Abstract
The aim of this study is to investigate the role of calcium-sensing receptor (CaSR) in the expression of inflammatory mediators of lipopolysaccharide (LPS)-treated human dental pulp cells (hDPCs). The expression profile of CaSR in LPS-simulated hDPCs was detected using immunofluorescence, real time quantitative PCR (RT-qPCR), and Western blot analyses. Then, its regulatory effects on the expression of specific inflammatory mediators such as interleukin (IL)-1β, IL-6, cyclooxygenase 2 (COX2)-derived prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, and IL-10 were determined by RT-qPCR and enzyme-linked immunosorbent assay (ELISA). LPS significantly downregulated the gene expression of CaSR, but upregulated its protein expression level in hDPCs. Treatments by CaSR agonist R568 or its antagonist Calhex231, and their combinations with protein kinase B (AKT) inhibitor LY294002 showed obvious effects on the expression of selected inflammatory mediators in a time-dependent manner. Meanwhile, an opposite direction was found between the action of R568 and Calhex231, as well as the expression of the pro- (IL-1β, IL-6, COX2-derived PGE2, and TNF-α) and anti-inflammatory (IL-10) mediators. The results provide the first evidence that CaSR-phosphatidylinositol-3 kinase (PI3K)-AKT-signaling pathway is involved in the release of inflammatory mediators in LPS-treated hDPCs, suggesting that the activation or blockade of CaSR may provide a novel therapeutic strategy for the treatment of pulp inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental pulp inflammation is a dynamic process characterized by changes in local blood flow, immunocompetent cell function, and neuronal activity, accompanying with the transition from an innate to an adaptive immune response [1]. There are now evidences that relative low inflammation could result in dentin formation, to “wall-off” the insult from the pulp, and is a prerequisite for tissue healing and pulp regeneration [2, 3]. In a slow-growing lesion, certain bacterial endotoxins, such as lipopolysaccharide (LPS), have been shown to be a potent inducer of pulpitis as well as dental pulp cells (DPCs)-mediated reparative events [4, 5]. LPS can activate immune activity and induce inflammatory reactions (e.g., the classical activation and polarization of macrophages, and secretion of proinflammatory cytokines) by various signals, including calcium-sensing receptor (CaSR) [6, 7].
The CaSR is extremely multifaceted due to its ability to participate in various different signaling pathways that are ligand and tissue specific, enabling this receptor to play a variety of critical roles in the physiology and pathophysiology of both Ca2+ regulation and other cellular functions which appear unrelated to Ca2+ homeostasis, e.g. secretion of digestive hormones and airway constriction [8,9,10]. Our previous study and other researches have identified the functional expression of CaSR in dental pulp tissue and cells and verified its involvement in the odontoblastic differentiation of DPCs through phosphatidylinositol-3 kinase/protein kinase B (PI3K/AKT) signals [11, 12]. Of note, CaSR is now thought to be an initiator and responder to the inflammation [13], and PI3K/AKT is an important and quite complex signaling pathway that mediates numerous cell processes, including cell division, metabolism, survival, and inflammation [14]. Thus, we speculated that CaSR and PI3K/AKT signaling pathways may be involved in the modulation of dental pulp inflammation.
During the process of pulp inflammation, a complex variety of pro-inflammatory and anti-inflammatory chemokines and cytokines, such as cyclooxygenase 2 (COX2)-derived prostaglandin E2 (PGE2), interleukin (IL)-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12 and tumor necrosis factor (TNF)-α, etc., have been reported to be involved in pulp response to infections [15,16,17,18]. However, the role of CaSR in the regulation of inflammatory mediator expression in dental pulp tissue has not been studied before. Here, we for the first time identified the expression of CaSR in LPS-treated hDPCs, and then investigated the effect of CaSR-PI3K/AKT on the expression of some specific pro-inflammatory and anti-inflammatory mediators through pharmacological activation.
Materials and methods
Cell culture and stimulation
Clinically healthy human premolars were obtained with informed consent of the patients between 18 and 25 years old who were undergoing orthodontics extractions to collected dental pulp tissues in accordance with the Ethics Committee of Guanghua School of Stomatology, Sun Yat-sen University, Guangzhou, Guangdong, China. Cells were isolated from dental pulp explants and cultured as previously described [12], and then used for stimulation experiments.
For the CaSR expression assay, hDPCs were cultured in α-MEM media with 0.1 and 1 µg/mL Escherichia coli LPS (Cat. no. L2880, Sigma, St. Louis, MO, USA) for 24, 48, and 72 h. The LPS concentrations were defined on the basis of our preliminary experiment and previous studies [4].
For the determination of inflammatory factors expression, cells were treated for 24 and 48 h using α-MEM media supplemented with different combinations of several stimulants as follows: (a) Blank control; (b) Positive control: 1 μg/mL LPS; (c) 1 μg/mL LPS + 1 μm R568 (Tocris Bioscience, Bristol, UK); (d) 1 μg/mL LPS + 1 μm R568 + 25 μm LY294002 (PI3K inhibitor; Cat. no. HY-10108, Shanghai, China); (e) 1 μg/mL LPS + 1 μm Calhex231 (Sigma-Aldrich, St Louis, MO); (f) 1 μg/mL LPS + 1 μm Calhex231 + 25 μm LY294002.
Immunofluorescence analysis
To identify CaSR-positive cells, 3 × 104 hDPCs were seeded into 6-well-chamber slides (NEST, Beijing, China) and exposed to 0.1 and 1 µg/mL E. coli LPS. The cells cultured in α-MEM media were used as positive controls, with omission of the first antibody as negative controls (data not shown). Then, cells were fixed and routinely processed using any of the previously described methods [12]. The anti-CaSR antibody (Cat. no. ab19347, Abcam) was diluted at 1:500 ratio. Images were captured by a fluorescence microscope (Axio Imager 2, ZEISS).
Real-time quantitative PCR (RT-qPCR) analysis
To detect the gene expression of CaSR and specific inflammatory mediators that include IL-1β, IL-6, COX2, TNF-α, and IL-10, total RNA was extracted from hDPCs exposed to different combinations of several stimulants described in section “Cell culture and stimulation” using Trizol Reagent (Invitrogen, Carlsbad, CA). The quantity and purity of isolated RNA were determined by a gel electrophoresis (Fig. 2a). Then, 1.5 μg of RNA was reverse transcribed into cDNA and PCR was performed using the Reverse Transcriptase M-MLV (RNase H-) (Takara Bio Inc, Shiga, Japan). 5 µL of diluted cDNA was added into 10 µL of SYBR® Premix Ex Taq™ (Tli RNaseH Plus, Takara Bio Inc, Shiga, Japan). The primer sets were shown in Table 1, and PCR conditions were as follows: 95 °C for 5 min, 95 °C for 10 s, and 60 °C for 30 s, 39 cycles. The housekeeping gene GAPDH was used as an internal control. The relative expression levels of mRNAs were calculated by the 2−ΔΔCt method.
Western blot
As alluded above, hDPCs were treated with 0.1 and 1 µg/mL LPS for 24 h, 48 h and 72 h to determine the protein level of CaSR. Cell lysates were prepared using the whole protein extraction kit (KGP2100; Keygen Biotech, Nanjing, China). Protein concentrations were estimated by bicinchoninic acid assay. After immunoblotting, the protein extracts were transferred to polyvinylidene fluoride membranes by a semi-dry transfer apparatus (Bio-Rad, Hercules, CA, USA). Western blot analysis was performed as described previously [12]. Primary antibodies were purchased from the following commercial sources: monoclonal antibodies against human CaSR [Cat. no. ab19347 (1:500 dilution), Abcam]; and monoclonal antibodies against-β-actin [Cat. no. ab8227 (1:1000 dilution), Abcam].
Enzyme-linked immunosorbent assay (ELISA)
To determine the action of CaSR on the expression of inflammatory factors, we used RT-qPCR as alluded in “Real time quantitative PCR (RT-qPCR) analysis” section to detect the gene expression of selected inflammatory mediators, and employed ELISA to monitor their secretion levels. In brief, the cells were cultured in 12-well plates at a density of 1 × 104 cells/well. After incubation of the cultures in experimental conditions, the medium of each sample was collected and assayed for IL-1β, IL-6, PGE2, TNF-α, and IL-10 synthesis according to the manufacturer’s protocol using a commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) and calibrated spectrophotometrically with a standard curve. The experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA). All results were expressed as the mean ± standard error of the mean. Multiple comparisons were performed using two-way analysis of variance (ANOVA) followed by Tukey test. A p value of < 0.05 was set a priori to identify significant differences between any two groups.
Results
LPS increases the protein level of CaSR in hDPCs
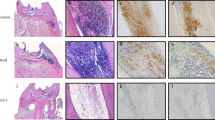
To measure the action of LPS on the CaSR expression in hDPCs, we used the immunohistochemical staining, RT-qPCR and Western blot assay. The results of immunohistochemical analysis showed an enhanced staining in LPS-treated hDPCs at 24 h (Fig. 1), 48 h and 72 h (data not shown). At the gene level, the 18S and 28S ribosomal RNA bands are clearly visible at about 1:2 ratio, indicating the intact RNA sample (Fig. 2a), and the CaSR expression of all experiment groups was significantly downregulated in all time points except 0.1 μg/mL LPS at 24 h (Fig. 2b). In contrast, an increased protein level of CaSR in LPS-stimulated cells was detected by Western blot assay, compared to LPS-free controls (Fig. 2c).
R568 and Calhex231 affect the gene expression of IL-6 and IL-10 in LPS-treated hDPCs (Table 2)
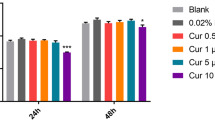
The results of RT-qPCR demonstrated that R568 alone didn’t affect the mRNA expression of selected inflammatory cytokines except its promotive action on the IL-6 levels in LPS-stimulated hDPCs, whereas Calhex231 alone can increase the gene level of proinflammatory cytokines IL-6 and anti-inflammatory cytokine IL-10 at 48 h (Fig. 3a–e). In addition, the significant difference was also found in the expression of IL-6 at 24 h and IL-10 at 48 h between R568- and Calhex231-treated cells (Fig. 3b, e).
The gene levels of selected inflammatory mediators after cell treatment for 24 and 48 h with 1.0 µg/mL LPS. Results were standardized to the housekeeping gene GAPDH, and expressed as relative fold change in mRNA levels. a IL-1βmRNA, b IL-6 mRNA, c COX2 mRNA, d TNF-α mRNA, and e IL-10 mRNA. Nc negative control; Pc positive control
R568 and Calhex231 regulate the secretion of inflammatory mediators in LPS-treated hDPCs (Table 2)
ELISA assay showed an increasing pattern with prolonged time in the secretion level of selected mediators. Treatment of hDPCs with R568 alone resulted in the reduction of LPS-stimulated pro-inflammatory cytokines production including IL-1β and TNF-α at 24 h, and IL-6 and PGE2 at 48 h, while increasing the production of IL-1β and TNF-α at 48 h, as well as anti-inflammatory cytokines IL-10 at 24 h. With regard to Calhex231 alone stimulation, IL-1β at 24 h and TNF-α and IL-10 at 48 h were highly expressed compared with the LPS only‐treated ones, but IL-6 at 48 h, TNF-α and IL-10 at 24 h were lowly expressed (Fig. 4). Similar to the situation in the gene expression, the secretive trend of IL-1β and IL-10 at 24 and 48 h, IL-6 at 24 h, and PGE2 at 48 h showed an opposite direction between R568 and Calhex231 treatment. Compared with the R568 stimulation, the release of IL-1β was elevated at 24 h but decreased at 48 h in the Calhex231-treated cells, whereas the IL-10 secretion was obviously suppressed at 24 h but increased at 48 h (Fig. 4a, e).
PI3K/AKT signal may be involved in CaSR-mediated inflammatory mediator production (Table 2)
Gene analysis revealed that LY294200 treatment (PI3K inhibitor) in R568-challenged hDPCs significantly reduced IL-1β expression at 24 h compared to parallel R568-stimulated controls, but increased IL-6 level at 24 h and COX2 and IL-10 at 48 h. In the cultures added with Calhex231, it can up-regulate IL-6 expression and TNF-α at 24 h, whereas down-regulate the IL-10 expression at 48 h (Fig. 3).
The protein level in culture supernatants determined by ELISA presented that LY294200 stimulation can enhance the production of IL-1β and IL-6 at 24 h, and PGE2 at 24 and 48 h, and IL-10 at 48 h, but suppress TNF-α secretion at 48 h in the cultures supplemented with R568. In Calhex231-stimulated cells, it was showed to promote the release of IL-1β (48 h), IL-6 (24 h), PGE2 (48 h), and IL-10 (24 h) compared to the treatment with Calhex231 alone at indicated time-points in brackets. In contrast, its inhibitive action was only found in the secretion of TNF-α at 48 h (Fig. 4).
Discussion
The understanding of the mechanisms that modulate inflammation in pulp tissue is key to preventing the consequences of pulpitis and achieving pulp repair or regeneration [18]. Previous studies have demonstrated that CaSR is involved in the release of inflammatory mediators in several LPS-treated cell linages, such as mouse bone marrow-derived macrophages and H292 airway epithelial cells [19, 20], indicating its key roles in inflammatory diseases [10, 21]. It is well known that LPS can activate oxidative metabolism and antimicrobial activity of macrophages and has been widely used to induce inflammatory response in numerous animal and in vitro models [7]. In the study regarding dental pulp tissue, LPS is usually topically administered to mimic pulp inflammation caused by cariogenic microorganisms to some degree. Thus, we first, investigated the CaSR expression in LPS-challenged hDPCs using immunofluorescent assay, and found an enhanced positive staining in all experimental groups, which may be attributed to the dose-dependent action of LPS that was indicated to promote the cell proliferation and to protect cells against apoptosis at 1 μg/mL level in mesenchymal stem cells [22], and human stem cells from the apical papilla at 0.1 μg/mL [23], accompanying by maximal induction of specific cytokines of 1 μg/mL LPS [24]. Therefore, LPS treatment with 1 μg/mL was adopted in the experiments on the expression of inflammatory mediators. The subsequent Western blot analysis also demonstrated a promotive action of LPS in defined concentrations on the protein level of CaSR in a dose-dependent manner, along with a decreased trend of CaSR expression level in the control that has been confirmed in our previous study [12]. The results are consistent with other researches on the role of CaSR in intestinal epithelial cells [25], and cardiomyocytes [6]. However, LPS unexpectedly decreased the mRNA level of CaSR, and the inconsistency between gene at the mRNA level and protein expression (also exists in the expression of selected inflammatory mediators) has already reported and discussed in previous studies [12, 26]; one possible reason is that gene expression is controlled at the translational level, but the expression of individual protein is differentiation stage specific through a posttranslational mechanism involving phosphorylation of key residues [27, 28].
As alluded above, some specific cytokines (e.g. IL-1β, IL-6, and TNF-α) are produced and secreted by a wide range of immune and non-immune cells and affect many interactions among these cells in the pulp inflammatory process [16, 17]. Hence, we monitored the role of CaSR in the release of selective mediators using its allosteric positive modulator NPS R-568 and negative allosteric modulator Calhex231 in LPS-stimulated hDPCs. In our present study, LPS alone did not promote the production of all selected mediators in all time-points as anticipated. The similar result has also been reported by other previous studies [29, 30], indicating a various regulative manner of LPS on the release of inflammatory cytokines at different time-points of the culture period. Similarly, both R568 and Calhex231 alone treatment showed different effects on the secretion of IL-1β, IL-6, TNF-α, and IL-10 at different time-points, suggesting a possible time-dependent regulative manner of CaSR on the inflammatory mediator secretion. We also found an antagonistic interaction between the CaSR agonist R568 and its antagonist Calhex 231 in hDPCs, which was indicated by an obvious opposite direction in the level of their induced IL-1β and IL-10 secretions. In addition, we showed a reverse direction between the pro- (IL-1β, IL-6, PGE2, and TNF-α) and anti-inflammatory (IL-10) cytokines expressions under R568 or Calhex231 stimulation. With regard to the phenomenon, some studies reported that IL-1 family cytokines are considered upstream of other inflammatory cytokines, such as IL-6, IL-2, and IL-12 that are stimulated by IL-1β and IL-18, which are mainly secreted by inflammatory cells; whereas IL-10 could inactivate macrophages and inhibit proinflammatory cytokine expressions, e.g., NF-κB, IL-8, and IL-6, in inflamed dental pulp [17]. Thus, we can conclude that CaSR may promote or attenuate the release of specific inflammatory mediators in dental pulp, depending on the situation.
CaSR can be activated by agonists (type I calcimimetics), and allosteric activators (type II calcimimetics; like NPS R-568, cinacalcet, and AMG 416) that have been proven clinically useful [31]. In hDPCs, we observed that R568 can inhibit IL-6 secretion but enhance the release of IL-1β, TNF-α and IL-10. The result was supported by a study of other researchers who reported an increasement of R568 on the TNF-α release in the rat peripheral blood T lymphocyte [32]. However, in rat renal tissue, R-568 showed a promotive effect on the secretion of IL-6, and an inhibitive action on the IL-10 level [33]. These findings partly support the notion that calcimimetics exhibit cell phenotype-dependent pharmacology [31]. Furthermore, the above findings on the action of R568 in hDPCs are also partially consistent with that in intestinal epithelial cells using γ-glutamyl cysteine and γ-glutamyl valine as CaSR agonists that reduced the expression of IL-1β, IL-6, and TNF-α, and increased the expression of IL-10 [34]. Conversely, CaSR activator cincalcet was verified to increase the secretion of IL-6, but decrease the production of IL-10 in rat peripheral blood polymorphonuclear neutrophils [35]. The agreement and disagreement among different researches may be related to various experimental conditions, such as cell linage, agonist, and measurement time-point, etc., and the tissue-specific actions of CaSR that mediated by biased signaling pathways upon agonist or allosteric activator stimulation (Chavez-Abiega et al. [9], Leach, Hannan et al. [10]). In addition, we may glean a clue for the differences from the properties of CaSR that tends to adopts multiple active conformations stabilized by different agonists to generate a set of distinct intracellular signals and biological effects, differing to many other GPCRs that exist in either an “on” or “off” conformation. Consequently, super-agonism (more than 100% efficacy) and biased-agonism (selective activation or inactivation of one function over others) may occur when a combination of different agonists is used to influence the receptor function [8].
In contrast to the positive effect of calcimimetics in CaSR activation, calcilytics (e.g. NPS 2143, Calhex231, and ATF 936) are used to inhibit CaSR activity. In the present study, we used Calhex231 to reduce the sensitivity of the CaSR and found a significant inhibitive effect of Calhex231 on the secretion of IL-6 in hDPCs; similarly, NPS 2143 (a novel and selective antagonist of CaSR) was reported to attenuate the releases of IL-6 LPS-stimulated H292 airway epithelial cells [19]. NPS 2143 and Calhex231 share the similar structure and target a common allosteric site within the seventh transmembrane domain of the CaSR, and thus likely show similar actions on the function of CaSR in several cell types [36, 37]. Moreover, we concluded a facilitative action of Calhex231 on the IL-1β production in LPS-treated hDPCs; in contrast, other studies indicated an inhibitive effect of Calhex231 on the release of IL-1β in rat peritoneal macrophages and human neutrophils [38, 39]. The discrepancy appears similar with the tissue-selective effects of calcimimetics, but still lacks sufficient evidences for such selectivity with calcilytics at present [31].
CaSR can activate multiple cellular signaling pathways, such as nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) signals, which were involved in many different physiological functions that includes the secretion of inflammatory mediators [9, 21]. In our previous study, we have demonstrated that CaSR was involved in the activation of PI3K/AKT pathway [12]. In this study, we found that PI3K/AKT pathway inhibitor LY294002 can intensify or attenuate the actions of R568 and Calhex231 in inflammatory cytokines production induced by LPS in a time-points- and cytokine types-dependent way, suggesting a possible involvement of CaSR-PI3K/AKT in dental pulp inflammation. In addition, LY294002 was also found to facilitate COX2 mRNA expression in R568-treated cells and PGE2 production in Calhex231-challenged cells, which indicated a possible interaction between the PI3K/AKT and COX2/PGE2 signals in the context of pulp inflammation. Future researches using gene knockout, gene silencing, RNA interference and specific inhibitors and agonists should be considered to clarify the roles of CaSR in the cross-talks among various signal pathways involved pulp inflammatory responses. Furthermore, due to its present and various key roles in many tissues and cells, such as parathyroid hormone secretion, bone turnover, vascular tone and blood pressure, and intestinal or renal reabsorption, etc., CaSR may mediate the physiological and pathological events of dental pulp through regulating vasodilator system and hormones secretion. Thus, we may explore this possible mechanism by which systemic diseases (e.g., diabetes mellitus and hypertension) affect dental pulp alterations through conducting some specific experiments, e.g., the role of CaSR in regulating the ration of blood urea nitrogen (BUN) and creatinine that usually indicate dehydration of tissues can be elucidated and used to monitor the functioning and performance of dental pulp in the patients with cardiovascular (hypertension) and metabolic diseases (diabetes) [40].
Conclusion
In summary, our present study demonstrated that CaSR is expressed in LPS-treated hDPCs, and also indicated a possible involvement of CaSR and PI3K-AKT pathway in the production of specific inflammatory mediators related to dental pulp inflammation. These findings imply that the activation or blockade of CaSR may provide a new research line for the treatment of pulp inflammatory diseases. In future, it will be significant to investigate the performance of more different CaSR activators and CaSR inhibitors and the underlying mechanisms in dental pulp tissue under physiological and pathological states.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Hahn CL, Liewehr FR (2007) Innate immune responses of the dental pulp to caries. J Endod 33(6):643–651. https://doi.org/10.1016/j.joen.2007.01.001
Goldberg M, Njeh A, Uzunoglu E (2015) Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediat Inflamm 2015:347649. https://doi.org/10.1155/2015/347649
Fawzy El-Sayed KM, Elsalawy R, Ibrahim N, Gadalla M, Albargasy H, Zahra N, Mokhtar S, El Nahhas N, El Kaliouby Y, Dorfer CE (2019) The dental pulp stem/progenitor cells-mediated inflammatory-regenerative axis. Tissue Eng B 25(5):445–460. https://doi.org/10.1089/ten.TEB.2019.0106
He W, Wang Z, Luo Z, Yu Q, Jiang Y, Zhang Y, Zhou Z, Smith AJ, Cooper PR (2015) LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. J Cell Physiol 230(3):554–561. https://doi.org/10.1002/jcp.24732
Chmilewsky F, Jeanneau C, Laurent P, About I (2015) LPS induces pulp progenitor cell recruitment via complement activation. J Dent Res 94(1):166–174. https://doi.org/10.1177/0022034514555524
Wang HY, Liu XY, Han G, Wang ZY, Li XX, Jiang ZM, Jiang CM (2013) LPS induces cardiomyocyte injury through calcium-sensing receptor. Mol Cell Biochem 379(1–2):153–159. https://doi.org/10.1007/s11010-013-1637-3
Orecchioni M, Ghosheh Y, Pramod AB, Ley K (2019) Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS−) vs. alternatively activated macrophages. Front Immunol 10:1084. https://doi.org/10.3389/fimmu.2019.01084
Owen JL, Cheng SX, Ge Y, Sahay B, Mohamadzadeh M (2016) The role of the calcium-sensing receptor in gastrointestinal inflammation. Semin Cell Dev Biol 49:44–51. https://doi.org/10.1016/j.semcdb.2015.10.040
Chavez-Abiega S, Mos I, Centeno PP, Elajnaf T, Schlattl W, Ward DT, Goedhart J, Kallay E (2020) Sensing extracellular calcium—an insight into the structure and function of the calcium-sensing receptor (CaSR). Adv Exp Med Biol 1131:1031–1063. https://doi.org/10.1007/978-3-030-12457-1_41
Leach K, Hannan FM, Josephs TM, Keller AN, Moller TC, Ward DT, Kallay E, Mason RS, Thakker RV, Riccardi D, Conigrave AD, Brauner-Osborne H (2020) International union of basic and clinical pharmacology. CVIII. Calcium-sensing receptor nomenclature, pharmacology, and function. Pharmacol Rev 72(3):558–604. https://doi.org/10.1124/pr.119.018531
Mizumachi H, Yoshida S, Tomokiyo A, Hasegawa D, Hamano S, Yuda A, Sugii H, Serita S, Mitarai H, Koori K, Wada N, Maeda H (2017) Calcium-sensing receptor-ERK signaling promotes odontoblastic differentiation of human dental pulp cells. Bone 101:191–201. https://doi.org/10.1016/j.bone.2017.05.012
Chen Y, Gao Y, Tao Y, Lin D, An S (2019) Identification of a calcium-sensing receptor in human dental pulp cells that regulates mineral trioxide aggregate-induced mineralization. J Endod 45(7):907–916. https://doi.org/10.1016/j.joen.2019.03.019
Hendy GN, Canaff L (2016) Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin Cell Dev Biol 49:37–43. https://doi.org/10.1016/j.semcdb.2015.11.006
Sun K, Luo J, Guo J, Yao X, Jing X, Guo F (2020) The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr Cartil 28(4):400–409. https://doi.org/10.1016/j.joca.2020.02.027
Miyauchi M, Takata T, Ito H, Ogawa I, Kobayashi J, Nikai H, Ijuhin N (1996) Immunohistochemical demonstration of prostaglandins E2, F2 alpha, and 6-keto-prostaglandin F1 alpha in rat dental pulp with experimentally induced inflammation. J Endod 22(11):600–602. https://doi.org/10.1016/s0099-2399(96)80029-x
Hirsch V, Wolgin M, Mitronin AV, Kielbassa AM (2017) Inflammatory cytokines in normal and irreversibly inflamed pulps: a systematic review. Arch Oral Biol 82:38–46. https://doi.org/10.1016/j.archoralbio.2017.05.008
Khorasani MMY, Hassanshahi G, Brodzikowska A, Khorramdelazad H (2020) Role(s) of cytokines in pulpitis: latest evidence and therapeutic approaches. Cytokine 126:154896. https://doi.org/10.1016/j.cyto.2019.154896
Duncan HF, Cooper PR (2020) Pulp innate immune defense: translational opportunities. J Endod 46(9S):S10–S18. https://doi.org/10.1016/j.joen.2020.06.019
Lee JW, Park HA, Kwon OK, Park JW, Lee G, Lee HJ, Lee SJ, Oh SR, Ahn KS (2017) NPS 2143, a selective calcium-sensing receptor antagonist inhibits lipopolysaccharide-induced pulmonary inflammation. Mol Immunol 90:150–157. https://doi.org/10.1016/j.molimm.2017.07.012
Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492(7427):123–127. https://doi.org/10.1038/nature11588
Klein GL, Castro SM, Garofalo RP (2016) The calcium-sensing receptor as a mediator of inflammation. Semin Cell Dev Biol 49:52–56. https://doi.org/10.1016/j.semcdb.2015.08.006
Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q, Gao X (2009) Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol Int 33(6):665–674. https://doi.org/10.1016/j.cellbi.2009.03.006
Liu J, Du J, Chen X, Yang L, Zhao W, Song M, Wang Z, Wang Y (2019) The effects of mitogen-activated protein kinase signaling pathways on lipopolysaccharide-mediated osteo/odontogenic differentiation of stem cells from the apical papilla. J Endod 45(2):161–167. https://doi.org/10.1016/j.joen.2018.10.009
Zhang J, Zhang Y, Lv H, Yu Q, Zhou Z, Zhu Q, Wang Z, Cooper PR, Smith AJ, Niu Z, He W (2013) Human stem cells from the apical papilla response to bacterial lipopolysaccharide exposure and anti-inflammatory effects of nuclear factor I C. J Endod 39(11):1416–1422. https://doi.org/10.1016/j.joen.2013.07.018
Liu H, Tan B, Huang B, Li J, Wang J, Liao P, Guan G, Ji P, Yin Y (2018) Involvement of calcium-sensing receptor activation in the alleviation of intestinal inflammation in a piglet model by dietary aromatic amino acid supplementation. Br J Nutr 120(12):1321–1331. https://doi.org/10.1017/S0007114518002891
Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H (2008) How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 40(5):426–436. https://doi.org/10.1111/j.1745-7270.2008.00418.x
Shui C, Spelsberg TC, Riggs BL, Khosla S (2003) Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J Bone Miner Res 18(2):213–221. https://doi.org/10.1359/jbmr.2003.18.2.213
Sudhakar S, Li Y, Katz MS, Elango N (2001) Translational regulation is a control point in RUNX2/Cbfa1 gene expression. Biochem Biophys Res Commun 289(2):616–622. https://doi.org/10.1006/bbrc.2001.6033
Widbiller M, Eidt A, Wolflick M, Lindner SR, Schweikl H, Hiller KA, Buchalla W, Galler KM (2018) Interactive effects of LPS and dentine matrix proteins on human dental pulp stem cells. Int Endod J 51(8):877–888. https://doi.org/10.1111/iej.12897
Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, Colic M (2011) Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev 20(4):695–708. https://doi.org/10.1089/scd.2010.0145
Nemeth EF, Goodman WG (2016) Calcimimetic and calcilytic drugs: feats, flops, and futures. Calcif Tissue Int 98(4):341–358. https://doi.org/10.1007/s00223-015-0052-z
Wu CL, Wu QY, Du JJ, Zeng JY, Li TT, Xu CQ, Sun YH (2015) Calcium-sensing receptor in the T lymphocyte enhanced the apoptosis and cytokine secretion in sepsis. Mol Immunol 63(2):337–342. https://doi.org/10.1016/j.molimm.2014.08.007
Hu B, Tong F, Xu L, Shen Z, Yan L, Xu G, Shen R (2018) Role of calcium sensing receptor in streptozotocin-induced diabetic rats exposed to renal ischemia reperfusion injury. Kidney Blood Press Res 43(1):276–286. https://doi.org/10.1159/000487685
Zhang H, Kovacs-Nolan J, Kodera T, Eto Y, Mine Y (2015) gamma-Glutamyl cysteine and gamma-glutamyl valine inhibit TNF-alpha signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochem Biophys Acta 1852(5):792–804. https://doi.org/10.1016/j.bbadis.2014.12.023
Zhai TY, Cui BH, Zou L, Zeng JY, Gao S, Zhao Q, Wang Y, Xie WL, Sun YH (2017) Expression and role of the calcium-sensing receptor in rat peripheral blood polymorphonuclear neutrophils. Oxid Med Cell Longev 2017:3869561. https://doi.org/10.1155/2017/3869561
Greenberg HZE, Jahan KS, Shi J, Vanessa Ho WS, Albert AP (2016) The calcilytics Calhex-231 and NPS 2143 and the calcimimetic Calindol reduce vascular reactivity via inhibition of voltage-gated Ca(2+) channels. Eur J Pharmacol 791:659–668. https://doi.org/10.1016/j.ejphar.2016.10.008
Yamamura A, Nayeem MJ, Sato M (2019) Calcilytics inhibit the proliferation and migration of human prostate cancer PC-3 cells. J Pharmacol Sci 139(3):254–257. https://doi.org/10.1016/j.jphs.2019.01.008
Liu W, Sun J, Guo Y, Liu N, Ding X, Zhang X, Chi J, Kang N, Liu Y, Yin X (2020) Calhex231 ameliorates myocardial fibrosis post myocardial infarction in rats through the autophagy-NLRP3 inflammasome pathway in macrophages. J Cell Mol Med. https://doi.org/10.1111/jcmm.15969
Ren Z, Yang K, Zhao M, Liu W, Zhang X, Chi J, Shi Z, Zhang X, Fu Y, Liu Y, Yin X (2020) Calcium-sensing receptor on neutrophil promotes myocardial apoptosis and fibrosis after acute myocardial infarction via NLRP3 inflammasome activation. Can J Cardiol 36(6):893–905. https://doi.org/10.1016/j.cjca.2019.09.026
Deng L, Qiu S, Wang C, Bian H, Wang L, Li Y, Wu B, Liu M (2019) Effects of the blood urea nitrogen to creatinine ratio on haemorrhagic transformation in AIS patients with diabetes mellitus. BMC Neurol 19(1):63. https://doi.org/10.1186/s12883-019-1290-x
Funding
This study was supported by the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515010072), and National Science Foundation of China (Grant No. 81700957).
Author information
Authors and Affiliations
Contributions
YC, YL and TY performed the experiments and analyzed the data. SA and YH designed and supervised this project jointly. SA analyzed the data, drafted the first manuscript and revised the manuscript finally.
Corresponding author
Ethics declarations
Conflict of interest
The authors deny any conflict of interest related to this study.
Ethical approval
All experimental protocols were approved by the Ethics Committee of Guanghua School of Stomatology, Sun Yat-sen University, Guangzhou, Guangdong, China (No. KQEC-2022-18-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
An, S., Chen, Y., Yang, T. et al. A role for the calcium-sensing receptor in the expression of inflammatory mediators in LPS-treated human dental pulp cells. Mol Cell Biochem 477, 2871–2881 (2022). https://doi.org/10.1007/s11010-022-04486-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04486-1