Abstract
This study aimed to analyze whether taurine has a nootropic effect on short-term and long-term memory in a model of sporadic dementia of the Alzheimer’s type (SDAT). Moreover, we evaluated the immunoreactivity and insulin receptor (IR) distribution and markers for neurons and glial cells in the hippocampus of rats with SDAT and treated with taurine. For this, Male Wistar rats received STZ (ICV, 3 mg/kg, bilateral, 5ul per site, aCFS vehicle) and were treated with taurine (100 mg/kg orally, 1 time per day, saline vehicle) for 25 days. The animals were divided into 4 groups: vehicle (VE), taurine (TAU), ICV-STZ (STZ) and ICV-STZ plus taurine (STZ + TAU). At the end of taurine treatment, short- and long-term memory were assessed by performance on object recognition and Y-maze tasks. Insulin receptor (IR) was evaluated by immunoperoxidase while mature neurons (NeuN), astrocytes (GFAP, S100B, SOX9), and microglia (Iba-1) were evaluated by immunofluorescence. STZ induced worse spatial and recognition memory (INDEX) in YM and ORT tasks. Taurine protected against STZ-induced memory impairment. SDAT reduced the population of mature neurons as well as increased astrocytic and microglial reactivity, and taurine protected against these STZ-induced effects, mainly in the CA1 region of the hippocampus. Taurine increases IR expression in the hippocampus, and protects against the reduction in the density of this receptor in CA1 induced by STZ. In conclusion, these findings demonstrate that taurine is able to enhance memory, up-regulates IR in the hippocampus, protects the neuron population, and reduces the astrogliosis found in SDAT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
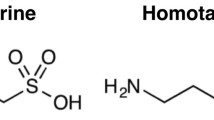
Taurine (2-aminoethansulfolic acid) is an amino acid formed by the decarboxylation of cysteine, abundant in animal tissues, especially in heart and brain tissues. Taurine is related to numerous physiological functions such as: maintaining membrane stability, osmoregulation, calcium mobilization, detoxification, neurotransmission, and anti-inflammatory properties among others [1]. In nervous tissue, taurine is beneficial, having its neuroprotective activity already proven in some models for stroke generated by ischemia and for inflammatory processes [2,3,4]. In the cerebellum, this compound has shown antiapoptotic activity by suppressing glial cell reactivity [5], as well as protecting neurons and memory in cases of neuropathy caused by hyperglycemia [6].
Alzheimer’s disease (AD) is characterized as dementia that progressively alters memory, thinking, and behavior, preventing the individual from performing everyday tasks [7, 8]. The biochemistry of the pathology is complex, involving a deficient vascular system, networks of excitatory and inhibitory neurons, microglia, astroglia, and oligodendrocytes that lose their homeostasis [9].
Astrocytes are versatile glial cells and are widely studied in AD. Astrocytes maintain numerous cytoplasmic processes that make contact with synapses in the hippocampus. Their prolongations also come into contact with blood vessels and express receptors for many neurotransmitters. It has been described that these astrocytic extensions have an active role in synapse formation: controlling, regulating, and integrating numerous processes involving glia and neuron [10]. In AD, reactive astroglia are observed, with hypertrophic astrocytes that end up expressing increased glial fibrillary acidic protein (GFAP), as well as calcium-binding protein (S100β), which when at high levels acts as a pro-inflammatory cytokine exacerbating neuroinflammation, and its increase has been related to AD. Astroglia express an important transcription factor called SOX9, a nuclear marker of adult astrocytes outside the neurogenic region, which allows more objective quantification of the population of adult astrocytes [11,12,13].
Changes in microglia are also observed in AD. These cells are responsible for phagocytosis and protection in the central nervous system (CNS), being directly related to neuroinflammation. It is known that microglia also maintain the plasticity of neuronal circuits, helping in the remodeling of synapses. When cell death occurs, or deposition of altered proteins, microglia migrate to the site of injury to perform cleaning and phagocytosis, and initiate an innate immune response process, increasing the expression of the ionized calcium-binding adapter molecule 1 (Iba-1) [14, 15]. The association of astrogliosis and increased microglial reactivity results in the production of numerous cytokines, interleukins, nitric oxide, and other potentially cytotoxic molecules. In addition, the presence of β-amyloid plaques exacerbates neuroinflammation, especially in the regions of the hippocampus, responsible for learning and memory [16, 17].
In AD, an impairment in glucose uptake and cerebral metabolism is observed, due to decreased signaling of insulin and glucose transporters in the brain [18]. Streptozotocin (STZ) is a diabetogenic agent used to cause a state of insulin resistance in the nervous tissue of animals, induce neuroinflammation, and even the appearance of amyloid plaques [19]. Based on this evidence, the intracerebroventricular (ICV) administration of STZ has been used as a preclinical model for AD, and mimics the initial stages of sporadic dementia, described as sporadic dementia of the Alzheimer type (SDAT), characterized by a worsening temporary impairment of memory and impairment of brain energy metabolism and changes in hippocampal neuronal populations [19, 20].
Here we investigate the underlying mechanism of cognitive improvement and neuronal and glial regulation by administration of taurine in a pre-clinic model of AD. We showed that taurine recovers memory and protects mature neurons from damage induced by SDAT. Subsequently, taurine improves the neuroinflammation environment in the hippocampus regulated by glial cells and increases per se the expression of the IR but not in SDAT. These findings implicate taurine protects against cognitive deficits, astrogliosis, and neuroinflammation present in SDAT.
Materials and methods
Chemicals
Taurine, streptozotocin (STZ), albumin, paraformaldehyde, and DAPI di-hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents used in the detailed experiments were of analytical grade and had the highest purity.
Animals
Adult male Wistar rats (60 days, 250–300 g) were provided by the Central Animal House of the Federal University of Health Sciences of Porto Alegre. The animals were kept in cages under standard temperature (23 ± 1 °C), relative humidity (45–55%), and lighting conditions (12 h light/dark cycle) and with free access to standard rodent pelleted diet and water ad libitum. This study was conducted under the policies stipulated in the Guide for the Care and Use of Laboratory Animals (NIH) from the Committee of Ethics and Animal Experimentation of the Federal University of Health Sciences of Porto Alegre, Brazil, under protocol number CEUA 488–2016, approved all animal procedures. The use of animals was following the Brazilian Guidelines for the Care and Use of Animals in Scientific Research Activities (DBCA), which is in agreement with the National Council of Control of Animal Experimentation (CONCEA). The animals were handled by international laws for the ethical care and handling of laboratory animals (Guidelines of the Council of the European Communities of 22 September 2010, 2010/63/EU and Law 11.794/08).
Intracerebroventricular Injection of Streptozotocin
The animals were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) for all surgical procedures. The head was placed in position in the stereotaxic apparatus, and a midline sagittal incision was made in the scalp of each animal. The stereotaxic coordinates for the lateral ventricle were measured accurately as anteroposterior − 0.8 mm, lateral 1.5 mm, and dorsoventral − 4.0 mm, relative to the bregma and ventral from the dura with the tooth bar set at 0 mm [21]. Through a skull hole, the piston of a 28-gauge Hamilton® syringe of 10 µl attached to a stereotaxic apparatus was lowered manually into each lateral ventricle. The SDAT groups received an ICV injection of STZ 3 mg/kg body weight dissolved in artificial cerebrospinal fluid (aCSF) (5 µl/ventricle). This dose/volume has already been pre-established in previous work of the authors as well as described by other research groups as being effective in the development of the dementia-related cognitive deficit [22, 23]. Vehicle animals received only aCSF (147 mM NaCl; 2.9 mM KCl; 1.6 mM MgCl, 1.7 mM CaCl, and 2.2 mM dextrose, pH 7.4). After, the animals regained consciousness after the surgery; they were placed back in the housing box with water and food ad libitum. During two consecutive days, the animals received analgesic treatment with Ketoprofen i.p. at a dose of 10–20 mg/kg to avoid any painful discomfort.
Taurine treatment
The animals were treated for 25 days with taurine, orally, once a day, at a dose of 100 mg/kg. Taurine was dissolved in 0.9% saline. The vehicle group received only saline solution orally and was administered in a volume equal to 1 ml/kg orally.
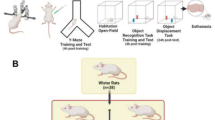
The animals were divided into four groups (n = 10 each): vehicle (VE); taurine (TAU, 100 mg/kg), STZ (3 mg/kg), and STZ + taurine (STZ + TAU) (Fig. 1). The body weight of the animals was evaluated weekly during the experimental period. The taurine dose was chosen based on previous studies indicating neuroprotection [5].
Scheme of the protocol used to induce the experimental model of sporadic dementia of Alzheimer’s type in rats treated for 25 days with taurine (orally, once a day). STZ: streptozotocin; aCFS: artificial cerebrospinal fluid; SDAT: sporadic dementia of Alzheimer’s type; ICV: intracerebroventricular PAF: paraformaldehyde; GFAP: glial fibrillary acidic protein; Iba-1: ionized calcium-binding adapter molecule 1; IR-α: insulin receptor; S100β: calcium-binding protein; SOX-9: transcription factor 9
Behavioral evaluation
Open-field test
Twenty-one days after the surgical procedure, the locomotor behavioral tests were performed using an open-field apparatus as described previously by Pacheco et al. (2018). The open-field test was realized in an apparatus consisting of a box with the floor of the arena divided into 16 equal squares (18 × 18 cm) and placed in a sound-free room. Animals were placed in the posterior left square and were allowed to freely explore for 5 min. The number of squares crossed with all paws (crossing) was counted manually. The apparatus was cleaned up with a 30% ethanol solution and dried after each rat session. This test was performed to identify motor disabilities that might influence the other behavioral tests performed.
Y-maze test
Spatial memory was evaluated in the apparatus that had three arms: the start arm, in which rats were placed to start to explore (always open); the novel arm, which was blocked during the training session, but open during the test session; and familiar arm (always open). In the training session, the animals were placed in the apparatus on the start arm and were free to explore only the start arm and familiar arm for 5 min. The novel arm remained blocked throughout the training session. After 24 h, the test session was performed with access to the novel arm the animal could freely explore all three arms over a 5-min period.
The apparatus was cleaned with 30% ethanol after each session. The time spent and the number of entries in each arm were determined, and the results were expressed as the number of entries and time spent on the arms [24].
Object recognition test (OR)
The object recognition test (OR) was performed to assess declarative memory in rodents, which is based on the animals’ natural tendency to explore the new object more than the familiar one, in a known context [25]. The task was performed in a wooden box 60 cm wide, 60 cm long, and 60 cm high with three walls painted black. The task consisted of habituation, training, and testing sessions. In habituation, the animals were placed in the apparatus to explore freely for 10 min in the absence of objects. Twenty-four hours later, in the training session, the animals were placed, for 10 min, in the presence of two identical objects (objects A1 and A2), where they were positioned on two adjacent sides. Exploration time was determined when the animal touched its nose or came close to the object (at a distance of less than 2 cm). Treatment with the vehicle (saline), taurine (100 mg/kg) was performed 2 h before training. The test session was performed 4 h (short-term memory) and 24 h after training (long-term memory) in the same session, the rats were left for 10 min in the apparatus to explore, and one of the familiar objects used during training was replaced by a new object (object A, B-4 h or C-24 h). All objects are of similar size and odorless plastic, but the color and shape are different for each test. Objects and fields were cleaned with a 30% ethanol solution. The total time spent smelling or touching each object with the nose and/or forepaws and the number of crossing and rearing were recorded. Recognition memory was evaluated by a discrimination index (%), calculated as the difference between the time spent exploring a new object (B or C) and the familiar one (A) x 100, divided by the sum between the times spent exploring the object new (B or C) and the familiar (A): ([(new T.B or C – familiar T.A) / (new T.B or C + familiar T.A)] /100).
Preparation of samples for immunohistochemistry assays
On day 25, the animals were deeply anesthetized with an i.p. injection of ketamine (80 mg/kg; Syntech) and xylazine (5 mg/kg; Syntech 2%) and transcardially perfused with saline 0.9% (during 10 min) and 4% paraformaldehyde (PFA, during 30 min) in 1% phosphate-buffered saline (PBS at pH 7.4). The brains were post-fixed in 4% PFA (24 h) and transferred to a solution of 30% sucrose in PBS-1% until total submersion [5]. Then, the frozen fixed brains were sectioned (5 and 16 μm coronal sections) using a cryostat Leica CM3050S (Leica Microsystem, German). Next, the sections were mounted on slides coated with 2% gelatin plus 0.08% chromalin (chromium and potassium sulfate, from Sigma-Aldrich, Brazil) and finally allowed to dry at room temperature for 24 h. At last, all sections were stored at -20 °C until use.
Immunohistochemistry and immunofluorescence analysis
Firstly, the samples, still frozen, were washed with cold acetone for 10 min, under agitation. Then, the acetone was allowed to evaporate; the sections were washed in PBS twice (10 min) and blocked/permeabilized with 5% bovine serum albumin (BSA) and PBS plus 0.1% Triton X-100 (Tx) for 2 h at room temperature (RT). Sections were incubated with primary antibodies diluted in 5% BSA solution: Rabbit anti-Iba-1 (sections of 16 μm, microglia marker, Abcam Cod. GTX100042) 1:500, overnight at 4 °C, followed by secondary antibody (goat anti-rabbit alexa 555, 1:1,000, 2 h RT in the dark, Thermo Fischer cod. A21429); Rabbit anti-GFAP (sections of 16 μm, astrocyte marker, Sigma Aldrich cod. G9269) 1:800, overnight at 4 °C, followed by secondary antibody (goat anti-rabbit alexa 555, 1:1,000, 2 h RT in the dark, Thermo Fischer cod. A21429); Mouse anti-S100β (sections of 16 μm, astrocyte marker, Abcam cod ab41548) 1:1,000, overnight at 4 °C, followed by secondary antibody (goat anti-rabbit alexa 555, 1:1,000, 2 h RT in the dark, Thermo Fischer cod. A21429); Mouse anti-NeuN (sections of 5 μm, mature neuronal marker, Millipore cod MAB377) 1:6,000, overnight at 4 °C, followed by secondary antibody (goat anti-mouse alexa fluor 488, 1:500, 2 h, RT in the dark, Thermo Fischer cod. A11001); Rabbit anti-SOX-9 (sections of 16 μm, mature astrocyte marker, Abcam cod ab185966) 1:1,000, overnight at 4 °C, followed by secondary antibody (goat anti-rabbit alexa 555, 1:1,000, 2 h RT in the dark, Thermo Fischer cod. A21429).
The DAPI dihydrochloride solution (1 µg/ml, Sigma Aldrich cod D9542) was prepared in PBS-Tx, and incubation was carried out for 10 min in the dark. Then, the sections were washed 4 times with PBS-Tx for 5 min each. Vectashield (antifade mounting medium) was added over the sections which were then overlaid with a coverslip.
To assess the presence of the insulin receptor in the hippocampus regions rabbit anti-IR (sections of 16 μm, insulin receptor, Abcam cod. ab500) 1:200, was incubated overnight at 4 °C, followed by secondary antibody (goat anti-mouse and anti-rabbit, ready for use, Dako EnVision™ +Dual Link System-HRP cod. K4063). Immunoreactions were developed with 0.06% 3,3’-diaminobenzidine (DAB) (Dako®, California, USA, product number: K3468) in PBS-tx for 5 min and then washed with PBS. Afterward, all samples were dehydrated with ethanol and xylol and mounted on slides with a mounting medium for microscopy Entellan™ (Merk Millipore, Darmstadt, Germany, product number: 107,960).
The images were acquired using a Leica DM6-B microscope, Leica DFC 7000-T camera, and Leica Las X software. Five sections were used for each rat. For each section, 6 images were acquired on the regions of the hippocampus (CA1, CA3, and Dentate Gyrus, DG). The slides were evaluated in a wide-field optical microscope for fluorescence observations (Leica DM6000-B) and the images obtained were captured by a camera (Leica DFC7000-T) attached to the microscope. The images were analyzed using the Leica LAS X and Image Pro-Plus 6.3 software to perform the densitometry of cells labeled with the aforementioned antibodies. The summarized procedures are in Fig. 1.
Statistical analysis
The normality analysis of the samples was performed by the Kolmogorov-Smirnov test. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for means comparison using Graph Pad Prism version 5.0 program (Intuitive Software for Science, São Diego, CA, USA). Differences with P ≤ 0.05 were considered statistically significant in the analysis. All data were expressed as mean ± standard error of the mean (SEM).
Results
Figure 2 shows the behavioral parameters for spontaneous locomotion (open field task), spatial memory (Y-maze task), and sensorial/recognition memory (novel object recognition test) (Panel A). Rats treated with TAU and STZ showed no significant differences in the time spent in the center (B) and periphery (C), crossing (D), or rearing (E) for the open field task. Concerning spatial memory (Fig. 2F-G), rats treated with taurine had a significant increase in the number of entries in the novel arm in TAU per se (5.0 ± 0.43; P = 0.0129) and STZ + TAU (5.0 ± 0.44; P = 0.0221) when compared to STZ (2.4 ± 0.67; P < 0.05), but not in comparison to STZ (2.4 ± 0.67; P < 0.05), versus VE (4.0 ± 0.70; P = 0.27) (Fig. 2F). No significant differences were observed in all groups when time spent in the novel arm was measured (Fig. 2G).
Effects of treatment with taurine (100 mg/kg) on locomotor activity (evaluated by the open-field task, OF), spatial memory (evaluated by the Y-maze Task), and recognition memory (evaluated by the Object Recognition Task, ORT) in rats with sporadic dementia of the Alzheimer’s type (SDAT, STZ 3 mg/kg). A illustration of behavioral tasks used; B time spent in the center in seconds in OF; C time spent in the periphery in seconds in OF; D number of crossing in OF; E number of rearing in OF; F number of entries in the novel arm in the Y-maze task; G time spent in the novel arm in the Y-maze task; H index for short-term memory (STM) in ORT; I index for long-term memory (LTM) in ORT. Statistical significance was determined using a One-way ANOVA (with Tukey post hoc comparison) at *P < 0.05 and **P < 0.01 (n = 6–10 animals per group)
Short-term and long-term memory index values can be seen in Fig. 2H-I, using the novel object recognition task. One-way ANOVA indicated a significant decrease in the short-term memory index in the STZ group (0.0019 ± 0.0007; P < 0.05) when compared to VE (0.0047 ± 0.0006; P = 0.0345) and TAU per se (0.0044 ± 0.0003; P = 0.0356) (Fig. 2H). However, the taurine treatment for 25 days showed a protective effect on the short-term memory index in the STZ + TAU (0.0049 ± 0.0004; P = 0.4520) versus STZ (0.0019 ± 0.0007; P < 0.05). In Fig. 2I, Tukey’s test evidenced a significant difference in long-term memory index between STZ (-0.00053 ± 0.00087; P < 0.05) versus STZ + TAU (0.0050 ± 0.0020; P = 0.0285). STZ (-0.00053 ± 0.00087; P < 0.05) versus VE (0.0012 ± 0.0010; P = 0.77); versus TAU per se (0.0039 ± 0.00096; P = 0.09) showed no significant difference (Fig. 2I).
Figure 3 shows the representative images for insulin receptor alpha (IR-α) immunoreactivity (Panel A) in the DG, CA1, and CA3 (Panel B) of rats submitted to SDAT and treated with taurine. Concerning IR-α in DG (Fig. 3C), rats treated with taurine had a significant increase in the immunoreactivity for IR-α between TAU per se (0.16 ± 0.007; P < 0.001) versus STZ (0.11 ± 0.007; P = 0.0009); versus STZ + TAU (0.13 ± 0.008; P = 0.0137). TAU per se (0.16 ± 0.007; P < 0.001) versus VE (0.13 ± 0.005; P = 0.0909) showed no significant difference (Fig. 3C). No significant differences were observed between the STZ and STZ + TAU groups. In the CA1 (Fig. 3D), a significant increase in the immunoreactivity for IR-α only in TAU per se (0.16 ± 0.008; P < 0.001) versus STZ (0.099 ± 0.008; P = 0.0031), but no significant differences were observed between VE (0.13 ± 0.012; P = 0.1499) or TAU + STZ (0.12 ± 0.010; P = 0.3788) versus STZ. In the CA3 (Fig. 3E), we observed a significant decrease in the immunoreactivity for IR-α between STZ (0.12 ± 0.005; P < 0.05) versus VE (0.14 ± 0.004; P = 0.0256); versus TAU (0.17 ± 0.004; P < 0.0001). STZ (0.12 ± 0.005; P < 0.05) versus TAU + STZ (0.13 ± 0.007; P = 0.1882) showed no significant difference. In addition, we observed a significant increase for the IR-α immunoreactivity in TAU per se (0.17 ± 0.004; P < 0.0001) versus TAU + STZ (0.13 ± 0.007; P = 0.0036) in the CA3, but not in comparison to the VE (0.13 ± 0.005; P = 0.0989) (Fig. 3E).
Effects of treatment with taurine (100 mg/kg) on immunoreactivity for insulin receptor (IR-α) in the hippocampus of rats with sporadic dementia of Alzheimer’s type (SDAT, STZ 3 mg/kg). A representative image for immunoreactivity of IR in the VE, TAU, STZ, and STZ + TAU groups; B rrepresentative image of the hippocampus region in relation to the brain; C immunohistochemistry staining for IR in DG; D immunohistochemistry staining for IR in CA1; E immunohistochemistry staining for IR in CA3. Statistical significance was determined using One-way ANOVA (with Tukey post hoc comparison) at *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 when compared with all the groups (n = 6–10 animals per group)
Figure 4 shows the representative images for NeuN immunoreactivity, a convenient marker of post-mitotic neurons (Panel A), in the regions of the DG, CA1, and CA3 (Panel B) of rats submitted to SDAT and treated with taurine. Concerning NeuN immunoreactivity in DG (Fig. 4C), we observed a significant decrease between STZ (1.39 ± 0.017; P < 0.001) versus VE (1.57 ± 0.021; P = 0.001); versus TAU per se (1.52 ± 0.012; P = 0.0048). STZ (1.39 ± 0.017; P < 0.001) versus STZ + TAU (1.45 ± 0.031; P = 0.2837) showed no significant difference. In addition, a significant difference was observed between VE (1.57 ± 0.021; P < 0.01) versus the STZ + TAU group (1.45 ± 0.031; P = 0.0048). In the CA1 (Fig. 4D), we observed a significant decrease in the NeuN immunoreactivity in STZ (1.38 ± 0.022; P < 0.001) versus VE (1.52 ± 0.017; P = 0.0002), versus TAU per se (1.49 ± 0.018; P = 0.0045) and versus STZ + TAU (1.46 ± 0.020; P = 0.0499). In the CA3 (Fig. 4E), we observed a significant decrease in the NeuN immunoreactivity in STZ (1.37 ± 0.014; P < 0.05) versus VE (1.51 ± 0.019; P < 0.0001); versus TAU per se (1.48 ± 0.017; P = 0.0007). STZ (1.37 ± 0.014; P < 0.05) versus STZ + TAU (1.40 ± 0.010; P = 0.6499) showed no significant difference. In addition, we observed a significant difference in the NeuN immunoreactivity in STZ + TAU (1.40 ± 0.010; P < 0.05) versus VE (1.51 ± 0.019; P = 0.011); and TAU per se (1.48 ± 0.017; P = 0.0111) (Fig. 4E).
Effects of treatment with taurine (100 mg/kg) on immunofluorescence for mature neurons staining with anti-NeuN (green) and counterstained with DAPI (blue) in the hippocampus of rats with sporadic dementia of Alzheimer’s type (SDAT, STZ 3 mg/kg). A representative image for immunoreactivity of anti-NeuN in the different regions of the hippocampus in the VE, TAU, STZ, and STZ + TAU groups; B representative image of the hippocampus region in relation to the brain; C immunostained of NeuN in DG; D immunostained of NeuN in CA1; E immunostained of NeuN in CA3. Statistical significance was determined using One-way ANOVA (with Tukey post hoc comparison) at *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with all the groups (n = 6–10 animals per group)
Figure 5 shows representative images for Iba-1 (ionized calcium-binding adapter molecule 1), which is used as a microglial marker (Panel A), in the DG, CA1, and CA3 (Panel B) of rats submitted to SDAT and treated with taurine. With respect to Iba-1in DG (Fig. 7C), we do not observe a significant difference between groups. In the CA1 (Fig. 5D), we observed a significant increase in Iba-1 immunoreactivity between STZ (0.80 ± 0.010; P < 0.05) versus VE (0.77 ± 0.005; P < 0.0239); versus TAU per se (0.73 ± 0.005; P < 0.0001); versus STZ + TAU (0.77 ± 0.003; P = 0.0347). In addition, we observed a significant decrease in Iba-1 immunoreactivity between TAU per se (0.73 ± 0.005; P < 0.05); versus VE (0.77 ± 0.005; P = 0.0087); versus STZ + TAU (0.77 ± 0.003; P = 0.0035) (Fig. 5D). In the CA3 (Fig. 5E), we observed a significant increase in the immunoreactivity for Iba-1 immunoreactivity in the STZ (0.80 ± 0.010; P < 0.05) versus VE (0.77 ± 0.010; P = 0.0483); versus TAU per se (0.74 ± 0.005; P = 0.0003); versus STZ + TAU (0.77 ± 0.002; P = 0.0445).
Effects of treatment with taurine (100 mg/kg) on immunostained microglia with an anti-Iba-1 antibody (magenta) and counterstained with DAPI (blue) in the hippocampus of rats with sporadic dementia of the Alzheimer’s type (SDAT, STZ 3 mg/kg). A representative image for immunoreactivity of anti-Iba-1 in the DG, CA1, and CA3 regions of the hippocampus in the VE, TAU, STZ, and STZ + TAU groups; B representative image of the hippocampus region in relation to the brain; C immunostained of Iba-1 in DG; D immunostained of Iba-1 in CA1; E immunostained of Iba-1 in CA3. Statistical significance was determined using One-way ANOVA (with Tukey post hoc comparison) at *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with all the groups (n = 6–10 animals per group)
Effects of treatment with taurine (100 mg/kg) on immunostained with an anti-S100β antibody (red) and counterstained with DAPI (blue) in the hippocampus of rats with sporadic dementia of the Alzheimer’s type (SDAT, STZ 3 mg/kg). A representative image for immunoreactivity of anti-S100β in the DG, CA1, and CA3 regions of the hippocampus in the VE, TAU, STZ, and STZ + TAU groups; B representative image of the hippocampus region in relation to the brain; C immunostained of S100β in DG; D immunostained of S100β in CA1; E immunostained of S100β in CA3. Statistical significance was determined using a One-way ANOVA (with Tukey post hoc comparison) at **P < 0.01 when compared with all the groups (n = 6–10 animals per group)
Figure 6 shows the representative images for GFAP immunoreactivity, an intermediate filament protein in astrocytes (Panel A), in the DG, CA1, and CA3 (Panel B) of rats submitted to SDAT and treated with taurine. Analyzes for optical density for GFAP revealed a significant increase in DG (Fig. 6B) between STZ (1.15 ± 0.094; P < 0.05) versus VE (0.88 ± 0.11; P = 0.0271); versus TAU per se (0.89 ± 0.019, P = 0.0286). STZ (1.15 ± 0.094; P < 0.05) versus STZ + TAU (1.03 ± 0.037; P = 0.4149) showed no significant difference (Fig. 6C). In the CA1 (Fig. 6D), the GFAP immunoreactivity was significantly increased in the STZ group (1.19 ± 0.11; P < 0.05) versus VE (0.87 ± 0.019; P = 0.0061); versus TAU per se (0.91 ± 0.029; P = 0.0092) or versus STZ + TAU (0.92 ± 0.026; P = 0.0145) (Fig. 4D). In the CA3 (Fig. 6E), we observed a significant increase in the GFAP immunoreactivity in STZ (1.08 ± 0.079; P < 0.05) versus VE (0.87 ± 0.021; P = 0.0457). STZ (1.08 ± 0.079; P < 0.05) versus TAU per se (0.90 ± 0.028; P = 0.0702) or versus STZ + TAU (0.96 ± 0.009; P = 0.3102) showed no significant differences.
Effects of treatment with taurine (100 mg/kg) on immunostained astrocytes with an anti-GFAP antibody (red) and counterstained with DAPI (blue) in the hippocampus of rats with sporadic dementia of the Alzheimer’s type (SDAT, STZ 3 mg/kg). A representative image for immunoreactivity of anti-GFAP in the DG, CA1, and CA3 regions of the hippocampus in the VE, TAU, STZ, and STZ + TAU groups; B representative image of the hippocampus region in relation to the brain; C immunostained of GFAP in DG; D immunostained of GFAP in CA1; E immunostained of GFAP in CA3. Statistical significance was determined using a One-way ANOVA (with Tukey post hoc comparison) at *P < 0.05 and **P < 0.01 when compared with all the groups (n = 6–10 animals per group)
Figure 7 shows representative images for S100β immunoreactivity, a late marker of astrocyte development and characterizing a mature stage (Panel A), in the DG, CA1, and CA3 (Panel B) of rats submitted to SDAT and treated with taurine. With respect to S100β in the DG (Fig. 7C), we do not observe a significant difference between groups. In the CA1 (Fig. 7D), we observed a significant increase for S100β immunoreactivity between STZ (0.87 ± 0.012; P < 0.05) versus VE (0.82 ± 0.012; P = 0.0081); versus TAU per se (0.83 ± 0.007; P = 0.0070) or versus STZ + TAU (0.82 ± 0.006; P = 0.0013). In the CA3 (Fig. 7E), we observed a significant increase in S100β immunoreactivity in the STZ (0.87 ± 0.013; P < 0.05) versus the VE group (0.80 ± 0.006; P = 0.0001); versus TAU per se (0.81 ± 0.005; P = 0.0017) or versus STZ + TAU (0.82 ± 0.007; P = 0.0052).
Figure 8 shows representative images for SOX-9 immunoreactivity, a nuclear-specific marker for adult astrocytes (Panel A), in the DG, CA1, and CA3 (Panel B) of rats submitted to SDAT and treated with taurine. Figure 8 C shows the analysis for the optical density of SOX-9 in the DG, we observed a significant increase between STZ (0.94 ± 0.006; P < 0.05) versus VE (0.82 ± 0.022; P = 0.0119); versus TAU per se (0.78 ± 0.044; P = 0.0015); versus STZ + TAU (0.79 ± 0.25; P = 0.0008). In the CA1 (Fig. 8D), we observed a significant increase for SOX-9 immunoreactivity between STZ (0.95 ± 0.016; P < 0.05) versus VE (0.82 ± 0.014; P = 0.0114); versus TAU per se (0.81 ± 0.034; P = 0.0065); versus STZ + TAU (0.79 ± 0.025; P = 0.0004). In the CA3 (Fig. 8E), we observed a significant increase for SOX-9 immunoreactivity between STZ (0.95 ± 0.017; P < 0.05) versus VE (0.82 ± 0.015; P = 0.0260); versus TAU per se (0.82 ± 0.035; P = 0.0169); versus STZ + TAU (0.76 ± 0.032; P = 0.0003).
Effects of treatment with taurine (100 mg/kg) on immunostained with an anti-SOX-9 antibody (red) and counterstained with DAPI (blue) in the hippocampus of rats with sporadic dementia of the Alzheimer’s type (SDAT, STZ 3 mg/kg). A representative image for immunoreactivity of anti-SOX-9 in DG, CA1, and CA3 regions of the hippocampus in the VE, TAU, STZ, and STZ + TAU groups; B representative image of the hippocampus region in relation to the brain; C immunostained of SOX-9 in DG; D immunostained of SOX-9 in CA1; E immunostained of SOX-9 in CA3. Statistical significance was determined using One-way ANOVA (with Tukey post hoc comparison) at *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with all the groups (n = 6–10 animals per group)
Discussion
Taurine is amino acid with many functions in the nervous system. In recent years, many studies have found relevant effects of taurine on cognitive function. This study was conducted to observe the protective effects of taurine on different hippocampal regions, such as the DG, CA1 and CA3, which were affected by the action of intracerebroventricular injections of STZ, a well-established model for the study of SDAT. STZ injections affect brain glucose uptake, generating important metabolic alterations and hippocampal neuroinflammation, which as a consequence lead to alterations in memory and learning [19].
In the behavioral tests performed, we found no significant difference regarding the locomotion activity. However, with respect to spatial memory assessed in the Y-maze task, it is possible to suggest that taurine has a positive effect on spatial memory. Besides, our results also showed that short- and long-term declarative memory was improved by the action of taurine in comparison to SDAT animals. Taurine has also been investigated for the improvement of psychiatric disorders in animals, such as memory problems associated with noise, where similar data for long-term memory were found by Haider et al.(2020) [26]. Javed et al.(2013), also shown that taurine can reverse memory deficits in mice in the Morris water maze test in SDAT animals. Reeta et al. (2017) showed that taurine protects against memory loss caused by ICV-STZ, showing that treated mice improved performance in memory behavioral tasks [27, 28]. The cited authors report that taurine is involved in mechanisms related to a decrease in oxidative stress in nervous tissue, thereby improving local neuroinflammation and consequently spatial and aversive memory impaired by SDAT [27, 28].
The SDAT causes changes in IR, leading a desensitization of them and compromising cerebral glucose uptake and energy metabolism in a prolonged manner [27]. Neurogenesis, which drives the formation of new neurons, happens in adults mainly in the subgranular region (SGZ) of the DG and is associated with the action of insulin signaling [29, 30]. It is worth noting that the neuronal IR may be affected by the more complex morphology of neurons compared to cells of classical insulin target organs, as well as the wide variety of lipids unique to the cell membranes of the nervous system [31]. Insulin also has a neuroprotective effect, assisting synaptic plasticity, memory production, and consolidation. IR are more expressed in neurons than in glial cells, and in regions such as the hippocampus, amygdala, hypothalamus, entorhinal cortex, and olfactory bulb, IR levels are affected in learning and memory processes [29, 30]. Some studies have shown that taurine can regulate insulin signaling, since taurine intake improves the endocrine function of the pancreas, increasing overall insulin production and, in response, there is an increase in the expression and excitability of insulin receptors in the hippocampal region [32]. Our results corroborate these findings, since we showed that taurine per se increased immunoreactivity for IR-α. However, STZ seems to reverse this regulatory effect on IR-α immunoreactivity in the STZ + TAU group.
In the analysis of mature neuron populations, our findings show a reduction in the density of NeuN-labelled cells in SDAT animals. However, the protective effect of taurine was observed only in the CA3 region. In agreement, Wang et al. (2021) observed that taurine was important to increase NeuN density in the hippocampus of rats exposed to paraquat, a classical model to induce Parkinson’s disease (PD) in animals [33]. Glutamatergic pyramidal neurons found in the cornu ammonis showed co-localization for glutamate, glutaminase and taurine [34]. In addition, taurine may reduce glutamatergic currents via its role as a partial agonist for glycine receptors [35, 36]. Indeed, Se Jong Oh et al. (2020) showed that taurine increased uptake of the metabolic glutamate receptor type 5 in hippocampal pyramidal neurons, contributing in the protection against glutamatergic excitotoxicity in 5xFAD transgenic mice [37]. The relay role of the CA3 field in the trisynaptic circuitry of the hippocampus is well established [38], has anatomical features that are ideal to allow CA3 to act as a self-associative network and an input balancing area in hippocampal formation, but the effects of taurine or SDAT animals on this region are still poorly understood. It is worth noting that CA3 neuronal networks play a crucial role in memory information processes, and temporal analysis in ventral CA3 showed several transcriptomic changes related to inflammation, neuronal differentiation and synaptic transmission [39].
Remembering that the model using intracerebroventricular injections of STZ causes changes in glucose uptake and thus generates an imbalance in hippocampal brain metabolism, producing many reactive oxygen species, which causes the release of numerous cytokines by the microglia, generating neuroinflammation [28]. The microglia are considered the first line of defense of the CNS, actively functioning against environmental disturbances and contributing to local homeostasis. With activation of the microglia against tissue insults, reactive astrocytosis occurs [40]. Thus, showing an intimate relationship between microglia and astrocytes, which in turn have already been described as cells with immune characteristics and receptors, thus contributing to the innate and adaptive immune responses in the CNS, besides performing many other functions already mentioned. In neuroinflammation, there is bidirectional communication between astrocytes and microglia, and one can activate the other [41]. After some insults, astrocytes undergo changes in the expression of multiple factors and modulate microglial phenotypes and functions through the production of cytokines, chemokines, calcium, complement proteins, and other inflammatory mediators, which can affect microglial functions such as activation, migration, and phagocytosis. On the other hand, the microglia can activate astrocytes, which in turn develop two different phenotypes’ phenotype 1 (A1), which is considered destructive to nerve cells, and phenotype 2 (A2), which is considered neuroprotective and reparative [40] [42].
Our finding evaluating the Iba-1 marker showed an increase in microglia reactivity in CA1 and CA3 regions, but not in DG hippocampus in SDAT animals. However, taurine treatment for 25 days played an important role in inhibiting the activation of microglia cells and perhaps could reduce the release of inflammatory factors. As an endogenous amino acid, taurine has long been recognized for its anti-inflammatory and neuroprotective effects [5, 43,44,45]. In addition, it has been reported that taurine depletion causes activation of microglia, emphasizing its protective role against neuroinflammation [44]. Liu et al. (2022) showed that taurine in vitro inhibited LPS-induced generation of inflammatory factors and increased ROS intensity in BV-2 cells. The same authors demonstrated that taurine inhibited microglial lysine demethylase 3a (KDM3a), promoting relief of microglia activation in LPS-treated mice [46]. Wang et al. (2021) also evaluated the immunoreactivity of Iba-1 in the hippocampus of rats with PD treated with taurine, noting that taurine was able to reduce the immunoreactivity of the microglia in these animals. The authors comment that one of the mechanisms that taurine uses to perform neuroprotection is through the inactivation of the microglia in the neuroinflammatory environment [33]. Since only the CA1 and CA3 regions were responsive to taurine treatment against the microglial reactivity insult provoked by STZ, it is possible to suggest from our findings that the microglia were more sensitive in responding in these regions. In addition, the DG region may possibly be associated with a delay in the anti-inflammatory feedback directed at the SDAT group and the animals that received taurine treatment, since the neuroinflammation, proliferation and survival of new cells in dentate gyrus of mammals, whether neurons or glial cells, is a complex process that is subject to numerous influences.
Considering that microglia are the main source of pro-inflammatory cytokines, they are believed to be key participants in the development of astrogliosis. Before the astrocyte response, the inflammatory reaction is initiated by an increase in microglia-derived mediators and activation of microglia [47]. Park et al. (2021) believe that activation of the NF-B signaling cascade by resident microglia increases the potential to convert resting astrocytes into reactive astrocytes, which may lead to pathophysiological transformation of astrocytes in a variety of neurodegenerative disorders, such as PD and AD disease, and preventing activation of the microglia-astrocyte activation circuit may help prevent these diseases [48]. Our data corroborate with other studies pointing to intense astrogliosis in SDAT rodents [49, 50] and interestingly taurine regulates this process, since both in DG and CA1 there was a reversal of the astrogliosis promoted by ICV-STZ.
The expression of glial fibrillary acidic protein (GFAP) and the use of taurine have quite controversial results, Kim and Cha (2014) in an experimental model with double transgenic mice (APP/PS1) for AD that took doses of taurine in water observed an increase in GFAP expression in taurine-treated animals [1]. Su et al. (2014) showed that after head trauma with intense inflammation and treatment with taurine for 7 days, GFAP immunoreactivity in the hippocampus was reduced, indicating that taurine reduces local neuroinflammation in the trauma model [51]. To extend the analyzes on astrocytes, we evaluated other complementary markers, such as the labeling of reactive astrocytes using calcium-binding protein β (S100β) and a nuclear marker of astrocytes such as transcription factor 9 (SOX-9).
Our results demonstrate that in CA1 and CA3 regions SDAT animals show a significant increase in S100β compared to that of the other groups, and taurine-treated animals reverted this effect. At nanomolar concentrations, S100β conveys neuroprotective and neurotrophic properties, while at higher concentrations it has been associated with deleterious effects, and may form the basis of neurodegenerative diseases such as AD [52, 53]. Besides, S100β effects are mediated through the receptor for advanced glycation end products (RAGE) [52] and a persistent RAGE activation causes neuronal death as a result of increased production of reactive oxygen species [54] it is possible to suppose that antioxidant effects of taurine may also be related to a reduction in S100β found in taurine-treated animals. S100β is also associated with cellular aging, where higher concentrations of the protein have been observed in the hippocampus and cortex related to age-related activation of astrocytes, which would aid in age-related cognitive deficits, and any imbalance in the regulation of S100β protein is a direct cause of hyperphosphorylation of tau, a common finding in the nervous tissue of AD patients [55, 56]. Intracellularly, S100B, as a calcium-sensing protein, is known to regulate a variety of activities, transferring second messenger signals and interacting with different molecules in different cell types [57]. In particular, S100B intervenes in cell proliferation, survival and differentiation [58, 59], participates in the regulation of cellular calcium homeostasis and enzymatic activities [60,61,62,63], but the data available to date do not allude to a clearly defined role or function in relation to different regions of the hippocampus. In our study, we found no significant difference in the DG region for S100β immunoreactivity. Although there are a few studies that have shown that the DG region is less susceptible to oxidative stress [64] compared to CA1 and CA3, it is possible to assume that these discrepancies found could be attributed to different concentrations of S100B (toxic) produced by STZ in the DG region that were not able to cause changes in S100β immunoreactivity.
Previous studies have suggested that the transcription factor SOX9 is highly enriched in astrocytes [65,66,67,68]. Sun et al. (2017) reported that comparisons of the transcriptomes of SOX9+ cells and glutamate-transporting astrocytic cells (GLT1) indicated that these two markers had a large overlap in gene expression. Like other currently used astrocytic markers, SOX9 specifically targets astrocytes outside neurogenic regions. However, SOX9 also had several advantages in this regard, including the fact that its expression does not decrease with age or functional status, that the use of a nuclear marker of the astrocytic phenotype allows a more conservative assessment of the astrocytic phenotype than the previous one using cytoplasmic markers [13]. Our findings showed that for all regions of the hippocampus, SDAT animals obtained a considerable increase in immunoreactivity for SOX-9. Interestingly, SDAT animals treated with taurine showed a significant reduction in SOX-9, that in fact, taurine can regulate astrogliosis both by evaluating a cytoplasmic marker for astrocytes (GFAP) and nuclear and this reveals that possible neuroprotective mechanisms of taurine can be associated with a reduction in astrocyte reactivity. However, despite these findings, the increase in SOX-9 found in SDAT animals in the DG may be associated with neural precursors of the astroglial lineage within the neurogenic zones, perhaps pointing to a compensation in the balance of new astrocyte formation once that marked reactive astrogliosis exists in these animals.
Overall, we found that taurine is able to improve spatial memory and both short and long-term episodic memory, increase adult neuronal population, considerably inhibit microglial activation and control astrogliosis in the hippocampus of animals with SDAT.
References
Kim C, Cha YN (2014) Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 46:89–100. https://doi.org/10.1007/s00726-013-1545-6
Wang GH, Jiang ZL, Fan XJ et al (2007) Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 52:1199–1209. https://doi.org/10.1016/j.neuropharm.2006.10.022
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429. https://doi.org/10.1007/s00726-010-0751-8
Sun M, Zhao Y, Gu Y, Xu C (2012) Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-jB. Amino Acids 42:1735–1747. https://doi.org/10.1007/s00726-011-0885-3
Silva SP, Zago AM, Carvalho FB et al (2021) Neuroprotective Effect of Taurine against Cell Death, Glial Changes, and Neuronal Loss in the Cerebellum of Rats Exposed to Chronic-Recurrent Neuroinflammation Induced by LPS. J Immunol Res 2021:. https://doi.org/10.1155/2021/7497185
Rahmeier FL, Zavalhia LS, Tortorelli LS et al (2016) The effect of taurine and enriched environment on behaviour, memory and hippocampus of diabetic rats. Neurosci Lett 630:84–92. https://doi.org/10.1016/j.neulet.2016.07.032
Möller H-J, Graeber MB (1998) The case described by Alois Alzheimer in 1911. Eur Arch Psychiatry Clin Neurosci 248:111–122. https://doi.org/10.1007/s004060050027
Benilova I, Karran E, De Strooper B (2012) The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15:349–357. https://doi.org/10.1038/nn.3028
De Strooper B, Karran E (2016) The Cellular phase of Alzheimer’s Disease. Cell 164:603–615. https://doi.org/10.1016/j.cell.2015.12.056
Allen NJ (2014) Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 30:439–463. https://doi.org/10.1146/annurev-cellbio-100913-013053
Sofroniew MV, Vinters HV (2010) Astrocytes: Biology and pathology. Acta Neuropathol 119:7–35. https://doi.org/10.1007/s00401-009-0619-8
Craft JM, Watterson DM, Marks A, Van Eldik LJ (2005) Enhanced susceptibility of S-100B transgenic mice to neuroinflammation and neuronal dysfunction induced by intracerebroventricular infusion of human β-amyloid. Glia 51:209–216. https://doi.org/10.1002/glia.20194
Sun W, Cornwell A, Li J et al (2017) SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci 37:4493–4507. https://doi.org/10.1523/JNEUROSCI.3199-16.2017
Liu Y, Walter S, Stagi M et al (2005) LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain 128:1778–1789. https://doi.org/10.1093/brain/awh531
Heneka MT, Carson MJ, Khoury J, El et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Furman JL, Sama DM, Gant JC et al (2012) Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci 32:16129–16140. https://doi.org/10.1523/JNEUROSCI.2323-12.2012
Parkhurst CN, Yang G, Ninan I et al (2014) Microglia promote learning-dependent synapse formation through BDNF. 155:1596–1609. https://doi.org/10.1016/j.cell.2013.11.030.Microglia
Chen Y, Liang Z, Tian Z et al (2014) Intracerebroventricular streptozotocin exacerbates alzheimer-like changes of 3xTg-AD mice. Mol Neurobiol 49:547–562. https://doi.org/10.1007/s12035-013-8539-y
Grünblatt E, Salkovic-Petrisic M, Osmanovic J et al (2007) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem 101:757–770. https://doi.org/10.1111/j.1471-4159.2006.04368.x
Kamat PK, Kalani A, Rai S et al (2016) Streptozotocin Intracerebroventricular-Induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer’s Disease (sAD)-Like Pathology. Mol Neurobiol 53:4548–4562. https://doi.org/10.1007/s12035-015-9384-y
Teixeira FC, Soares MSP, Blödorn EB et al (2022) Investigating the Effect of Inosine on Brain Purinergic receptors and neurotrophic and neuroinflammatory parameters in an experimental model of Alzheimer’s Disease. Mol Neurobiol 59:841–855. https://doi.org/10.1007/s12035-021-02627-z
Teixeira FC, Gutierres JM, Soares MSP et al (2020) Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacology 237:811–823. https://doi.org/10.1007/s00213-019-05419-5
Gutierres JM, Carvalho FB, Schetinger MRC et al (2014) Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci 96:7–17. https://doi.org/10.1016/j.lfs.2013.11.014
Pacheco SM, Soares MSP, Gutierres JM et al (2018) Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J Nutr Biochem 56:193–204. https://doi.org/10.1016/j.jnutbio.2018.02.014
Dodart JC, Mathis C, Ungerer A (1997) Scopolamine-induced deficits in a two-trial object recognition task in mice. NeuroReport 8:1173–1178. https://doi.org/10.1097/00001756-199703240-00023
Haider S, Sajid I, Batool Z, Madiha S, Sadir S, Kamil N et al (2020) Supplementation of Taurine insulates against oxidative stress, confers Neuroprotection and attenuates memory impairment in noise stress exposed male Wistar rats. Neurochem Res 45:2762–2774. https://doi.org/10.1007/s11064-020-03127-7
Javed H, Khan A, Vaibhav K et al (2013) Taurine ameliorates neurobehavioral, neurochemical and immunohistochemical changes in sporadic dementia of Alzheimer’s type (SDAT) caused by intracerebroventricular streptozotocin in rats. Neurol Sci 34:2181–2192. https://doi.org/10.1007/s10072-013-1444-3
Reeta KH, Singh D, Gupta YK (2017) Chronic treatment with taurine after intracerebroventricular streptozotocin injection improves cognitive dysfunction in rats by modulating oxidative stress, cholinergic functions and neuroinflammation. Neurochem Int 108:146–156. https://doi.org/10.1016/j.neuint.2017.03.006
Dou JT, Chen M, Dufour F et al (2005) Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem 12:646–655. https://doi.org/10.1101/lm.88005
Ronaghi A, Zibaii MI, Pandamooz S et al (2019) Entorhinal cortex stimulation induces dentate gyrus neurogenesis through insulin receptor signaling. Brain Res Bull 144:75–84. https://doi.org/10.1016/j.brainresbull.2018.11.011
Palmano K, Rowan A, Guillermo R et al (2015) The role of gangliosides in neurodevelopment. Nutrients 7:3891–3913. https://doi.org/10.3390/nu7053891
El Idrissi A (2019) Taurine regulation of neuroendocrine function. Adv Exp Med Biol 1155:977–985. https://doi.org/10.1007/978-981-13-8023-5_81
Wang K, Shi Y, Liu W et al (2021) Taurine improves neuron injuries and cognitive impairment in a mouse Parkinson’s disease model through inhibition of microglial activation. Neurotoxicology 83:129–136. https://doi.org/10.1016/j.neuro.2021.01.002
Kowall NW, Beal MF (1991) Glutamate-, glutaminase‐, and taurine‐immunoreactive neurons develop neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 29:162–167. https://doi.org/10.1002/ana.410290208
Du J, Lü W, Wu S et al (2015) Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526:224–229. https://doi.org/10.1038/nature14853
Yu J, Zhu H, Lape R et al (2021) Mechanism of gating and partial agonist action in the glycine receptor. Cell 184:957–968e21. https://doi.org/10.1016/j.cell.2021.01.026
Oh SJ, Lee HJ, Jeong YJ et al (2020) Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-72755-4
Toma V, Al, Farcas AD, Parvu M et al (2017) CA3 hippocampal field: Cellular changes and its relation with blood nitro-oxidative stress reveal a balancing function of CA3 area in rats exposed to repetead restraint stress. Brain Res Bull 130:10–17. https://doi.org/10.1016/j.brainresbull.2016.12.012
Azevedo H, Khaled NA, Santos P et al (2018) Temporal analysis of hippocampal CA3 gene coexpression networks in a rat model of febrile seizures. DMM Dis Model Mech 11. https://doi.org/10.1242/dmm.029074
Jha MK, Jo M, Kim JH, Suk K (2019) Microglia-Astrocyte Crosstalk: an intimate Molecular Conversation. Neuroscientist 25:227–240. https://doi.org/10.1177/1073858418783959
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. https://doi.org/10.1016/j.it.2007.01.005
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372. https://doi.org/10.1038/nrn3880
Gupte R, Christian S, Keselman P et al (2019) Evaluation of taurine neuroprotection in aged rats with traumatic brain injury. Brain Imaging Behav 13:461–471. https://doi.org/10.1007/s11682-018-9865-5
Vargas-Castro V, Gomez-Diaz R, Blanco-Alvarez VM et al (2021) Long-term taurine administration improves motor skills in a tubulinopathy rat model by decreasing oxidative stress and promoting myelination. Mol Cell Neurosci 115. https://doi.org/10.1016/j.mcn.2021.103643
Wang K, Zhang B, Tian T et al (2022) Taurine protects dopaminergic neurons in paraquat-induced Parkinson’s disease mouse model through PI3K/Akt signaling pathways. Amino Acids 54:1–11. https://doi.org/10.1007/s00726-021-03104-6
Liu K, Zhu R, Jiang H et al (2022) Taurine inhibits KDM3a production and microglia activation in lipopolysaccharide-treated mice and BV-2 cells. Mol Cell Neurosci 122:103759. https://doi.org/10.1016/j.mcn.2022.103759
Quintas C, Vale N, Gonçalves J, Queiroz G (2018) Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front Pharmacol 9:1–12. https://doi.org/10.3389/fphar.2018.00418
Park JS, Kam TI, Lee S et al (2021) Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol Commun 9:1–15. https://doi.org/10.1186/s40478-021-01180-z
Villarreal A, Vidos C, Monteverde Busso M et al (2021) Pathological Neuroinflammatory Conversion of reactive astrocytes is Induced by Microglia and involves chromatin remodeling. Front Pharmacol 12:1–15. https://doi.org/10.3389/fphar.2021.689346
Zhang H, Wang D, Gong P et al (2019) Formyl peptide receptor 2 Deficiency improves cognition and attenuates tau hyperphosphorylation and astrogliosis in a mouse model of Alzheimer’s Disease. J Alzheimer’s Dis 67:169–179. https://doi.org/10.3233/JAD-180823
Su Y, Fan W, Ma Z et al (2014) Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 266:56–65. https://doi.org/10.1016/j.neuroscience.2014.02.006
Donato R, Sorci G, Riuzzi F et al (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta - Mol Cell Res 1793:1008–1022. https://doi.org/10.1016/j.bbamcr.2008.11.009
Wartchow KM, Rodrigues L, Swierzy I et al (2021) Amyloid-β processing in aged s100b transgenic mice is sex dependent. Int J Mol Sci 22. https://doi.org/10.3390/ijms221910823
Huttunen HJ, Kuja-Panula J, Sorci G et al (2000) Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275:40096–40105. https://doi.org/10.1074/jbc.M006993200
Ma Rhong, Zhang Y, Hong X, yue et al (2017) Role of microtubule-associated protein tau phosphorylation in Alzheimer’s disease. J Huazhong Univ Sci Technol - Med Sci 37:307–312. https://doi.org/10.1007/s11596-017-1732-x
Langeh U, Singh S (2020) Targeting S100B protein as a surrogate biomarker and its role in various neurological Disorders. Curr Neuropharmacol 19:265–277. https://doi.org/10.2174/1570159x18666200729100427
Michetti F, D’Ambrosi N, Toesca A et al (2019) The S100B story: from biomarker to active factor in neural injury. J Neurochem 148:168–187. https://doi.org/10.1111/jnc.14574
Shimamoto S, Tsuchiya M, Yamaguchi F et al (2014) Ca2+/S100 proteins inhibit the interaction of FKBP38 with Bcl-2 and Hsp90. Biochem J 458:141–152. https://doi.org/10.1042/BJ20130924
Li D, Li K, Chen G et al (2016) S100B suppresses the differentiation of C3H/10T1/2 murine embryonic mesenchymal cells into osteoblasts. Mol Med Rep 14:3878–3886. https://doi.org/10.3892/mmr.2016.5697
Tsoporis JN, Overgaard CB, Izhar S, Parker TG (2009) S100B modulates the hemodynamic response to norepinephrine stimulation. Am J Hypertens 22:1048–1053. https://doi.org/10.1038/ajh.2009.145
Wen XH, Duda T, Pertzev A et al (2012) S100B serves as a ca 2 + sensor for ROS-GC1 guanylate cyclase in cones but not in rods of the murine retina. Cell Physiol Biochem 29:417–430. https://doi.org/10.1159/000338496
Agam G, Almog O (2015) Calbindin D28k and S100B have a similar interaction site with the lithium-inhibitable enzyme inositol monophosphatase-1: a new drug target site. J Med Chem 58:2042–2044. https://doi.org/10.1021/jm5019324
Gógl G, Alexa A, Kiss B et al (2016) Structural basis of ribosomal S6 kinase 1 (RSK1) inhibition by S100B protein: modulation of the extracellular signal-regulated kinase (ERK) signaling cascade in a calcium-dependent way. J Biol Chem 291:11–27. https://doi.org/10.1074/jbc.M115.684928
Lee SG, Yoo DY, Jung HY et al (2015) Neurons in the hippocampal CA1 region, but not the dentate gyrus, are susceptible to oxidative stress in rats with streptozotocin-induced type 1 diabetes. Neural Regen Res 10:451–456. https://doi.org/10.4103/1673-5374.153695
Zhang Y, Chen K, Sloan SA et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. https://doi.org/10.1523/JNEUROSCI.1860-14.2014
Farmer WT, Abrahamsson T, Chierzi S et al (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Sci (80-) 351:849–854. https://doi.org/10.1126/science.aab3103
Nagao M, Ogata T, Sawada Y, Gotoh Y (2016) Zbtb20 promotes astrocytogenesis during neocortical development. Nat Commun 7:1–14. https://doi.org/10.1038/ncomms11102
Zhang Y, Sloan SA, Clarke LE et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. https://doi.org/10.1016/j.neuron.2015.11.013.Purification
Acknowledgements
The authors are grateful for all the support offered by the technician of the Pathology Research Laboratory, Teresinha Stein.
Author information
Authors and Affiliations
Contributions
F.H, A.M.Z, G.N.S and J.M.G performed stereotactic surgery, drug preparation and animal care; G.N.S and F.H performed behavioral tests; F.H, A.M.Z and G.N.S performed immunofluorescence assays; M.C.F, F.H and L.F.K performed the optical density microscopic analysis; J.M.G, M.C.F and F.H wrote the main text of the manuscript and J.M.G and F.H prepared scheme 1 and Figs. 1, 2, 3, 4, 5, 6 and 7. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Founding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors would like to thank the scholarships that allowed the execution of this project. These scholarships were funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) Finance Code 001. Fernanda Huf, Gabrielle N. da Silva, Luiz Felipe C. Koenig and Adriana M. Zago received PhD scholarships from the CAPES program, and Jessié M. Gutierres was supported by the National Postdoctoral Program (PNPD/CAPES).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huf, F., Gutierres, J.M., da Silva, G.N. et al. Neuroprotection elicited by taurine in sporadic Alzheimer-like disease: benefits on memory and control of neuroinflammation in the hippocampus of rats. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04872-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04872-3