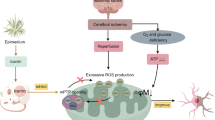
Abstract
Cerebral ischemia reperfusion injury (CIRI) is still a serious problem threatening human health. Salidroside (SAL) is a natural phenylpropanoid glycoside compound with antioxidant, anti-inflammatory, and anti-ischemic properties. This study investigated the protective mechanism of SAL on middle cerebral artery occlusion (MCAO)- and oxygen-glucose deprivation/reoxygenation (OGD/R) model-induced CIRI via regulating the nuclear factor erythroid 2-related factor 2 (Nrf2)/thioredoxin 1 (Trx1) axis. The results indicated that SAL (50 mg/kg or 100 mg/kg, intraperitoneal injection) not only effectively alleviated infarction rate, improved histopathological changes, relieved apoptosis by strengthening the suppression of cleaved caspase-3 and Bax/Bcl-2 proteins and decreased malondialdehyde (MDA) formation, but also increased superoxide dismutase (SOD) and catalase (CAT) activities and upregulated the expressions of Nrf2 and Trx1 on MCAO-induced CIRI rats. SAL also efficiently inhibited apoptosis and decreased oxidative stress in OGD/R-stimulated PC12 cells. Furthermore, blocking the Nrf2/Trx1 pathway using tretinoin, an Nrf2 inhibitor, significantly reversed the protective effect of SAL on OGD/R-induced oxidative stress. Moreover, SAL reduced the expression of apoptosis signal-regulating kinase-1 (ASK1) and mitogen-activated protein kinase (MAPK) family proteins. These results demonstrated that SAL inhibited oxidative stress through Nrf2/Trx1 signaling pathway, and subsequently reduced CIRI-induced apoptosis by inhibiting ASK1/MAPK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia or ischemic stroke is an acute cerebrovascular disease with high rates of mortality and disability, which has caused a huge burden to humans (Roth et al. 2020). It is caused by a lack of blood supply, which results in the immediate depletion of oxygen and glucose in brain tissues. At present, the effective clinical treatment for this disease is thrombolysis (Group. 1995), but the rapid recovery of blood flow and nutrients during thrombolysis may aggravate brain injury and lead to cerebral ischemia/reperfusion injury (CIRI). The development of CIRI involves complex pathophysiological events, including free radical injury, oxidative stress, inflammatory response, intracellular calcium overload, and apoptosis (Meng et al. 2019; Wang et al. 2016; Ye et al. 2012; Wu et al. 2020). Therefore, finding a more effective treatment for CIRI has been a hot and challenging topic in this field.
Apoptosis, also known as programmed cell death, caused by oxidative stress and excitotoxicity, plays a crucial role in CIRI (Fangma et al. 2021). Moreover, excessive reactive oxygen species in brain tissues after reperfusion will cause oxidative stress. Nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidative responses, can play a protective role by coordinating the basal and stress-inducible activation of multiple cytoprotective genes (Vomund et al. 2017). It is reported that caffeic acid phenethyl ester 4-O-glucoside could inhibit oxidative stress to ameliorate neurodegenerative diseases by activating the Nrf2 pathway (Wan et al. 2019). Furthermore, Nrf2 could also alleviate CIRI by promoting antioxidant activity. A previous study has shown that Nrf2 played a protective role by regulating the expression of thioredoxin 1 (Trx1) during CIRI (Hou et al. 2018). Trx1 is a cellular redox enzyme that plays an indispensable role in cell redox homeostasis. After CIRI, Trx1 silencing increased the production of reactive oxygen species (ROS), where its expression level was probably in part regulated by Nrf2 (Li et al. 2015). Trx1 can interact with apoptosis signal-regulating kinase-1 (ASK1), and the knockdown of Trx1 could promote phosphorylation of ASK1 to induce apoptosis (Wu et al. 2015). ASK1 is a crucial effector in ROS-induced cell death, and blocking the ASK1/MAPK signaling pathway is considered to be able to inhibit apoptosis (Yoon et al. 2020). The Nrf2/Trx1 axis is a specific target of therapeutic intervention. Consequently, targeting the Nrf2/Trx1 axis is an attractive therapeutic strategy for stroke prevention and reversal.
Salidroside (SAL, Fig. 1A shows the chemical structure of SAL) is a phenylpropanoid glycoside compound isolated from Rhodiola rosea L. growing in high-altitude areas. Additionally, SAL has extensive pharmacological activities, such as anti-inflammatory, antioxidant, anti-apoptosis, and cardioprotective (Chen et al. 2019; Huang et al. 2019; Rong et al. 2020; Wu et al. 2019). Many studies have shown that SAL has protective effects on cell damage and apoptosis through different cell signal transduction pathways. For example, SAL could protect human umbilical vein endothelial cells (HUVECs) from oxidized low-density lipoprotein (ox-LDL) damage by inhibiting oxidative stress (Dongming et al. 2019). A recent study has indicated that SAL could alleviate hepatic oxidative stress induced by hypoxia through the Nrf2 signaling pathway (Xiong et al. 2021). Here, SAL could cross the blood-brain barrier when given intraperitoneally (Zuo et al. 2018). Moreover, SAL could be used to treat neurodegenerative diseases, and its neuroprotective effect is involved in the Nrf2 pathway (Zhong et al. 2018). This study aims to explore whether SAL plays an antioxidant stress role through the Nrf2/Trx1 pathway in CIRI, thus playing an anti-apoptosis role.
Materials and methods
Animals
All the animal experiments were approved by the Animal Ethics Committee of Shenyang Pharmaceutical University. Experiments were performed with SPF male, 50 Sprague-Dawley (SD) rats weighing 250–300 g from the Central Animal House of Shenyang Pharmaceutical University (Shenyang, China). Rats were kept in plastic cages in standard laboratory conditions (temperature 23 ± 2℃, 12 h light/dark cycle) accessing to adequate food and water, and they were meanwhile allowed to adjust the environment for 7 days before the experiment.
Middle cerebral artery occlusion (MCAO) injury operation
All groups of rats except for rats in the sham group were treated with modified Longa’s method (Longa. 1989). The rats were anesthetized and placed on a surgical plate. Subsequently, an incision was made along the median line of the neck and the right common, external (ECA) and internal (ICA) carotid arteries were carefully exposed and separated. After the ligature of the distal end of the ECA and its branches, a silicone-coated nylon monofilament (Beijing Cinontech Co., Ltd., China) was inserted through the ECA stump into the ICA and gently advanced to block the middle cerebral artery. After 2 h of MCAO, the filament was removed to restore cerebral perfusion. The body temperature was maintained at 37 ± 0.5℃ with a heating lamp throughout the surgery and occlusion period. In the sham group, all surgical procedures were the same as above except for the insertion of the nylon monofilament.
Groupings
The experimental procedures of the in vivo experiments are shown in Fig. 1B. All rats were randomly divided into 5 groups (n = 10) including: sham, MCAO, MCAO + 100 mg/kg SAL (purity > 98% by HPLC, Cat. #MB5843, Meilun Biotech, China) (Zhong et al. 2019), MCAO + 50 mg/kg SAL, MCAO + 5 mg/kg edaravone (ED, positive control group) (purity > 98% by HPLC, Cat. #MB1441, Meilun Biotech, China), no rats were dead. Seven days before MCAO, all groups of rats except for the sham group were injected intraperitoneally once a day. SAL and ED were dissolved in normal saline solution, respectively before use. The sham group was given normal saline.
2,3,5-Triphenyltetrazolium (TTC) staining
Rat brains were quickly removed and coronally sectioned to a thickness of 2 mm. Subsequently, the sections were quickly immersed in a solution containing 2% TTC (Meilun Biotech, China) at 37 °C in the dark for 20 min and fixed in 4% paraformaldehyde overnight. TTC staining of each brain section of rats in each group (the unstained area was identified as infarct area) was used to quantitatively evaluate the percentage of infarct volume with Image-Pro Plus software.
Neurological deficits assessment
Neurological deficits were determined using Longa’s methods (Longa. 1989) with minor revision, and the score ranged from 0 to 4 (0, no obvious nerve injury; 1, failed to stretch forepaw fully; 2, circling to the contralateral side; 3, falling to the left or no spontaneous motor activity; 4, do not walk spontaneously). The neurological test was conducted at 24 h after MCAO by three blinded observers.
Tissue preparation and hematoxylin and eosin (HE), Nissl, and TdT-mediated dUTP nick end labeling (TUNEL) staining
Deeply anesthetized rats were perfused first with cold normal saline (4 °C) until forelimbs became pale and the brains were collected gently and fixed in cold 4% paraformaldehyde for 24 h at 4 °C. After dehydration in gradient ethanol, the samples were made transparent with xylene, and the brain cortex was embedded in paraffin. Subsequently, the coronal slices (3–4 μm) were stained with HE staining, Nissl, and TUNEL staining. For histological staining, sections were stained with hematoxylin and eosin (Servicebio, China) for HE staining and Nissl staining solution (Servicebio, China) for Nissl staining, respectively. Apoptosis was assessed histologically and with a TUNEL reagent kit (Thermo Fisher Scientific, USA), according to the manufacturer’s instructions. A microscope (Nikon, Tokyo, Japan) was used to collect images.
Cell culture
The PC12 cells (Shanghai lnstitute of Biochemistry and Cell Biology, Shanghai, China) were cultured in RPMI-1640 (Meilun Biotech, China) complete medium containing 10% FBS (Meilun Biotech, China), 100 U/mL penicillin, and 100 mg/mL streptomycin (Meilun Biotech, China). Cells were cultured at 37℃ with 5% CO2 in a humidified atmosphere. SAL, ED and tretinoin were dissolved in DMSO to make 200 mM stock solution. The stock solution was then diluted with RPMI-1640 complete medium so that the concentration of DMSO was kept below 0.1% to avoid toxic effects on cells in culture. Cells were pretreated with freshly prepared SAL, ED (10 µM) and tretinoin (12.5 µM) (Meilun Biotech, China) for 12 h, before oxygen-glucose deprivation (OGD) and during OGD/reoxygenation (OGD/R).The experimental procedures of the in vitro experiments are shown in Fig. 1C.
OGD/R treatment
Before OGD, the PC12 cells were washed three times with PBS and were then replaced with glucose-free RPMI-1640 (Meilun Biotech, China) and then incubated with 95% N2/5% CO2 for 6 h. Cells were then reoxygenated by placing them in fresh normal RPMI-1640 and an incubator with 95% air/5% CO2 (normoxia) for 1 h, respectively. Control cells were incubated with a normal medium for 6 h and replaced with a normal medium with normoxic conditions in a manner identical to that for OGD/R (Wang et al. 2020).
Cell viability assay
MTT assay was conducted to determine the cell viability of PC12 cells. In brief, PC12 cells were seeded into 96-well cell culture microplates at an approximate density of 8 × 103 cells per well and incubated overnight. After the treatment with SAL and ED for 12 h, OGD/R was carried out as described above. The cells were incubated with an additional 20 µL of MTT (Meilun Biotech, China) solution (5 mg/mL) at 37 °C for 3 h. After that, 100 µL of a solution containing 10% SDS, 5% isopropanol and 0.12 M HCl was added to each well to dissolve the formazan crystals. Finally, the absorbance of each well was read on a microplate reader (Spectra maxm2, Molecular Devices, Sunnyvale, CA, USA) at 570 nm.
Detection of LDH, SOD, MDA, CAT
The activities of lactate dehydrogenase (LDH) assay kit (Cat. # A020, Nanjing Jiancheng Bioengineering Institute, China), superoxide Dismutase (SOD) assay kit (Cat. # S0103, Beyotime, China), catalase (CAT) assay kit (Cat. # S0051, Beyotime, China), and malondialdehyde (MDA) assay kit (Cat. # S0131, Beyotime, China) in the brain cortex and PC12 cells were measured according to the protocol recommended.
Reactive oxygen species production assay
2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Meilun Biotech, China) staining method was applied to measure the generation of reactive oxygen species. PC12 cells were incubated with 10 µM DCF-DA for 20 min at 37 °C in the dark. The cell suspension was loaded into the flow-specific tube and analyzed by flow cytometry at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
Hoechst 33,342 staining
PC12 cells were seeded into 12-well plates at a density of 10,000 cells per well and cultured for 24 h. Then, the cells were treated with SAL and/or tretinoin for 12 h before OGD/R. Subsequently, cells were washed with cold PBS once. Hoechst 33,342 (Meilun Biotech, China) was added to cells for 20 min incubation at 37 °C in dark. The apoptotic cells with the fragmented nuclei were observed under a fluorescence microscope (MCTIO, Xiamen, China).
Western blot
Total proteins extracted from the ischemic side cerebral cortex and PC12 cells were collected and fractionated on SDS-PAGE gels. Then, the protein was incubated with primary antibodies against Bax (CAT. # 50,599, Proteintech, China), Bcl-2 (CAT. # 26,593, Proteintech, China), cleaved caspase-3 (CAT. # 19,677, Proteintech, China), p-ASK1 (CAT. # 320,030, Zen-Bioscience, China), ASK1 (CAT. # 28,201, Proteintech, China), p-JNK (CAT. # 80,024, Proteintech, China), JNK (CAT. # 66,210, Proteintech, China), Nrf2 (CAT. # 16,396, Proteintech, China), Trx1 (CAT. # 14,999, Proteintech, China), p-ERK1/2 (CAT. # 50,599, Proteintech, China), ERK1/2 (CAT. # 11,257, Proteintech, China), p38 (CAT. # 66,234, Proteintech, China), p-p38 (CAT. # 310,069, Zen-Bioscience, China), and β-actin (CAT. # 20,536, Proteintech, China). After that, the membranes were incubated with the corresponding secondary antibodies (CAT. # SA00001, Proteintech, China) and scanned using the Tamon imaging system (Shanghai, China). The relative band intensity was normalized to that of β-actin. Band intensities were quantified using Image-Pro 6.0 software (Media Cybernetics, Baltimore, MD, USA).
Statistical analysis
Data are expressed as means ± SEM. Differences between the groups were analyzed by one-way ANOVA with Tukey’s multiple comparisons test by GraphPad Prism (SanDiego, CA, USA). The differences among the groups were considered statistically significant at P < 0.05.
Results
Neuroprotective effects of SAL against CIRI
To study the potential neuroprotective effect of SAL on CIRI, the histopathological changes in the brain tissues were evaluated using TTC staining following treatment of MCAO rats with SAL. The largest visible infarct area was found in the MCAO group (Fig. 2 A-B), which was significantly reduced with the treatment of high-dose SAL (P < 0.001). As shown in Fig. 2 C, the sham group showed no symptoms of neurological impairment, and the neurological function score was 0. On the contrary, the neurological deficit score of the MCAO group was significantly higher than that of the sham group (P < 0.001), suggesting that the neurological function of the rats was severely damaged and the modeling was successful. The symptoms of the SAL group were less than those of the MCAO group (P < 0.001), suggesting that SAL could ameliorate neurological impairment. The effects of SAL on neuronal morphology of cortical neurons in rats after MCAO were determined by HE staining and Nissl staining (Fig. 2D-E). The structure of brain tissue was normal, the cells were arranged orderly with clear cell outline, necrotic cells were not observed, Nissl bodies, nuclei and nucleoli were clearly distinguishable in the sham group. Instead, in the MCAO group, the brain tissues were arranged irregularly, stained unevenly, the intercellular space increased, the number of cells decreased obviously, Nissl bodies shrank and were distributed disorderly, and the nuclei were small and even disappeared. However, SAL could reduce damage by reducing the number of damaged cells (P < 0.001). Taken together, these results suggested that SAL had neuroprotective effects on CIRI.
Neuroprotective effects of SAL against CIRI. (A and B) Cerebral infarction volume in each group was evaluated by using TTC staining. Normal brain tissue (red) and infarction area (white), scale bar = 2 mm. (C) Statistical graph of neurological deficit score after reperfusion. Histopathological changes in the brain tissues were evaluated using HE staining (D) and Nissl staining (E) images. (F) was quantitative analysis of HE staining. (G) was quantitative analysis of Nissl staining Magnification, x200. Values are presented as mean ± SEM (n = 6). ###P < 0.001 versus sham group; *P < 0.05, **P < 0.01 and ***P < 0.001 versus MCAO group
Effects of SAL on cellular apoptosis in the brain of rats in CIRI
Apoptosis was detected by the TUNEL assay. Compared with the sham group, the number of apoptotic cells was increased after MCAO in brain tissue (P < 0.001) (Fig. 3 A-B). The cell apoptosis induced by MCAO was better reduced by high-dose SAL (P < 0.001). The ratio of Bax/Bcl-2 and the expression of cleaved caspase-3 increased in the MCAO group (P < 0.001) (Fig. 3 C-E), while SAL obviously reversed their expression compared with the MCAO group (Bax/Bcl-2, P < 0.001; cleaved caspase-3, P < 0.05). These results indicated that SAL could inhibit CIRI-induced apoptosis.
Effects of SAL on cellular apoptosis in the brain of rats after MCAO. (A-B) The apoptotic neurons were detected by TUNEL (green)/DAPI (blue) staining. The proportion of apoptotic cells was analyzed quantitatively. Magnification, x200. (C-E) The expression of Bax, Bcl-2, and cleaved caspase-3 proteins. Values are presented as mean ± SEM. ###P < 0.001 versus sham group; *P < 0.05 and ***P < 0.001 versus MCAO group
Effects of SAL on oxidative stress in the brain of rats in CIRI
As shown in Fig. 4 A, compared with the sham group, the activities of SOD and CAT were decreased, and the activity of MDA was increased after MCAO, while high-dose SAL treatment better reversed MCAO-induced changes in brain tissues (P < 0.001). Meanwhile, it was found that the expression of Nrf2 and its downstream factor Trx1 were significantly reduced in the MCAO group in brain tissues of rats compared with the sham group. SAL obviously increased their expression compared with the MCAO group, and the effect of high-dose SAL was better (P < 0.001) (Fig. 4B-D). Our results showed that SAL attenuated oxidative stress in the brain of rats after MCAO.
Effects of SAL on oxidative stress in the brain of rats after MCAO. (A) SOD, CAT, and MDA commercial kits were used to detect the activities of SOD, CAT, and MDA in the brain of rats. (B-D) Expression of Nrf2 and Trx1 proteins. Values are presented as mean ± SEM. ###P < 0.001 versus sham group; **P < 0.01 and ***P < 0.001 versus MCAO group
Effects of SAL on OGD/R induced apoptosis of PC12 cells
The effects of SAL on the survival of PC12 cells were examined using the MTT assay. The results showed that PC12 cells treated with 0–50 µM SAL did not affect cells viability (Fig. 5 A). LDH assay was then performed for the detection of cytotoxicity, and the result showed that SAL led to a marked decrease in the cytotoxicity of OGD/R induced by PC12 cells (P < 0.001) (Fig. 5B). Besides, the expression of apoptosis-related proteins was detected by western blot. The results showed that PC12 cells treated with OGD/R significantly increased the ratio of Bax/Bcl-2 and the expression of cleaved caspase-3 (P < 0.001) (Fig. 5 C-E). Their expression induced by OGD/R were reversed by SAL, with the most significant effect at the dose of 50 µM (P < 0.001). These results showed that SAL played an anti-apoptosis role in vitro.
Effects of SAL on OGD/R induced apoptosis of PC12 cells. (A) Cell viability of PC12 cells treated with SAL (0–50 µM). (B) The LDH release of PC12 cells treated with SAL (0–50 µM) followed by OGD/R stimulation. (C-E) The expression of Bax, Bcl-2, and cleaved caspase-3 proteins. Values are presented as mean ± SEM. ###P < 0.001 versus control group; **P < 0.01 and ***P < 0.001 versus OGD/R group
Effects of SAL on oxidative stress in OGD/R treated PC12 cells
To further explore the potential effects of SAL on OGD/R-induced PC12 cells, indicators of oxidative stress were examined in vitro. As shown in Fig. 6 A, compared with the control group, the SOD and CAT activities of the OGD/R group significantly decreased, and the MDA activity increased, while this change was significantly reversed after SAL treatment, and the reversal effect at the dose of 50 µM was even more pronounced (P < 0.001). Subsequently, the level of ROS in PC12 cells was detected by a DCFH-DA probe. OGD/R was shown to increase the level of ROS in PC12 cells, while treatment with SAL decreased ROS production (P < 0.001) (Fig. 6B-C). Meanwhile, it was found that OGD/R significantly reduced the expression of Nrf2 and its downstream factor Trx1 in PC12 cells compared with the control, but after treatment with SAL, their expression increased significantly (Nrf2, P < 0.01; Trx1, P < 0.001) (Fig. 6D-E). The results demonstrated that SAL inhibited oxidative stress in OGD/R-treated PC12 cells.
Effects of SAL on oxidative stress in OGD/R treated PC12 cells. (A) SOD, CAT, and MDA commercial kits were used to detect the activities of SOD, CAT, and MDA in PC12 cells. (B-C) The intracellular ROS levels in PC12 cells were measured by flow cytometry. (D-E) Expression of Nrf2 and Trx1 proteins. Values are presented as mean ± SEM. ###P < 0.001 versus control group; *P < 0.05, **P < 0.01 and ***P < 0.001 versus OGD/R group
Effects of SAL on oxidative stress in OGD/R treated PC12 cells by Nrf2/Trx1 pathway
To determine whether the Nrf2 pathway is involved in the inhibitory effect of SAL on OGD/R, we exposed PC12 cells to tretinoin, an Nrf2 inhibitor, to detect the downstream related indicators. Indicators of oxidative stress in PC12 cells were detected (Fig. 7 A-C). OGD/R was shown to reduce the activities of SOD and CAT and increase the activity of MDA, thus producing oxidative stress, while SAL treatment showed an oppositive effect. Moreover, tretinoin eliminated the inhibitory effect of SAL on oxidative stress (P < 0.001). And it was found that SAL reversed the downregulation of Nrf2 and Trx1 expression induced by OGD/R, while tretinoin inhibited the SAL-activated Nrf2/Trx1 pathway (P < 0.001) (Fig. 7D-E). The results further indicated that SAL inhibited oxidative stress in PC12 cells by the Nrf2/Trx1 pathway.
Effects of SAL on oxidative stress in OGD/R treated PC12 cells by Nrf2/Trx1 pathway. (A-C) SOD, CAT, and MDA commercial kits were used to detect the activities of SOD, CAT, and MDA in PC12 cells. (D-E) Expression of Nrf2 and Trx1 proteins. Values are presented as mean ± SEM. ###P < 0.001 versus control group; ***P < 0.001 versus OGD/R group; $$P < 0.01, $$$P < 0.001 versus OGD/R + SAL group
Effects of SAL mediated Nrf2/Trx1 signaling on oxidative stress-driven apoptosis
Finally, we determined whether the mechanism behind how SAL reduced apoptosis driven by oxidative stress was directly involved in Nrf2 up-regulation. As shown in Fig. 8 A, compared with the SAL group, tretinoin resulted in extensive apoptotic nuclear fragmentation of PC12 cells. Excessive ROS accumulation stimulates the activation of ASK1, which can induce apoptosis through the MAPK signaling pathway. Since MAPK signaling is a well-recognized canonical apoptosis signaling pathway, thus we further examined the activation of MAPK family proteins, including JNK, ERK, and p38 signaling pathways. As shown in Fig. 8B-H, the results showed that OGD/R significantly increased the phosphorylation expression of JNK, ERK, and p38 (P < 0.001). After pre-treatment with SAL, it was found that the expression levels of p-JNK (P < 0.05), p-ERK (P < 0.001), and p-p38 (P < 0.001) decreased significantly, while tretinoin could reverse this situation (P < 0.001). In addition, tretinoin decreased the ratio of Bax/Bcl-2 and the expression of cleaved caspase-3 (P < 0.001). The data indicated that SAL-mediated Nrf2/Trx1 signaling suppressed oxidative stress-driven apoptosis.
Effects of SAL mediated Nrf2/Trx1 signaling on oxidative stress-driven apoptosis. (A) Apoptosis was detected by Hoechst 33,342 staining (200 × magnification, Scale bar = 50 μm). (B-H) Expression of p-ASK1, ASK1, p-JNK, JNK, p-p38, p38, p-ERK, ERK, Bcl-2, Bax, and cleaved caspase-3 proteins. Values are presented as mean ± SEM. ###P < 0.001 versus control group; *P < 0.05, ***P < 0.001 versus OGD/R group; $$$P < 0.001 versus OGD/R + SAL group
Discussion
This study investigated the role of SAL in CIRI-induced oxidative stress and apoptosis in vivo and in vitro. The MCAO and OGD/R models were used to mimic the pathophysiology of ischemia/reperfusion to investigate the neuroprotective effects of SAL both in vivo and in vitro. We found that SAL and ED could equally reduce infarct size, decrease neurological severity score, improve pathological changes, and reduce LDH release, and that high-dose SAL was more effective, suggesting that SAL has neuroprotective effects on CIRI induced by MCAO and OGD/R. The present study showed that SAL treatment inhibited oxidative stress by reducing ROS production, thereby reducing apoptosis caused by CIRI, which might be mediated by the regulation of the Nrf2/Trx1 axis.
During CIRI, a great number of neuron cells is apoptotic, which mainly occurs in the penumbra around the necrotic core of stroke focus. The Bcl-2 protein family and its members play significant roles in regulating and controlling apoptosis. Also, when cell apoptosis occurs, caspase-3 will turn into its active form cleaved caspase-3 (Porter et al. 1999). This study mainly focused on Bax, Bcl-2, and cleaved caspase-3, and the results demonstrated that, under both in vivo and in vitro conditions, high-dose SAL better regulated their expression to exert anti-apoptotic effects. Oxidative stress is an essential factor and plays a vital role in CIRI. The mass-produced ROS, which cannot be scavenged, can induce neuronal apoptosis (Davies. 1999). Therefore, reducing the damage caused by oxidative stress can effectively alleviate the occurrence of CIRI. Here, biochemical parameters related to oxidative stress in the brain and PC12 cells were tested. SAL and ED equally reversed the MCAO- and OGD/R-induced decrease in the activities of SOD and CAT and the increase in the activity of MDA and the level of ROS, and the therapeutic effect of the high-dose SAL was more significant than that of ED. These results indicated that SAL could first enhance antioxidant capacity and then inhibit oxidative stress.
Nrf2 is a key molecule responsible for properly regulating the antioxidant system, which can induce the expression of antioxidant enzymes and play a pivotal role in the protection of antioxidant stress (Vomund et al. 2017). Previous studies have demonstrated that SAL could improve hypoxia-induced oxidative stress through the Nrf2 signaling pathway (Xiong et al. 2021). Trx1, a cell redox protein, is the key protein in maintaining the steady-state of cell redox level, thus protecting cells from oxidative stress (Li et al. 2021). In addition, many studies show that the expression of Trx1 can be up-regulated by enhancing the Nrf2 signal (Im et al. 2012). Trx1 protects oxidative stress-induced neuronal cell death through the ASK1/MAPK signaling pathway (Yeo et al. 2021). The results of the present study showed that SAL could regulate the expression of Nrf2 and Trx1 proteins in vivo and in vitro. To further evaluate how SAL inhibited oxidative stress and apoptosis activated by CIRI, tretinoin, an Nrf2 inhibitor (Zhang et al. 2020), was used to inhibit the expression of Nrf2 in PC12 cells to explain the potential relationship and determine whether apoptosis was directly related to the activation of the Nrf2 signaling pathway. Tretinoin could reverse the antioxidant effects of SAL, suggesting that SAL inhibited oxidative stress by the Nrf2/Trx1 pathway.
Trx1 binds directly to the N-terminal region of ASK1. Under the condition of oxidative stress, excessive ROS accumulation stimulates the activation of ASK1 and activates many downstream signaling pathways, including the MAPK pathway. MAPK family proteins include ERK, JNK, and p38 (You et al. 2019), which are involved in cell growth, proliferation and apoptosis. The MAPK signaling pathway is activated by ASK1, and it is the key signaling pathway leading to neuronal cell death (Yeo et al. 2021; Chen et al. 2021; Lin et al. 2013). In this study, the specific mechanism of SAL on oxidative stress-induced apoptosis was explored. SAL reduced the increase in ASK1/MAPK induced by OGD/R, while tretinoin could reverse this situation, indicating that SAL plays an antioxidant role by up-regulating the Nrf2/Trx1 pathway, and subsequently inhibits apoptosis by inhibiting the ASK1/MAPK signaling pathway.
Conclusion
Our results showed that SAL had a neuroprotective effect by inhibiting CIRI-induced oxidative stress and apoptosis. This study showed that SAL reduced cell apoptosis caused by CIRI by inhibiting ASK1/MAPK, which might be through inhibiting oxidative stress regulated by Nrf2/Trx1 signaling pathway. This present study expanded the current understanding of the pathophysiology of brain injury, thereby providing a new therapeutic option for CIRI.
Data Availability
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
References
Chen P, Liu J, Ruan H et al (2019) Protective effects of Salidroside on cardiac function in mice with myocardial infarction. Sci Rep 9(1):1–11
Chen X, Ma W, Yao Y et al (2021) Serum deprivation-response protein induces apoptosis in hepatocellular carcinoma through ASK1-JNK/p38 MAPK pathways. Cell Death Dis 12(5):425
Davies KJA (1999) The Broad Spectrum of Responses to Oxidants in Proliferating Cells: A New Paradigm for Oxidative Stress. IUBMB Life 48(1):41–47
Zhao D, Sun X, Lv S et al (2019) Salidroside attenuates oxidized low–density lipoprotein–induced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med 43(6):2279–2290
Fangma Y, Zhou H, Shao C et al (2021) Hydroxysafflor Yellow A and Anhydrosafflor Yellow B Protect Against Cerebral Ischemia/Reperfusion Injury by Attenuating Oxidative Stress and Apoptosis via the Silent Information Regulator 1 Signaling Pathway. Front Pharmacol 12:739864
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke.New England Journal of Medicine 333:1581–1588
Hou Y, Wang Y, He Q et al (2018) Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav Brain Res 336:32–39
Huang Z, Fang Q, Ma W et al (2019) Skeletal Muscle Atrophy Was Alleviated by Salidroside Through Suppressing Oxidative Stress and Inflammation During Denervation. Front Physiol 10:997
Im J-Y, Lee K-W, Woo J-M et al (2012) DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet 21(13):3013–3024
Li L, Zhu K, Liu Y et al (2015) Targeting thioredoxin-1 with siRNA exacerbates oxidative stress injury after cerebral ischemia/reperfusion in rats. Neuroscience 284:815–823
Li W, Xu X, Dong D et al (2021) Up-regulation of thioredoxin system by puerarin inhibits lipid uptake in macrophages. Free Radic Biol Med 162(31):542–554
Lin T, Chen Y, Ding Z et al (2013) Novel insights into the synergistic interaction of a thioredoxin reductase inhibitor and TRAIL: the activation of the ASK1-ERK-Sp1 pathway. PLoS ONE 8(5):e63966
Longa E Z, Weinstein P R, Carlson S et al (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 19(1):84–91
Meng XL, Zhang D, L.andSui SH (2019) Acute remote ischemic preconditioning alleviates free radical injury and inflammatory response in cerebral ischemia/reperfusion rats. Experimental and therapeutic medicine 18(3):1953–1960
Porter A G, Jänicke R U (1999) Emerging roles of caspase-3 in apoptosis.Cell Death & Differentiation 6(2):99–104
Rong L, Li Z, Leng X et al (2020) Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother 122:109726
Roth GA, Mensah GA, Johnson CO et al (2020) Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol 76(25):2982–3021
Vomund S, Schafer A, Parnham MJ et al (2017) Nrf2, the Master Regulator of Anti-Oxidative Responses. Int J Mol Sci 18(12):2772
Wan T, Wang Z, Luo Y et al (2019) FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Protects against Oxidative Stress-Mediated Neuronal Cell Apoptosis and Scopolamine-Induced Cognitive Impairment by Activating Nrf2/HO-1 Signaling. Oxidative Medicine and Cellular Longevity 2019
Wang G, Wang T, Hu Y et al (2020) NMMHC IIA triggers neuronal autophagic cell death by promoting F-actin-dependent ATG9A trafficking in cerebral ischemia/reperfusion. Cell Death Dis 11(6):428
Wang H, Zheng S, Liu M et al (2016) The Effect of Propofol on Mitochondrial Fission during Oxygen-Glucose Deprivation and Reperfusion Injury in Rat Hippocampal Neurons. PLoS ONE 11(10):e0165052
Wu C, Tang L, Ni X et al (2019) Salidroside Attenuates Denervation-Induced Skeletal Muscle Atrophy Through Negative Regulation of Pro-inflammatory Cytokine. Front Physiol 10:665
Wu L, Xiong X, Wu X et al (2020) Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front Mol Neurosci 13:28
Wu X, Li L, Zhang L et al (2015) Inhibition of thioredoxin-1 with siRNA exacerbates apoptosis by activating the ASK1-JNK/p38 pathway in brain of a stroke model rats. Brain Res 1599:20–31
Xiong Y, Wang Y, Xiong Y et al (2021) Protective effect of Salidroside on hypoxia-related liver oxidative stress and inflammation via Nrf2 and JAK2/STAT3 signaling pathways. Food Sci Nutr 9(9):5060–5069
Ye R, Yang Q, Kong X et al (2012) Sevoflurane preconditioning improves mitochondrial function and long-term neurologic sequelae after transient cerebral ischemia: role of mitochondrial permeability transition. Crit Care Med 40(9):2685–2693
Yeo EJ, Eum WS, Yeo HJ et al (2021) Protective Role of Transduced Tat-Thioredoxin1 (Trx1) against Oxidative Stress-Induced Neuronal Cell Death via ASK1-MAPK Signal Pathway. Biomolecules & Therapeutics 29(3):321–330
Yoon YC, Fang Z, Lee JE et al (2020) Selonsertib Inhibits Liver Fibrosis via Downregulation of ASK1/ MAPK Pathway of Hepatic Stellate Cells. Biomol Ther (Seoul) 28(6):527–536
You Z, Liu SP, Du J et al (2019) Advancements in MAPK signaling pathways and MAPK-targeted therapies for ameloblastoma: A review. ournal of Oral Pathology & Medicine 48(3):201–205
Zhang X, Wang J, Gong G et al (2020) Spinosin Inhibits Abeta1-42 Production and Aggregation via Activating Nrf2/HO-1 Pathway. Biomolecules & Therapeutics 28(3):259–266
Zhong Z, Han J, Zhang J et al (2018) Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des Dev Therapy 12:1479–1489
Zhong ZF, Han J, Zhang JZ et al (2019) Neuroprotective Effects of Salidroside on Cerebral Ischemia/Reperfusion-Induced Behavioral Impairment Involves the Dopaminergic System. Front Pharmacol 10:1433
Zuo W, Yan F, Zhang B et al (2018) Salidroside improves brain ischemic injury by activating PI3K/Akt pathway and reduces complications induced by delayed tPA treatment. Eur J Pharmacol 830:128–138
Funding
This research was supported by National Natural Science Foundation of China (No. 82173961), Key Laboratory of polysaccharide bioactivity evaluation of TCM of Liaoning Province, Liaoning Distinguished Professor Project for Ying Jia (2017), High-level innovation and entrepreneurship team of Liaoning Province (XLYC2008029), Liaoning Provincial Department of Education Fund (LJKZ0911, LJKZ0950).
Author information
Authors and Affiliations
Contributions
Fuyuan Li: Methodology, Project administration, Writing - original draft. Qianqian Mao: Data curation and investigation. Jinyu Wang: Methodology. Xiaoying Zhang: Methodology. Xinyan Lv: Methodology. Bo Wu: Project administration. Tingxu Yan: Methodology, Project administration, Writing - review & editing, Funding acquisition. Ying Jia: Methodology, Project administration, Writing - review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Ethics approval
The study was carried out in compliance with the National Institutes of Health and Institutional Guidelines for the Humane Care of Animals and was approved by the Animal Care Committee of Shenyang Pharmaceutical University (protocol no.: SYPU-IACUC- C2019- 11-2-301).
Consent for publication
All authors approved the submission of the manuscript for publication.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, F., Mao, Q., Wang, J. et al. Salidroside inhibited cerebral ischemia/reperfusion-induced oxidative stress and apoptosis via Nrf2/Trx1 signaling pathway. Metab Brain Dis 37, 2965–2978 (2022). https://doi.org/10.1007/s11011-022-01061-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01061-x