2/HO-1/GPX4 pathway plays a crucial role in preventing liver damage caused by Maresin-1. maresin-1 can effectively prevent ferroptosis by activating the Nrf2/HO-1 pathway, which inhibits the production of ROS and boosts the expression of GPX4 [61]. Researchers discovered that SIRT6 controls the expression of p-Nrf2 and NCOA4, while melatonin prevents ferroptosis in mirror epithelial cells by activating the SIRT6/p-Nrf2/GPX4 pathway when exposed to antioxidant stress [62].
To summarize, it is clear that Nrf2/GPX4 has a significant impact on ferroptosis in many tissues and organs, making it a valuable target for the development of pioneering drugs that could benefit humans. For example, wogonin is a flavonoid extracted from Scutellaria baicalensis, which has a variety of pharmacological effects, including anticancer and anti-inflammatory effects. Wogonin induces ferroptosis in pancreatic cancer cells by increasing the levels of intracellular ferrous ions and superoxide radicals, decreasing the level of GSH, and inhibiting the expression of the Nrf2/GPX4 pathway [63].
NAD(P)H quinone oxidoreductase 1 (NQO1)
NQO1, an antioxidant enzyme, has been discovered to impede the ferroptosis response. In cases where cellular autophagy is compromised, P62 tends to accumulate. P62 then competes with Nrf2 for Keap1 binding, leading to an increase in the expression of antioxidant proteins such as HO1, FTH1, and NQO1 by facilitating the translocation of Nrf2 [64, 65]. NQO1 plays multiple roles in ferroptosis. Specifically, utilizing the PharmMapper database and molecular docking technology, it was discovered that plumbagin(PLB) can bind to NQO1 during PLB-induced ferroptosis. This binding significantly increased NQO1 activity, leading to cell death through an NQO1-dependent pathway. Furthermore, when NQO1 was silenced, the effectiveness of PLB in inhibiting cell growth was notably reduced. These findings strongly suggest that NQO1 plays a crucial role in PLB-induced cell death in ferroptosis [64, 66].
It has been observed that propofol can increase the levels of Nrf2, which in turn can protect against lung injury caused by ventilators, myocardial toxicity due to ischemia/reperfusion, and kidney injury. As a result, there is an increase in the levels of Nrf2 and its downstream target genes, including NQO1 and SLC7A11. NQO1 plays a crucial role in controlling the redox balance within cells and protecting them from oxidative harm [67, 68]. These effects are important in various drug applications and development. The use of imatinib (IMA) may harm the heart by triggering ferroptosis. However, it was discovered that berberine offers protection against IMA-induced cardiac damage, partly due to the action of Nrf2/NQO1 [69]. A different research study found that tanshinones have protective effects, mediated by NQO1, against ferroptosis-related damage. Additionally, NQO1 gene defects weakened the protective effects of 15-047 (a compound) against liver injury induced by concanavalin A and myocardial ischemia–reperfusion injury in mice [70]. A study reported for the first time about the high expression of transcription factor AP2 alpha (TFAP2A) in gallbladder cancer in terms of a malignant phenotype and the regulation of ferroptosis. Specifically, inhibition of TFAP2A decreased proliferation, migration, and invasion of gallbladder cancer cells while promoting ferroptosis. Silencing TFAP2A led to down-regulation of Nrf2 and its target genes (NQO1, etc.) [71].
Recent studies have revealed important pathways related to HO-1 and NQO1. The Nrf2/HO-1/NQO1 signaling pathway plays a crucial role in regulating ovarian cancer cells. Additionally, eriodictyol has been found to have a positive impact on cell viability, ferroptosis effects, and mitochondrial function by regulating this signaling pathway. In a mouse transplantation tumor model, eriodictyol inhibited tumor growth, exacerbated mitochondrial dysfunction, and decreased Nrf2 levels, which in turn affected the action of NQO1 [72]. Furthermore, the anticancer effect of neferine in thyroid cancer is related to the Nrf2/HO-1/NQO1 pathway. NQO-1 can neutralize oxidative stress by enhancing the ability to scavenge ROS. On the other hand, NQO-1 can inhibit the cancerous process by stabilizing the P53 tumor suppressor [73].
In conclusion, the target gene of Nrf2, NQO1, has a vital role in future research and tumor drug development.
SLC7A11
The SLC7A11 protein is essential for transporting cystine and GSH within cells. This amino acid transporter protein performs a crucial antioxidant function by supplying cystine to synthesize GSH, which helps cells fight against oxidative stress [74, 75]. SLC7A11 is involved in the regulation of ferroptosis by transporting cystine. It was also found that the expression of SLC7A11 during ferroptosis is regulated by factors such as Nrf2 [74, 76]. Highly activated Nrf2 induces the expression of SLC7A11 by directly binding to its promoter region. A study found that Nrf2 overactivation induces its expression through direct binding to the promoter region of SLC7A11, which promotes resistance to radiation therapy and reduces radiation therapy-induced levels of lipid peroxidation, prostaglandin-endoperoxide synthase 2 expression, and morphological features from radiation therapy-associated ferroptosis [76]. It is worth noting that SLC7A11 plays a crucial role in mediating Nrf2-related resistance to radiation therapy through the inhibition of ferroptosis. This presents an exciting avenue for the future targeting of therapeutic resistance biomarkers through the Nrf2/SLC7A11/ferroptosis axis. In lung epithelial cell experiments, the Nrf2/SLC7A11/HO-1 pathway was essential in OGD/R-induced ferroptosis, highlighting the dual role of SLC7A11 in regulating cell death and redox homeostasis, as well as nutrient dependence. By increasing SLC7A11 expression, Nrf2 activation helps to reduce OGD/R-induced ferroptosis, leading to an increase in intracellular cystine concentration and a decrease in oxidative stress [77]. Another study found that Nrf2 and signal transducer and activator of transcription 3 (STAT3) are involved in the regulation of ferroptosis by affecting the expression of SLC7A11 and play an important role in acute lung injury [78]. Nrf2 and STAT3 ameliorate pathological processes associated with acute lung injury by regulating SLC7A11 [78, 79].
In addition to its role in lung injury, the Nrf2/SLC7A11/HO-1 axis plays an important role in liver ischemic injury. Mice and cell models treated with dimethyl fumarate (DMF) showed that DMF could inhibit ferroptosis in injured liver cells by activating the Nrf2/SLC7A11/HO-1 pathway, thereby protecting the liver from injury [80]. When cerebral hypoxia–ischemia occurs, the expression of SLC7A11 is inhibited, leading to a decrease in GPX4 activity and an increase in the accumulation of lipid peroxides, which induces ferroptosis. However, activation of the Nrf2/SLC7A11/GPX4 signaling pathway by the application of kaempferol, a natural antioxidant, enhances antioxidant capacity, reduces the accumulation of lipid peroxides, and resists OGD/R-induced ferroptosis [81].
A study offered valuable insights into the potential benefits of activating the SLC7A11/GPX4 pathway to inhibit ferroptosis and safeguard the brain against stroke damage. Its results showed that rats who underwent treadmill training exhibited lowered levels of malondialdehyde and iron ions, as well as increased expression of GSH, SLC7A11, and GPX4 proteins compared with those in the stroke group. However, inhibiting SLC7A11 appeared to reverse this effect [82].
In a model of acute kidney injury induced by hypercapnia or ischemia–reperfusion, melatonin enhances the binding of Nrf2 to the SLC7A11 promoter, promotes the transcriptional expression of SLC7A11, and restores the protein level of Nrf2; this attenuates the cellular damage caused by acute kidney injury [83].
Therefore, various studies have demonstrated that Nrf2-SLC7A11 has a pivotal role in safeguarding cells against ferroptosis.
Glutamate-cysteine ligase catalytic subunit (GCLC) and glutamate-cysteine ligase modifier subunit (GCLM)
GCLC catalyzes the attachment of cysteine to glutamic acid in the initial step of GSH production [84, 85]. GCLC is highly expressed in cancers such as breast cancer, hepatocellular carcinoma (HCC), and colon cancer. High expression of GCLC in breast cancer enhances GSH biosynthesis and reduces intracellular ROS accumulation [84]. Nrf2 activates GCLC transcription by interacting with related cis-acting elements upstream of the GCLC transcription start site. Actin-like protein 6A regulates the transcriptional activity of GCLC and participates in the regulation of ferroptosis by interacting with Nrf2 [85, 86]. Moreover, when cells are activated by Nrf2, GCLC can further prevent ferroptosis by controlling the buildup of glutamate through the synthesis of γ-glutamyl peptide. This not only helps maintain glutamate balance but also plays a non-traditional, GSH-dependent role in safeguarding against ferroptosis [87].
Nrf2 also regulates the expression of several genes related to iron metabolism, including GCLM. GCLM is the regulatory subunit of GSH synthetase, which is an essential component of the GSH synthesis pathway. Nrf2's role in regulating these genes helps cells better adapt to oxidative stress and protects them from ferroptosis [87, 88]. Reduced expression of GCLM is responsible for reduced glutamate-cysteine ligase activity. In experiments with hypoxia-reperfusion injury and ischemia–reperfusion injury, GCLM was in an inhibited state, which further led to a decrease in the synthesis of GSH. In addition, other studies have found GSH is an essential factor in the prevention of lipid peroxidation and the protection of cells from ferroptosis [89]. Furthermore, GCLM is highly expressed in bladder cancer and is associated with poor prognosis, and GCLM may promote the survival and growth of bladder cancer cells [90]. In GSH homeostasis, both GCLC and GCLM play essential roles in regulating the process of ferroptosis under the regulation of Nrf2; this should be further followed up by relevant studies.
Glucose-6-phosphate dehydrogenase (G6PD)
G6PD is a vital enzyme that participates in cellular metabolic processes and, in particular, plays a vital role in maintaining cellular redox homeostasis. It is produced mainly in intracellular barosomes (small organelles) and stabilizes the membrane structure of erythrocytes. In addition, G6PD is an essential enzyme in the pentose phosphate pathway, primarily responsible for the production of nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is a crucial coenzyme that plays a vital role in various cellular reduction reactions. Individuals with deficient or decreased levels of G6PD in their red blood cells are more susceptible to hemolytic anemia [91,92,93].
A study on the functional mechanism of G6PD in HCC and its role in ferroptosis found that G6PD expression is upregulated in HCC and that high expression is associated with poorer survival. Further experimental results demonstrated that higher expression of G6PD inhibited ferroptosis and thus promoted tumor cell survival [93]. G6PD is one of the vital ferroptosis-associated genes and is closely associated with HCC development and immune function [94].
Nrf2-regulated G6PD is critical for maintaining cellular redox balance and growth. Deletion or pharmacological inhibition of G6PD leads to an increase in the NADP+/NADPH ratio, resulting in oxidation [95]. Activation of Nrf2 enhances G6PD expression and promotes the proliferation and migration of breast cancer cells, and there is no obvious explanation for how Nrf2 regulates G6PD. However, studies have shown that bromodomain-containing protein 4 (BRD4) plays a vital role in regulating G6PD expression. BRD4 can directly bind to the G6PD promoter and activate its transcription while decreasing the stability of Nrf2, thus inhibiting G6PD expression [96].
Yes-association protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) in ferroptosis
YAP/TAZ are effectors of the Hippo signaling pathway that influence ferroptosis by regulating iron metabolism and ROS-producing proteins in a variety of biological settings [97]. YAP/TAZ activation is found in multiple tumor types and plays an important role in tumor biology. Since YAP/TAZ activation is associated with apoptotic escape, tumor progression, metastasis, and drug resistance, the use of YAP/TAZ-induced ferroptosis as a therapeutic strategy may have therapeutic potential for YAP/TAZ-activated chemoresistant and metastatic tumor cells [98] (Fig. 3).
One thing needs to be emphasized here. In some specific types of cancer, YAP/TAZ inhibits ferroptosis. In this case, YAP/TAZ inhibitors can be utilized to induce ferroptosis. Some drugs, such as zoledronic acid, inhibit the entry of YAP/TAZ into the nucleus by maintaining them in a phosphorylated state. Verteporfin restricts the transcriptional activity of TEAD (TEA/ATTS domain) by inhibiting its binding to YAP/TAZ. The BET protein inhibitor JQ-1 inhibits YAP/TAZ by inhibiting BRD4, an essential component of the YAP/TAZ-TEAD complex, thereby inhibiting YAP-mediated transcriptional activity. In esophageal squamous cell carcinoma cells, arsenic-iron oxide nano complexes degrade YAP and increase the sensitivity of esophageal squamous cell carcinoma cells to radiotherapy and chemotherapy [99,100,101].
The impact of YAP/TAZ on ferroptosis is a complex matter and cannot be oversimplified. Overexpression of discoidin domain receptor tyrosine kinase 2 in breast tumor cells with mesenchymal features is responsible for their high sensitivity to ferroptosis, and knockdown of discoidin domain receptor tyrosine kinase 2 inhibits the YAP/TAZ signaling pathway, yet protects cells from ferroptosis [102]. YAP/TAZ is involved in the regulation of the uptake of cystine, a precursor of GSH, through the glutamate-cystine antiporter. GPX4 catalyzes the reduction of lipid peroxidation to the corresponding alcohols through GSH [99, 100].
In an experiment, it was discovered that changes in cell density affect ferroptosis. When the cell density is low and there is a loss of intercellular contact, YAP and TAZ are activated, which further promotes ferroptosis. Conversely, high cell density helps maintain intercellular contact and inhibits ferroptosis [103]. Epithelial membrane protein 1 (EMP1) is a direct target gene of TAZ and affects susceptibility to ferroptosis by regulating the expression of EMP1. TAZ silencing resulted in a significant down-regulation of EMP1 mRNA, and EMP1 silencing resulted in increased cellular resistance to ferroptosis [104]. It was also shown that the activation state of TAZ plays an important role in the regulation of cell density-associated ferroptosis, affecting the levels of NADPH oxidase 4 and lipid peroxidation. Higher levels of NADPH oxidase 4 enhanced lipid peroxidation and sensitized cells to ferroptosis [104].
For YAP, it has been shown that S-phase kinase-associated protein 2, a downstream target gene of YAP, is a critical factor in the regulation of YAP-enhanced ferroptosis and lipid peroxidation. Inhibition of S-phase kinase-associated protein 2 has been associated with tumorigenesis and progression and is involved in cell cycle progression. Inhibition of S-phase kinase-associated protein 2 expression attenuated the ferroptosis-promoting effect of YAP [105].
ACSL4 in ferroptosis
According to recent research, ACSL4 has been identified as a critical component in the process of ferroptosis. This study provides evidence that excessive activation of ACSL4 can play a critical role in the induction of ferroptosis in skeletal muscle cells during exercise-induced heat stroke. This is achieved by altering lipid peroxidation levels in the cells [106]. Experimental evidence has shown that the activation of ferroptosis in skeletal muscle cells can be inhibited by either pharmacological or genetic inhibition of ACSL4. This inhibition can also reduce the tissue damage and inflammatory response associated with ferroptosis. Moreover, it has been observed that YAP expression is significantly increased during exercise heat stroke. Overexpression of YAP results in the upregulation of ACSL4, while gene repression of YAP leads to the downregulation of ACSL4 [106] (Fig. 4).
Under ischemic conditions, the expression of ACSL4 increases, which subsequently triggers the process of ferroptosis following ischemia/reperfusion. Inhibition of ACSL4 can hinder this process. ACSL4 inhibition can be achieved pharmacologically using drugs like rosiglitazone or genetically using siRNA. This protective measure inhibits ferroptosis and protects the cells under ischemic or hypoxic conditions [107]. For example, ACSL4 is a significant trigger for ferroptosis in acute kidney injury, and ACSL4 inhibition protects the kidneys from ischemia/reperfusion-induced kidney injury [108]. In acute cerebral ischemia–reperfusion injury, using animal models and cellular experiments, researchers found that ACSL4 mediates thrombin-induced neurotoxicity and is involved in ferroptosis. ACSL4 contributes to ferroptosis by converting free arachidonic acid to arachidonoyl coenzyme A, which generates lipid peroxides [109]. ACSL4 was downregulated in brain tissue early after ischemia and similarly in primary cortical neurons and microglia. Further studies showed that inhibition of ACSL4 expression attenuated brain injury and exerted a protective effect by suppressing ferroptosis and neuroinflammation within neurons [110].
ACSL4 was also found to be involved in hepatic inflammation, disorders of hepatic lipid metabolism, and cell proliferation processes. Deficiency of ACSL4 reduced hepatocyte sensitivity to ferroptosis and decreased lipid peroxidation. In transhepatic lipid accumulation disease and non-alcoholic steatohepatitis models, inhibition of ACSL4 attenuates lipotoxicity-induced liver injury [111]. It has been observed that tumor cells with deficient ACSL4 exhibit greater resistance to ferroptosis, in contrast to normal ACS4-expressing tumor cells. This disparity may contribute to the distinct response of these cells to T cells and their potential to affect antitumor immunity [112].
Additionally, in patients with colorectal cancer, the enzyme cytochrome P450 1B1 (CYP1B1) plays a crucial role in ferroptosis. CYP1B1 is also closely linked with a fatty acid metabolizing enzyme called long-chain family member 4. CYP1B1 promotes the polyubiquitination and degradation of ACSL4 by participating in the 20-HETE-activated protein kinase C pathway, which in turn increases the expression of FBXO10. This degradation of ACSL4 leads to the inhibition of ferroptosis, thus triggering tolerance in colorectal cancer cells [113].
Recently, it was found that ACSL4 is a phosphorylated substrate of cyclin-dependent kinase 1 (CDK1), and by directly phosphorylating ACSL4, CDK1 binds to and breaks down ACSL4 proteins. This suggests that ACSL4 degradation requires that the phosphorylated motif created by CDK1 be recognized by ubiquitin-protein ligase E3 component N-recognin 5. Further studies revealed that CDK1 promotes oxaliplatin resistance by inhibiting ACSL4-mediated ferroptosis [114]. In conclusion, CDK1 and CYP1B1 play an important role in promoting the phosphorylation of ACSL4 and its subsequent degradation by polyubiquitination. These findings provide important clues and rationale for further investigation of therapeutic strategies targeting ACSL4 and ferroptosis.
Heat shock factor (HSF1) in ferroptosis
HSF1 plays a crucial role in regulating the heat shock response. Its involvement in the regulation of ferroptosis, which is induced by iron overload in cancer cells, is of great significance [115]. Erastin is a specific ferroptosis-inducing compound and induces the expression of heat shock protein family B member 1 (HSPB1) via an HSF1-dependent pathway. In turn, overexpression of HSPB1 inhibited erastin-induced ferroptosis [116, 117]. HSF1 affects intracellular iron levels and maintains intracellular iron homeostasis by regulating the expression of heat shock proteins such as HSPB1 [118] (Fig. 5).
An overview of the role of HSF1 on Ferroptosis. Abbreviations: HSF1, Heat shock factor 1; HSPB1, Heat shock protein family B member 1; HSPE1, Heat shock protein family E member 1; FTH1, Ferritin heavy chain 1; ROS, Reactive oxygen species; SLC40A1, Solute carrier family 40 member 1; TFRC, Transferrin receptor
Recent studies have demonstrated that HSF1 plays a crucial role in mitigating iron overload induced by palmitic acid. It achieves this by regulating the uptake, storage, and efflux of iron. Conversely, a deficiency of HSF1 exacerbates the effects of palmitic acid-induced ferroptosis, endoplasmic reticulum stress, and iron metabolism disorders in the heart. In addition, HSF1 is involved in the regulation of GSH/oxidized GSH ratio and heavy metal-induced stress response as an indirect effect on ferroptosis [118].
Ferroptosis is a type of cell death that is associated with mitochondria, and it increases levels of ROS in vivo. HSF1, a protein, may impact the OXPHOS system in mitochondria by regulating heat shock protein family E member 1(HSPE1). In prostate cancer cells, HSF1 helps maintain metabolism and ROS balance by increasing the levels of HSPE1. If HSF1 is inhibited, the expression of HSPE1 decreases, which can lead to increased ROS levels and greater susceptibility to ferroptosis induced by RSL3 [119]. The induction of heat shock proteins was enhanced by co-treatment with erastin and celastrol, which led to the phosphorylation of HSF1, prompted nuclear translocation of HSF1, and recruited it to the heat shock protein’s promoter. By analyzing Hs578t cells, researchers observed that HSF1 inhibition increased the sensitivity of cancer cells to the ferroptosis inducers RSL3 and imidazole ketone erastin [120].
Recent studies have suggested that targeting the HSF1 gene can enhance ferroptosis in cervical sarcoma cells, leading to improved sensitivity to adriamycin and gemcitabine. This implies that HSF1 plays a crucial role as a regulatory molecule in the occurrence of ferroptosis and is a key factor in determining the sensitivity of cervical sarcoma patients to drug therapy [121, 122]. Protein expression of HSF1 is up-regulated in colorectal cancer tissues, and studies have shown that HSF1 binding sites are more open on DNA in colorectal cancer tissues than in normal tissues and are associated with the process of ferroptosis. It is worth noting that ferroptosis is a specific type of death associated with the cell death process, and its mechanism is intertwined with other programmed cell deaths, such as necrosis, autophagy, and apoptosis. It was also found that HSF1 is involved in critical genes linking ferroptosis to these three types of programmed cell death. The relationship between HSF1 and cellular ferroptosis in colorectal cancer tissues requires further study to determine the specific relationship [123]. In summary, the use of HSF1 as a therapeutic target has a promising future in cancer treatment.
STAT3 in ferroptosis
STAT3 is a transcription factor that has been extensively studied in the development and progression of a wide range of diseases [124]. STAT3 is a protein that functions as a signal transducer and transcriptional activator, commonly found to be activated in various types of cancer. Its activation is associated with promoting several cellular processes, including cell growth, apoptosis, self-renewal, stem cell development, and differentiation. Additionally, STAT3 plays a vital role in enhancing the antioxidant capacity of cells by activating the Nrf2 protein. This activation, in turn, helps protect cells from oxidative damage, which can have detrimental effects on cellular health [125,126,127,128,129] (Fig. 6).
An overview of the role of STAT3 on Ferroptosis. Abbreviations: DMF, Dimethyl fumarate; GPX4, Glutathione peroxidase 4; JAK, Janus kinase; MCL1, Myeloid cell leukemia-1; NEDD4L, Neural precursor cell expressed developmentally downregulated 4-like; Nrf2, Nuclear factor erythroid 2-related factor 2; STAT3, Signal transducer and activator of transcription 3; Tyk2, Tyrosine kinase 2
A study was conducted to investigate the effects of a compound known as DMF on lymphomas. DMF was effective in inhibiting NF-κB and JAK/STAT3 survival signaling in the ABC subtype of diffuse large B-cell lymphomas. Additionally, it efficiently induced ferroptosis in germinal center B cell-like lymphomas. Moreover, the study hypothesized a possible relationship between ferroptosis and STAT3 [126]. By interfering with the expression of STAT3, it was found to exacerbate hypoxia/reoxygenation-induced cellular ferroptosis, whereas overexpression of STAT3 attenuated cellular ferroptosis; STAT3 regulated the ferroptosis process by regulating the expression of genes such as SLC7A11 [78].
Experimental observations revealed that ferroptosis was increased in induced colitis, colitis caused by Salmonella typhimurium infection, and hydrogen peroxide-induced IEC-6 cells. The phosphorylation level of STAT3 was downregulated in H2O2-treated IEC-6 cells, and ferrostatin-1, an iron ion inhibitor, could reactivate the phosphorylation level of STAT3. From these results, we can infer that there is a relationship between STAT3 activation and cellular ferroptosis [124].
Thiostrepton is a compound with anticancer properties. It inhibits the proliferation and clone formation of pancreatic cancer cell lines in a dose-dependent manner and is characterized by the induction of cell ferroptosis. Thiostrepton significantly inhibited the protein expression of STAT3 and correspondingly reduced the phosphorylation level of STAT3 [130]. Ferroptosis is inhibited in cisplatin-resistant osteosarcoma cells. However, under combined treatment with cisplatin and the ferroptosis promoters erastin or RSL3, ferroptosis was reactivated, sensitizing the cells to increased sensitivity to cisplatin. In addition, by adding STAT3 inhibitors, the researchers observed a decrease in the protein levels of p-STAT3, Nrf2, and GPX4 in the cells, which increased ferroptosis and sensitivity to cisplatin. Thus, these results suggest that in cisplatin-resistant osteosarcoma cells, activation of the STAT3/Nrf2 signaling pathway increases the antioxidant capacity of the cells by inhibiting ferroptosis, which leads to resistance to cisplatin [125].
STAT3 also plays an important role in sorafenib-induced cell death in HCC. Exploring the mechanism, researchers found that sorafenib downregulated the expression of the anti-apoptotic molecule myeloid cell leukemia-1 (MCL1) by modulating the activity of STAT3. MCL1 binds to beclin-1 and initiates the autophagy process by releasing beclin-1. Therefore, sorafenib decreased the binding of MCL1 and beclin-1 and promoted the binding of beclin-1 and SLC7A11. Since the binding of beclin-1 and SLC7A11 inhibits the activity of systemic Xc-, sorafenib, therefore, inhibits the function of systemic Xc- through this mechanism [131].
According to recent research, inhibiting STAT3 through different pathways can cause growth arrest and apoptosis in glioma cells. The activation of STAT3 is linked to low survival and poor prognosis in glioma patients. The study also found that paeoniflorin may be involved in glioma cell death through ferroptosis. Paeoniflorin was able to increase the expression of NEDD4L and trigger ferroptosis in glioma cells by reducing the expression of Nrf2 GPX4. The researchers also discovered that NEDD4L (ubiquitinase) regulates STAT3 levels negatively by mediating the ubiquitination of STAT3. Further studies indicated that paeoniforin could trigger ferroptosis by inhibiting tumor growth in gliomas through the activation of the NEDD4L/STAT3/Nrf2/GPX4 signaling pathway [132].
In summary, the activation of STAT3 will inhibit ferroptosis to a certain extent, ensuring cell survival. However, some antitumor drugs have varying antitumor efficacy depending on their ability to inhibit the STAT3 signaling pathway.
Others
Research continues to discover the association of many factors with ferroptosis. This section discusses a few additional proteins whose roles have been less characterized so far.
Lymphoid-specific helicase (LSH) is a chromatin remodeling protein that has been found to play a significant role in inhibiting ferroptosis in colorectal cancer. LSH interacts with ubiquitin-specific protease 11, which stabilizes LSH by deubiquitination, resulting in the enhanced resistance of colorectal cancer cells to ferroptosis. This discovery could potentially lead to novel therapeutic targets for colorectal cancer. In addition, it also regulates intracellular Ca2+ levels and lipid peroxidation by modulating the transcription of cytochrome P450 family 24 subfamily A member 1, which enhances the resistance of cells to ferroptosis [133].
Activation transcription factor 3 (ATF3) is a member of the ATF/CREB family of transcription factors whose expression is rapidly induced by a variety of cellular stresses such as DNA damage, oxidative stress, and cellular injury. Recent research has discovered that ATF3 plays a crucial role in promoting ferroptosis by inhibiting the expression of SLC7A11, which is an important protein that helps protect cells from oxidative stress damage by maintaining intracellular GSH levels through the system Xc- transporter system. ATF3 has the ability to directly bind to the promoter of human SLC7A11 and inhibit its expression. Furthermore, ATF3 can regulate the response of cells to ferroptosis by reducing the expression of the transcription factor Nrf2 [134,135,136].
The signaling molecule Smad is a key component of the transforming growth factor beta (TGF-β)/Smad signaling pathway. TGF-β plays a crucial role in the onset and progression of lung fibrosis. TGF-β triggers a cellular signaling cascade by binding to its receptor TGF-β R-II. This binding process initiates the phosphorylation of two proteins, Smad2 and Smad3, which then translocate to the nucleus. Recent studies have revealed that the activity of the TGF-β/Smad signaling pathway can regulate the occurrence of cellular ferroptosis [137, 138]. The signaling pathways related to ferroptosis are being continuously explored and updated, and fully elucidating their mechanisms is of great significance for antitumor therapy, ischemic heart disease, and other diseases.
Conclusion and perspectives
Ferroptosis is a distinct mode of cell death that occurs through iron-dependent lipid peroxidation. This process is different from apoptosis, necrosis, and other non-apoptotic forms of cell death in terms of its morphology, biochemistry, and genetics [6, 7, 11]. Ferroptosis is characterized by the oxidation of cell membrane phospholipids; this results in oxidation of the polyunsaturated fatty acid content, variable reduction of iron availability, and loss of capacity for lipid peroxide repair. This process is involved in physiological functions and a variety of human diseases, such as ischemic organ damage, neurodegenerative diseases, and cancer [11]. The ferroptosis signaling pathway has been studied to gain insight into this novel mode of cell death and its potential role in various diseases [7].
A variety of genes play important roles in regulating ferroptosis. Numerous studies are currently underway to explain its mechanisms. The impact of P53 on ferroptosis is complex. While P53 can boost the expression of SLC7A11, which defends cells against ROS, it can also increase cell sensitivity to oxidative stress and lead to ferroptosis by activating SAT1 [18, 21, 139]. Additionally, the P53-ALOX12 signaling axis plays a crucial role in triggering ferroptosis in various cancer cells. Notably, the P53/P21 signaling pathway can help maintain redox homeostasis and elevate GSH levels, which can prevent cancer cells from experiencing ferroptosis when serine is absent [23, 34]. P53 also has an effect in terms of H2B ubiquitination as well as intracellular levels of ferric ions, which affect ferroptosis.
Nrf2 is a transcription factor closely related to intracellular iron metabolism and plays an important role in ferroptosis. Nrf2 regulates intracellular ferroptosis through the regulation of several downstream genes (FTH, FPN, FECH, etc.) and also plays an important role in GSH homeostasis, specifically through the regulation of SLC7A11, GCLC, and GCLM; this shows that Nrf2 plays an important role in the regulation of ferroptosis [55]. Nrf2/GPX4 and Nrf2/NQO1 effectively regulate ferroptosis in cells through redox regulation, and the anticancer effects of various drugs are related to these two pathways [8, 66]. The regulatory role of Nrf2 in ferroptosis is still in full swing. Research has indicated that the activation of YAP/TAZ is responsible for safeguarding multiple cancer cell types and hindering the process of ferroptosis. Therefore, the development of YAP/TAZ inhibitors as a therapeutic approach for treating related cancers is considered a highly effective strategy [99].
ACSL4 plays a crucial role in neuroinflammation and acute kidney injury by regulating the onset of ferroptosis. The inhibition of ACSL4 expression has been found to be effective in attenuating brain injury and exerting a protective effect in neuroinflammation by preventing the onset of intra-neuronal ferroptosis [110].
HSF1 is a key factor in the regulation of the heat shock response and has a non-negligible role in the occurrence of cellular ferroptosis through the regulation of HSPE1, which in turn affects intracellular ROS levels. In addition, HSF1 also regulates intracellular iron levels and GSH/oxidized GSH ratio. A variety of antitumor drugs have targeted HSF1 to regulate the onset of ferroptosis in cancer cells, resulting in promising efficacy outcomes.
The involvement of STAT3 in ferroptosis has been the subject of significant research in recent years. Its activation can have various effects on multiple downstream signaling pathways, including the regulation of iron homeostasis, redox, GSH homeostasis, lipid metabolism. These findings suggest that STAT3 plays a crucial role in the complex process of ferroptosis and its regulation [124].
The phenomenon of ferroptosis is intricately linked to a range of human diseases, including ischemic organ damage, neurodegenerative disorders, and cancer [7, 11]. A comprehensive understanding of ferroptosis is essential toward the advancement of global public health. Notably, certain types of cancers exhibit mechanisms that enable them to maintain iron homeostasis, potentially through the autophagy pathway. One such example is pancreatic ductal adenocarcinoma, where cancer cells utilize high levels of autophagy to degrade ferritin, a protein that stores iron in the body, to release available iron ions. This process is crucial for the development and progression of pancreatic ductal adenocarcinoma [140, 141].
So far, scientists have discovered numerous genes and signaling pathways that control the intricate process of ferroptosis. Future research will delve deeper into uncovering new signaling pathways. Previous studies have demonstrated that activating different signaling pathways can have varying effects on ferroptosis regulation. Therefore, developing a multitargeted drug that aims to regulate the balance of each signaling pathway is a promising area for further investigation. It is important to note that while the above discussion and summary of mechanisms are comprehensive and cover a broad range of areas, ferroptosis is a complex process and therefore improved, more detailed mechanisms are needed in the future.
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- ACSL4:
-
Acyl-CoA synthetase long chain family member 4
- ALOX12:
-
Arachidonic 12-lipoxygenase
- ALOX15:
-
Arachidonic 15-lipoxygenase
- ATF3:
-
Activation transcription factor 3
- Bach1:
-
BTB and CNC homology 1
- BRD4:
-
Bromodomain-containing protein 4
- CDK1:
-
Cyclin-dependent kinase 1
- CYP1B1:
-
Cytochrome P450 1B1
- Cys:
-
Cystine
- DMF:
-
Dimethyl fumarate
- DPP4:
-
Dipeptidyl peptidase 4
- EMP1:
-
Epithelial membrane protein 1
- FECH:
-
Ferrochelatase
- FPN:
-
Ferroportin
- FPN1:
-
Ferroportin 1
- FTH:
-
Ferritin heavy chain
- FTH1:
-
Ferritin heavy chain 1
- FTL:
-
Ferritin light chain
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- GCLC:
-
Glutamate-cysteine ligase catalytic subunit
- GCLM:
-
Glutamate-cysteine ligase modifier subunit
- GPX4:
-
Glutathione peroxidase 4
- GSH:
-
Glutathione
- H2Bub1:
-
H2B ubiquitination modification
- HCC:
-
Hepatocellular carcinoma
- HO-1:
-
Heme oxygenase-1
- HSF1:
-
Heat shock factor 1
- HSPB1:
-
Heat shock protein family B member 1
- HSPE1:
-
Heat shock protein family E member 1
- IMA:
-
Imatinib
- JAK:
-
Janus kinase
- LSH:
-
Lymphoid-specific helicase
- MCL1:
-
Myeloid cell leukemia-1
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NCOA4:
-
Nuclear receptor co-activator protein 4
- NEDD4L:
-
Neural precursor cell expressed developmentally downregulated 4-like
- NQO1:
-
NAD(P)H quinone oxidoreductase 1
- Nrf2:
-
Nuclear factor-erythroid 2-related factor 2
- OGD/R:
-
Oxygen-glucose deprivation and reoxygenation
- PLB:
-
Plumbagin
- ROS:
-
Reactive oxygen species
- SAT1:
-
Spermidine/spermine N1-acetyltransferase 1
- SIRT6:
-
Sirtuin 6
- SKP2:
-
S-phase kinase-associated protein 2
- SLC40A1:
-
Solute carrier family 40 member 1
- SLC7A11:
-
Solute carrier family 7 member 11
- STAT3:
-
Signal transducer and activator of transcription 3
- TAZ:
-
Transcriptional coactivator with PDZ-binding motif
- TEAD:
-
TEA/ATTS domain
- TFAP2A:
-
Transcription factor AP2 alpha
- TFRC:
-
Transferrin receptor
- TGF-β:
-
Transforming growth factor beta
- THSWD:
-
Tao Hong Si Wu Tang
- TNFAIP1:
-
TNF alpha-induced protein 1
- Tyk2:
-
Tyrosine kinase 2
- Ub:
-
Ubiquitin
- Usp7:
-
Ubiquitin-specific protease 7
- YAP:
-
Yes-Association protein
References
Gaschler MM, Hu F, Feng H et al (2018) Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem Biol 13:1013–1020. https://doi.org/10.1021/acschembio.8b00199
Von Krusenstiern AN, Robson RN, Qian N et al (2023) Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol 19:719–730. https://doi.org/10.1038/s41589-022-01249-3
Lei P, Bai T, Sun Y (2019) Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol 10:139. https://doi.org/10.3389/fphys.2019.00139
Javadov S (2022) Mitochondria and ferroptosis. Curr Opin Physiol 25:100483. https://doi.org/10.1016/j.cophys.2022.100483
Gao M, Yi J, Zhu J et al (2019) Role of mitochondria in ferroptosis. Mol Cell 73:354-363.e3. https://doi.org/10.1016/j.molcel.2018.10.042
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Cao JY, Dixon SJ (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73:2195–2209. https://doi.org/10.1007/s00018-016-2194-1
Yang WS, SriRamaratnam R, Welsch ME et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331. https://doi.org/10.1016/j.cell.2013.12.010
Chen C, Chen W, Zhou X et al (2022) Hyperbaric oxygen protects HT22 cells and PC12 cells from damage caused by oxygen-glucose deprivation/reperfusion via the inhibition of Nrf2/System Xc-/GPX4 axis-mediated ferroptosis. PLoS ONE 17:e0276083. https://doi.org/10.1371/journal.pone.0276083
Fang X, Ardehali H, Min J et al (2023) The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20(1):7–23
Battaglia AM, Chirillo R, Aversa I et al (2020) Ferroptosis and cancer: mitochondria meet the “Iron Maiden” cell death. Cells 9:1505. https://doi.org/10.3390/cells9061505
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30(1):11–26
Stockwell BR, Friedmann Angeli JP, Bayir H et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. https://doi.org/10.1016/j.cell.2017.09.021
Mandal PK, Saharan S, Tripathi M, Murari G (2015) Brain glutathione levels—a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol Psychiatry 78:702–710. https://doi.org/10.1016/j.biopsych.2015.04.005
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15:234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Xie Y, Zhu S, Song X et al (2017) The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 20:1692–1704. https://doi.org/10.1016/j.celrep.2017.07.055
Wang S-J, Li D, Ou Y et al (2016) Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep 17:366–373. https://doi.org/10.1016/j.celrep.2016.09.022
Maddocks ODK, Vousden KH (2011) Metabolic regulation by p53. J Mol Med 89:237–245. https://doi.org/10.1007/s00109-011-0735-5
Jiang L, Kon N, Li T et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520:57–62. https://doi.org/10.1038/nature14344
Tang Y, Zhao W, Chen Y et al (2008) Acetylation Is Indispensable for p53 Activation. Cell 133:612–626. https://doi.org/10.1016/j.cell.2008.03.025
Ou Y, Wang S-J, Li D et al (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1607152113
Luci D, Jameson JB, Yasgar A, et al (2010) Discovery of ML355, a potent and selective inhibitor of human 12-Lipoxygenase. In: Probe reports from the NIH molecular libraries program. National Center for Biotechnology Information (US), Bethesda (MD)
Chu B, Kon N, Chen D et al (2019) ALOX12 is required for p53-mediated tumor suppression through a distinct ferroptosis pathway. Nat Cell Biol 21(5):579–591
Liu C, Shen Y, Cavdar O et al (2023) Angiotensin II-induced vascular endothelial cells ferroptosis via P53-ALOX12 signal axis. Clin Exp Hypertens 45:2180019. https://doi.org/10.1080/10641963.2023.2180019
Li W, Li W, Li X et al (2023) Effect of P53 nuclear localization mediated by G3BP1 on ferroptosis in acute liver failure. Apoptosis 28:1226–1240. https://doi.org/10.1007/s10495-023-01856-y
Han C, Sheng J, Pei H et al (2023) Environmental toxin chlorpyrifos induces liver injury by activating P53-mediated ferroptosis via GSDMD-mtROS. Ecotoxicol Environ Saf 257:114938. https://doi.org/10.1016/j.ecoenv.2023.114938
Wang Y, Yang L, Zhang X et al (2019) Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep 20:e47563. https://doi.org/10.15252/embr.201847563
Tang L-J, Zhou Y-J, Xiong X-M et al (2021) Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med 162:339–352. https://doi.org/10.1016/j.freeradbiomed.2020.10.307
Xu R, Wang W, Zhang W (2023) Ferroptosis and the bidirectional regulatory factor p53. Cell Death Discov 9:197. https://doi.org/10.1038/s41420-023-01517-8
Zhang F, Wang W, Tsuji Y et al (2008) Post-transcriptional modulation of iron homeostasis during p53-dependent growth arrest. J Biol Chem 283:33911–33918. https://doi.org/10.1074/jbc.M806432200
Faniello MC, Di Sanzo M, Quaresima B et al (2008) p53-Mediated downregulation of H ferritin promoter transcriptional efficiency via NF-Y. Int J Biochem Cell Biol 40:2110–2119. https://doi.org/10.1016/j.biocel.2008.02.010
Zhang J, Chen X (2019) p53 tumor suppressor and iron homeostasis. FEBS J 286:620–629. https://doi.org/10.1111/febs.14638
Lee J-H, Jang H, Cho E-J, Youn H-D (2009) Ferritin binds and activates p53 under oxidative stress. Biochem Biophys Res Commun 389(3):399–404
Venkatesh D, Stockwell BR, Prives C (2020) p21 can be a barrier to ferroptosis independent of p53. Aging 12:17800–17814. https://doi.org/10.18632/aging.103961
Xie T (2023) Inhibition of ferroptosis ameliorates hypertensive nephropathy through p53/Nrf2/p21 pathway by Taohongsiwu decoction: based on network pharmacology and experimental validation. J Ethnopharmacol 312:116506
Gao Y, Chen B, Wang R et al (2022) Knockdown of RRM1 in tumor cells promotes radio-/chemotherapy induced ferroptosis by regulating p53 ubiquitination and p21-GPX4 signaling axis. Cell Death Discov 8:343. https://doi.org/10.1038/s41420-022-01140-z
Zhang W, Gai C, Ding D et al (2018) Targeted p53 on small-molecules-induced ferroptosis in cancers. Front Oncol 8:507. https://doi.org/10.3389/fonc.2018.00507
Gao H, Xie R, Huang R et al (2022) CIRBP regulates pancreatic cancer cell ferroptosis and growth by directly binding to p53. J Immunol Res 2022:2527210
Kang R, Kroemer G, Tang D (2019) The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med 133:162–168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
Lee J, Hyun D-H (2023) The interplay between intracellular iron homeostasis and neuroinflammation in neurodegenerative diseases. Antioxidants (Basel) 12(4):918
Han K, Jin X, Guo X et al (2021) Nrf2 knockout altered brain iron deposition and mitigated age-related motor dysfunction in aging mice. Free Radic Biol Med 162:592–602. https://doi.org/10.1016/j.freeradbiomed.2020.11.019
Namgaladze D, Fuhrmann DC, Brüne B (2022) Interplay of Nrf2 and BACH1 in inducing ferroportin expression and enhancing resistance of human macrophages towards ferroptosis. Cell Death Discov 8:327. https://doi.org/10.1038/s41420-022-01117-y
Zhao X, Liu Z, Gao J et al (2020) Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 440:152489. https://doi.org/10.1016/j.tox.2020.152489
Cheng H, Wang P, Wang N et al (2023) Neuroprotection of NRF2 against ferroptosis after traumatic brain injury in mice. Antioxidants 12(3):731
Arber CE, Li A, Houlden H et al (2016) Review: insights into molecular mechanisms of disease in neurodegeneration with brain iron accumulation: unifying theories. Neuropathol Appl Neurobiol 42(3):220–241
Zhao Y, Lu J, Mao A et al (2021) Autophagy inhibition plays a protective role in ferroptosis induced by alcohol via the p62–Keap1–Nrf2 pathway. J Agric Food Chem 69:9671–9683. https://doi.org/10.1021/acs.jafc.1c03751
Li Y et al (2022) Inhibition of CISD2 promotes ferroptosis through ferritinophagy-mediated ferritin turnover and regulation of p62–Keap1–NRF2 pathway. Cell Mol Biol Lett 27(1):81
Liu J, Ren Z, Yang L et al (2022) The NSUN5-FTH1/FTL pathway mediates ferroptosis in bone marrow-derived mesenchymal stem cells. Cell Death Discov 8:99. https://doi.org/10.1038/s41420-022-00902-z
Sandberg M, Patil J et al (2014) NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79:298–306
Xiong L et al (2022) Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicol Environ Saf Ecotoxicol Environ Saf 234:113373
Poli A, Schmitt C, Moulouel B et al (2021) Iron, heme synthesis and erythropoietic porphyrias: a complex interplay. Metabolites 11:798. https://doi.org/10.3390/metabo11120798
Yang C et al (2022) Flavonoid 4,4′-dimethoxychalcone induced ferroptosis in cancer cells by synergistically activating Keap1/Nrf2/HMOX1 pathway and inhibiting FECH. Free Radic Biol Med 188:14–23
Xue W et al (2023) Knockdown of SETD2 promotes erastin-induced ferroptosis in ccRCC. Cell Death Dis Cell Death Dis 14(8):539
He F, Ru X, Wen T (2020) NRF2, a transcription factor for stress response and beyond. Int J Mol Sci 21:4777. https://doi.org/10.3390/ijms21134777
Kerins MJ, Ooi A (2018) The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal 29:1756–1773. https://doi.org/10.1089/ars.2017.7176
Pillai R, Hayashi M, Zavitsanou A-M et al (2022) NRF2: KEAPing tumors protected. Cancer Discov 12(3):625–643
Zhang Y, Lan J, Zhao D et al (2023) Netrin-1 upregulates GPX4 and prevents ferroptosis after traumatic brain injury via the UNC5B/Nrf2 signaling pathway. CNS Neurosci Ther 29:216–227. https://doi.org/10.1111/cns.13997
Zhang Q, Qu H, Chen Y et al (2022) Atorvastatin induces mitochondria-dependent ferroptosis via the modulation of Nrf2-xCT/GPx4 axis. Front Cell Dev Biol 10:806081. https://doi.org/10.3389/fcell.2022.806081
Xiong L, Zhang J, Shi H et al (2022) Downregulation of TNFAIP1 alleviates OGD/R-induced neuronal damage by suppressing Nrf2/GPX4-mediated ferroptosis. Exp Ther Med 25:25. https://doi.org/10.3892/etm.2022.11724
Zhu L, Chen D, Zhu Y et al (2021) GPX4-regulated ferroptosis mediates S100-induced experimental autoimmune hepatitis associated with the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev 2021:1–16. https://doi.org/10.1155/2021/6551069
Yang W, Wang Y, Zhang C et al (2022) Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Front Pharmacol 13:865689. https://doi.org/10.3389/fphar.2022.865689
Mi Y, Wei C, Sun L et al (2023) Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed Pharmacother 157:114048. https://doi.org/10.1016/j.biopha.2022.114048
Liu X, Peng X, Cen S et al (2023) Wogonin induces ferroptosis in pancreatic cancer cells by inhibiting the Nrf2/GPX4 axis. Front Pharmacol 14:1129662
Li X, Chen J, Yuan S et al (2022) Activation of the P62-Keap1-NRF2 pathway protects against ferroptosis in radiation-induced lung injury. Oxid Med Cell Longev 2022:1–16. https://doi.org/10.1155/2022/8973509
Komatsu M, Kurokawa H, Waguri S et al (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223. https://doi.org/10.1038/ncb2021
Zhan S, Lu L, Pan S et al (2022) Targeting NQO1/GPX4-mediated ferroptosis by plumbagin suppresses in vitro and in vivo glioma growth. Br J Cancer 127:364–376. https://doi.org/10.1038/s41416-022-01800-y
Zhang B, Hou Q, Zhang X et al (2023) Anesthetic propofol inhibits ferroptosis and aggravates distant cancer metastasis via Nrf2 upregulation. Free Radic Biol Med 195:298–308. https://doi.org/10.1016/j.freeradbiomed.2022.12.092
Liu Y, Mi Y, Wang Y et al (2023) Loureirin C inhibits ferroptosis after cerebral ischemia reperfusion through regulation of the Nrf2 pathway in mice. Phytomedicine 113:154729. https://doi.org/10.1016/j.phymed.2023.154729
Song C, Li D, Zhang J, Zhao X (2023) Berberine hydrochloride alleviates imatinib mesylate—induced cardiotoxicity through the inhibition of Nrf2-dependent ferroptosis. Food Funct 14:1087–1098. https://doi.org/10.1039/D2FO03331C
Wang T-X, Duan K-L, Huang Z-X et al (2023) Tanshinone functions as a coenzyme that confers gain of function of NQO1 to suppress ferroptosis. Life Sci Alliance 6:e202201667. https://doi.org/10.26508/lsa.202201667
Huang HX, Yang G et al (2020) TFAP2A is a novel regulator that modulates ferroptosis in gallbladder carcinoma cells via the Nrf2 signalling axis. Eur Rev Med Pharmacol Sci 24(9):4745–4755
Wang X, Chen J, Tie H et al (2023) Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J Biochem Mol Toxicol 37:e23368. https://doi.org/10.1002/jbt.23368
Li S, Zhang Y, Zhang J et al (2022) Neferine exerts ferroptosis-inducing effect and antitumor effect on thyroid cancer through Nrf2/HO-1/NQO1 inhibition. J Oncol 2022:1–16. https://doi.org/10.1155/2022/7933775
Fiore A, Zeitler L, Russier M et al (2022) Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 82:920-932.e7. https://doi.org/10.1016/j.molcel.2022.02.007
Liu X, Chen C, Han D et al (2022) SLC7A11/GPX4 inactivation-mediated ferroptosis contributes to the pathogenesis of triptolide-induced cardiotoxicity. Oxid Med Cell Longev 2022:1–16. https://doi.org/10.1155/2022/3192607
Feng L, Zhao K, Sun L et al (2021) SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl Med 19:367. https://doi.org/10.1186/s12967-021-03042-7
Dong H, Qiang Z, Chai D et al (2020) Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging 12:12943–12959. https://doi.org/10.18632/aging.103378
Qiang Z, Dong H, Xia Y et al (2020) Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by regulating SLC7A1. Oxid Med Cell Longev 2020:5146982
Dong H, Xia Y, Jin S et al (2021) Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis 12:1027. https://doi.org/10.1038/s41419-021-04307-1
Qi D, Chen P, Bao H et al (2023) Dimethyl fumarate protects against hepatic ischemia-reperfusion injury by alleviating ferroptosis via the NRF2/SLC7A11/HO-1 axis. Cell Cycle 22:818–828. https://doi.org/10.1080/15384101.2022.2155016
Yuan Y, Zhai Y, Chen J et al (2021) Kaempferol ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal ferroptosis by activating Nrf2/SLC7A11/GPX4 axis. Biomolecules 11:923. https://doi.org/10.3390/biom11070923
Liu T, Cui Y, Dong S et al (2022) Treadmill training reduces cerebral ischemia-reperfusion injury by inhibiting ferroptosis through activation of SLC7A11/ GPX4. Oxid Med Cell Longev. https://doi.org/10.1155/2022/8693664
Huang Y, Jiang L, Liu X et al (2022) Melatonin alleviates acute kidney injury by inhibiting NRF2/Slc7a11 axis-mediated ferroptosis. Oxid Med Cell Longev 2022:1–24. https://doi.org/10.1155/2022/4776243
Luo L, Zhang Z, Weng Y, Zeng J (2022) Ferroptosis-related gene GCLC Is a novel prognostic molecular and correlates with immune infiltrates in lung adenocarcinoma. Cells 11:3371. https://doi.org/10.3390/cells11213371
Yang Z, Zou S, Zhang Y et al (2023) ACTL6A protects gastric cancer cells against ferroptosis through induction of glutathione synthesis. Nat Commun 14:4193. https://doi.org/10.1038/s41467-023-39901-8
Xu Y, Li Y, Li J, Chen W (2022) Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol 53:102349. https://doi.org/10.1016/j.redox.2022.102349
Kang YP, Mockabee-Macias A, Jiang C et al (2021) Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab 33:174-189.e7. https://doi.org/10.1016/j.cmet.2020.12.007
Nishizawa H, Matsumoto M et al (2020) Ferroptosis is controlled by the coordinated transcriptional regulation of glutathione and labile iron metabolism by the transcription factor BACH1. J Biol Chem 295(1):69–82
Gong S, Zhang A, Yao M et al (2023) REST contributes to AKI-to-CKD transition through inducing ferroptosis in renal tubular epithelial cells. JCI Insight 8:e166001. https://doi.org/10.1172/jci.insight.166001
Wang S, Wang H, Zhu S, Li F (2022) Systematical analysis of ferroptosis regulators and identification of GCLM as a tumor promotor and immunological biomarker in bladder cancer. Front Oncol 12:1040892. https://doi.org/10.3389/fonc.2022.1040892
Khan U, Hadid T (2016) RUSH for G6PD! Blood 128:2742–2742. https://doi.org/10.1182/blood-2016-07-730119
Meng Q, Zhang Y, Hao S et al (2022) Recent findings in the regulation of G6PD and its role in diseases. Front Pharmacol 13:932154. https://doi.org/10.3389/fphar.2022.932154
Cao F, Luo A, Yang C (2021) G6PD inhibits ferroptosis in hepatocellular carcinoma by targeting cytochrome P450 oxidoreductase. Cell Signal 87:110098. https://doi.org/10.1016/j.cellsig.2021.110098
Zhang Y, Ren H, Zhang C et al (2022) Development and validation of four ferroptosis-related gene signatures and their correlations with immune implication in hepatocellular carcinoma. Front Immunol 13:1028054. https://doi.org/10.3389/fimmu.2022.1028054
Ding H, Chen Z, Wu K et al (2021) Activation of the NRF2 antioxidant program sensitizes tumors to G6PD inhibition. Sci Adv 7:eabk1023. https://doi.org/10.1126/sciadv.abk1023
Lv Y, Lv X, Zhang J et al (2022) BRD4 targets the KEAP1-Nrf2-G6PD axis and suppresses redox metabolism in small cell lung cancer. Antioxidants 11:661. https://doi.org/10.3390/antiox11040661
Yang W-H, Chi J-T (2020) Hippo pathway effectors YAP/TAZ as novel determinants of ferroptosis. Mol Cell Oncol 7:1699375. https://doi.org/10.1080/23723556.2019.1699375
Sun T, Chi J-T (2021) Regulation of ferroptosis in cancer cells by YAP/TAZ and Hippo pathways: the therapeutic implications. Genes Dis 8:241–249. https://doi.org/10.1016/j.gendis.2020.05.004
Magesh S, Cai D (2022) Roles of YAP/TAZ in ferroptosis. Trends Cell Biol 32:729–732. https://doi.org/10.1016/j.tcb.2022.05.005
Zheng Y, Pan D (2019) The Hippo signaling pathway in development and disease. Dev Cell 50:264–282. https://doi.org/10.1016/j.devcel.2019.06.003
Gao R, Kalathur RKR, Coto-Llerena M et al (2021) YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med 13:e14351. https://doi.org/10.15252/emmm.202114351
Lin C-C, Yang W-H, Lin Y-T et al (2021) DDR2 upregulation confers ferroptosis susceptibility of recurrent breast tumors through the Hippo pathway. Oncogene 40:2018–2034. https://doi.org/10.1038/s41388-021-01676-x
Setayeshpour Y, Chi J-T (2021) Editorial: novel insights into ferroptosis. Front Cell Dev Biol 9:754160. https://doi.org/10.3389/fcell.2021.754160
Yang W-H, Ding C-KC, Sun T et al (2019) The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep 28:2501-2508.e4. https://doi.org/10.1016/j.celrep.2019.07.107
Yang W-H, Lin C-C, Wu J et al (2021) The Hippo pathway effector YAP promotes ferroptosis via the E3 ligase SKP2. Mol Cancer Res 19:1005–1014. https://doi.org/10.1158/1541-7786.MCR-20-0534
He S, Li R, Peng Y et al (2022) ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke. J Cachexia Sarcopenia Muscle 13:1717–1730. https://doi.org/10.1002/jcsm.12953
Li Y, Feng D, Wang Z et al (2019) Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 26:2284–2299. https://doi.org/10.1038/s41418-019-0299-4
Wang Y, Zhang M, Bi R et al (2022) ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol 51:102262. https://doi.org/10.1016/j.redox.2022.102262
Tuo Q, Liu Y, Xiang Z et al (2022) Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther 7:59. https://doi.org/10.1038/s41392-022-00917-z
Cui Y, Zhang Y, Zhao X et al (2021) ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun 93:312–321. https://doi.org/10.1016/j.bbi.2021.01.003
Grube J, Woitok MM, Mohs A et al (2022) ACSL4-dependent ferroptosis does not represent a tumor-suppressive mechanism but ACSL4 rather promotes liver cancer progression. Cell Death Dis 13:704. https://doi.org/10.1038/s41419-022-05137-5
Liao P, Wang W, Wang W et al (2022) CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40:365-378.e6. https://doi.org/10.1016/j.ccell.2022.02.003
Chen C, Yang Y, Guo Y et al (2023) CYP1B1 inhibits ferroptosis and induces anti-PD-1 resistance by degrading ACSL4 in colorectal cancer. Cell Death Dis 14:271. https://doi.org/10.1038/s41419-023-05803-2
Zeng K, Li W, Wang Y et al (2023) Inhibition of CDK1 overcomes oxaliplatin resistance by regulating ACSL4-mediated ferroptosis in colorectal cancer. Adv Sci. https://doi.org/10.1002/advs.202301088
Aolymat I, Hatmal MM, Olaimat AN (2023) The emerging role of heat shock factor 1 (HSF1) and heat shock proteins (HSPs) in ferroptosis. Pathophysiology 30:63–82. https://doi.org/10.3390/pathophysiology30010007
Sun X, Ou Z, Xie M et al (2015) HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 34:5617–5625. https://doi.org/10.1038/onc.2015.32
Zhang B, Fan Y, Cao P, Tan K (2021) Multifaceted roles of HSF1 in cell death: A state-of-the-art review. Biochim Biophys Acta BBA - Rev Cancer 1876:188591. https://doi.org/10.1016/j.bbcan.2021.188591
Wang N, Ma H, Li J et al (2021) HSF1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. J Mol Cell Cardiol 150:65–76. https://doi.org/10.1016/j.yjmcc.2020.10.010
Jia G, Wu W, Chen L et al (2023) HSF1 is a novel prognostic biomarker in high-risk prostate cancer that correlates with ferroptosis. Discov Oncol 14:107. https://doi.org/10.1007/s12672-023-00715-1
Brown CW, Chhoy P, Mukhopadhyay D et al (2021) Targeting prominin2 transcription to overcome ferroptosis resistance in cancer. EMBO Mol Med 13:e13792. https://doi.org/10.15252/emmm.202013792
Han S, Liu Q, Yang Z et al (2022) Identification of ferroptosis-related gene prognostic signature and HSF1 for reversing doxorubicin and gemcitabine resistance in uterine carcinosarcoma. Dis Markers 2022:1–16. https://doi.org/10.1155/2022/6400227
Liu M, Fan Y, Li D et al (2021) Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS–mitochondrial fission–mitophagy axis. Mol Oncol 15:2084–2105. https://doi.org/10.1002/1878-0261.12936
Zhong Y, Zhang W, Yu H et al (2022) Multi-platform-based characterization of ferroptosis in human colorectal cancer. iScience 25:104750. https://doi.org/10.1016/j.isci.2022.104750
Huang F, Zhang S, Li X et al (2022) STAT3-mediated ferroptosis is involved in ulcerative colitis. Free Radic Biol Med 188:375–385. https://doi.org/10.1016/j.freeradbiomed.2022.06.242
Liu Q, Wang K (2019) The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int 43:1245–1256. https://doi.org/10.1002/cbin.11121
Schmitt A, Xu W, Bucher P et al (2021) Dimethyl fumarate induces ferroptosis and impairs NF-kB/STAT3 signaling in DLBCL. Blood 138(10):871–884
Huang Q, Li J, Ma M et al (2023) High-throughput screening identification of a small-molecule compound that induces ferroptosis and attenuates the invasion and migration of hepatocellular carcinoma cells by targeting the STAT3/GPX4 axis. Int J Oncol 62:42. https://doi.org/10.3892/ijo.2023.5490
Luo L, Deng L, Chen Y et al (2023) Identification of lipocalin 2 as a ferroptosis-related key gene associated with hypoxic-ischemic brain damage via STAT3/NF-κB signaling pathway. Antioxidants 12:186. https://doi.org/10.3390/antiox12010186
Ouyang S, Li H, Lou L et al (2022) Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol 52:102317. https://doi.org/10.1016/j.redox.2022.102317
Zhang W, Gong M, Zhang W et al (2022) Thiostrepton induces ferroptosis in pancreatic cancer cells through STAT3/GPX4 signalling. Cell Death Dis 13:630. https://doi.org/10.1038/s41419-022-05082-3
Huang C-Y, Chen L-J, Chen G et al (2022) SHP-1/STAT3-signaling-axis-regulated coupling between BECN1 and SLC7A11 contributes to sorafenib-induced ferroptosis in hepatocellular carcinoma. Int J Mol Sci 23:11092. https://doi.org/10.3390/ijms231911092
Nie X-H, Qiu S, Xing Y et al (2022) Paeoniflorin regulates NEDD4L/STAT3 pathway to induce ferroptosis in human glioma cells. J Oncol 2022:1–15. https://doi.org/10.1155/2022/6093216
Duan J, Huang D, Liu C et al (2023) USP11-mediated LSH deubiquitination inhibits ferroptosis in colorectal cancer through epigenetic activation of CYP24A1. Cell Death Dis 14:402. https://doi.org/10.1038/s41419-023-05915-9
Li Y, Yan J, Zhao Q et al (2022) ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front Pharmacol 13:904314. https://doi.org/10.3389/fphar.2022.904314
Wang L, Liu Y, Du T et al (2020) ATF3 promotes erastin-induced ferroptosis by suppressing system Xc–. Cell Death Differ 27:662–675. https://doi.org/10.1038/s41418-019-0380-z
Ye J, Zhang F, Li B et al (2023) Knockdown of ATF3 suppresses the progression of ischemic stroke through inhibiting ferroptosis. Front Mol Neurosci 15:1079338. https://doi.org/10.3389/fnmol.2022.1079338
Ling H, Xiao H, Luo T et al (2023) Role of ferroptosis in regulating the epithelial-mesenchymal transition in pulmonary fibrosis. Biomedicines 11:163. https://doi.org/10.3390/biomedicines11010163
Bao R, Wang Q, Yu M et al (2023) AAV9-HGF cooperating with TGF-beta/Smad inhibitor attenuates silicosis fibrosis via inhibiting ferroptosis. Biomed Pharmacother 161:114537. https://doi.org/10.1016/j.biopha.2023.114537
Yang Y, Ma Y, Li Q et al (2022) STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death Dis 13:530. https://doi.org/10.1038/s41419-022-04971-x
Mukhopadhyay S, Encarnación-Rosado J, Lin EY et al (2023) Autophagy supports mitochondrial metabolism through the regulation of iron homeostasis in pancreatic cancer. Sci Adv 9:eadf9284. https://doi.org/10.1126/sciadv.adf9284
Santana-Codina N, Del Rey MQ, Kapner KS et al (2022) NCOA4-mediated ferritinophagy is a pancreatic cancer dependency via maintenance of iron bioavailability for iron-sulfur cluster proteins. Cancer Discov 12:2180–2197. https://doi.org/10.1158/2159-8290.CD-22-0043
Funding
A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD).
Author information
Authors and Affiliations
Contributions
Lai and Hu were responsible for the main manuscript preparation, while Yue carried out the main proofreading and literature collection. Images 1-6 were produced by Tan,Tao and Zhai. Li carried out direction and topic related guidance. Tan and Lai make subsequent revisions to the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lai, L., Tan, M., Hu, M. et al. Important molecular mechanisms in ferroptosis. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-05009-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-05009-w