Abstract
Increasing evidence indicates that anoikis resistance is a critical process for metastasis of cancer cells, making it the attractive therapeutic target for cancer benefit. Anoikis resistance is widely regulated by various factors, such as signaling pathways, integrins switch, and non-coding RNAs (ncRNAs). ncRNAs composed of microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), are frequently dysregulated in a variety of human malignancies and are closely related to anoikis resistance of cancer cells. Based on the available literature, we reviewed the molecular basis underlying ncRNAs modulating cancer cells anoikis resistance, which may contribute to a better understanding of cancer metastasis and provide new beneficial therapeutic strategies against cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
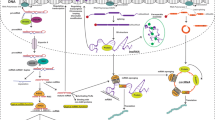
Anoikis resistance is a crucial cellular program that enables carcinoma cells to escape apoptosis in the absence of attachment to extracellular matrix (ECM) or upon cell adhesion to an inappropriate location [1,2,3]. The ability to resist apoptosis under the loss of ECM attachment endows cancer cells to detach from the primary tumor site, invade a distant site and establish a metastatic lesion [3, 4]. Therefore, it has been believed that anoikis resistance is a critical process for tumor cell metastasis [5], and overcoming anoikis resistance may have important therapeutic value for cancers [3, 6].
Previous studies have shown that cancer cells can achieve anoikis resistance through multiple factors or mechanisms including integrins switch, growth proteins, oxidative stress, autophagy, epithelial-mesenchymal transition (EMT), metabolism, and signaling pathway [3, 5, 7]. For instance, the interplay between integrin-α2β1/-α5β1 and EGFR enhanced anoikis resistance in colon cancer cells through activating downstream effectors ERK and AKT and suppressing Caspase-3 activation [8]. Upregulation of LC3B induced by oxidative stress attenuated anoikis resistance in contrast to regulating autophagy in ovarian cancer cells [9]. Moreover, CPT1A-mediated fatty acid oxidation activation led to colorectal cancer cells resisting anoikis and increase metastatic capacity [10]. These studies suggest that anoikis resistance in cancer cells is far more complicated than expected and warrants further investigation.
Non-coding RNAs (ncRNAs), such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), are a group of RNA transcripts that do not own the protein-coding ability but are involved in the regulation of physiological and pathological functions [11,12,13]. Ample evidence indicated that ncRNAs have been identified as oncogenic drivers or tumor suppressors in various malignancies [14]. Notably, multiple ncRNAs are associated with anoikis resistance in various cancers [1]. Considering the rapid development of the ncRNA field, a more detailed understanding of the molecular mechanisms underlying ncRNAs modulating cancer cells anoikis resistance is urgently needed. In this review, we focused on the critical roles of ncRNAs in modulating anoikis resistance in cancers.
Discussion
MiRNAs and anoikis resistance
MiRNAs are a kind of ~ 22-nucleotide, evolutionarily conserved ncRNAs, which modulate target gene expression through binding to the 3’-untranslated region (3’-UTR) of target mRNA molecules at the posttranscriptional level [15, 16]. Amounting evidence has shown that miRNA is involved in various processes, such as cell growth, metastasis, therapy resistance, and immune escape [17,18,19]. Interestingly, a variety of miRNAs can be oncogenic or tumor-suppressive and have repeatedly been implicated for their roles in regulating anoikis resistance [1, 20].
MiRNAs act as positive regulators of anoikis resistance
A growing body of evidence shows that aberrant miRNAs enhanced anoikis resistance of cancer cells through the regulation of pathways, adhesion molecules, or apoptosis-associated proteins (Table 1, Fig. 1).
Emerging data have indicated that several signaling pathways are abnormally activated or inactivated in cancers, and are vital mediators and drivers in anoikis resistance [7]. The Hippo signaling, tumor-suppressive signaling, has been frequently identified to be inactivated in multiple cancers, and the inactivation of Hippo signaling exerts a pleiotropic role in the progression and metastasis of cancers [21]. A previous study showed that miR-424-5p overexpression promoted, while miR-424-5p knockdown inhibited, anoikis resistance and lung metastasis of thyroid cancer cells in vitro and in vivo by inactivating the activity of Hippo signaling via directly targeting WWC1, SAV1, and LAST2[21]. As a key node of the Hippo signaling pathway, Yes-associated protein 1 (YAP1) has been implicated to regulate anoikis. Yu et al. noted that miR-200a overexpression promoted whereas miR-200a inhibition suppressed anoikis resistance in breast cancer cells by the downregulation of YAP1, which resulted in decreased pro-apoptotic protein expression [22]. Recent research showed that enforced expression of miR-141 enhances cell proliferation, anchorage-independent capacity, anoikis resistance, tumor growth and peritoneal metastases of ovarian cancer cells through the regulation of KLF12/Sp1/survivin axis [23]. What’s more, MiR-G-10, a novel miRNA identified in G-rich RNA sequence binding protein (GRSF1) complex, promote migration/invasion and anoikis resistance in vitro and lung metastasis in vivo, by upregulating PIK3R3 expression to activate the AKT/NF-κB signal pathway and suppressing TIMP3/MMP9 pathway [24]. Hida et al. used a public tumor endothelial cell microarray database and found that miR-145, increased in tumor endothelial cell, signifiacntly elevated cell adhesion and anoikis resistance in human microvascular endothelial cells by enhancing the BCl-2 and Bcl-xL expresion via the ERK1/2 pathway [25].
Cell surface adhesion molecules have been implicated in the acquisition of anoikis resistance of cancer cells [26, 27]. Penna et al. found that miR-214 was involved in the modulation of survival to anoikis in melanoma [28]. Using a melanoma metastatic model, miR-214 was found to be highly expressed in metastatic (high) cells compared with parental (low) cells. Moreover, the introduction of miR-214 expression contributed to melanoma cell movement and survival to anoikis in vitro as well as extravasation from blood vessels and lung metastasis formation in vivo through targeting TFAP2C, which is a member of the AP-2 transcription factor family and involved in the activation or repression of cell movement and adhesion molecules [28]. Additionally, miR-145 overexpression obviously enhanced cell invasion and anoikis resistance of esophageal adenocarcinoma cell lines (OE33, FLO-1, SK-GT-4) [29]. Moreover, upregulation of miR-145 induced resistance to anoikis and invasion potential in esophageal adenocarcinoma cells was associated with the downregulation of c-Myc, which led to the integrins subunits α5 and β3 expression [30].
Given that anoikis is a special form of apoptosis, the dysregulation of apoptosis-associated genes is observed to play a pivotal role in anoikis resistance of cancer cells [31]. Dysregulated TGF-β signaling was demonstrated to drive late-stage breast cancer metastasis. MiR-181a induced by TGF-β promoted epithelial-mesenchymal transition, migration, and invasion in breast cancer cells [32]. Mechanistically, miR-181a down-regulation significantly increased the expression of Bim, a proapoptotic protein molecule that sensitized metastatic cells to anoikis [32]. Zhao et al. hypothesized that miR-21, one of the most commonly observed aberrant miRNAs in human cancers, could contribute to tumor metastasis by regulating anoikis in human esophageal adenocarcinoma. In fact, transfection of miR-21 mimics significantly enhanced the resistance to anoikis in esophageal adenocarcinoma cells by targeting PDCD4 and PTEN, which were involved in the regulation of many basic cellular functions including cell apoptosis [33].
MiRNAs act as negative regulators of anoikis resistance
Next, we reviewed the effect of miRNAs, function as tumor suppressor gene, on anoikis resistance in cancers (Table 2, Fig. 1).
Experimental evidence reported that tumor-suppressive miRNAs also can modulate anoikis resistance of cancer cells via triggering or inhibiting signaling pathways [1, 20]. For example, the introduction of miR-525-5p, which acts as a tumor suppressor gene, dramatically hampered anchorage-independent growth and anoikis resistance of cervical cancer cells via blocking ubiquitin-conjugating enzyme E2C (UBE2C)/ZEB1/2 signal axis [34]. In pancreatic cancer, miR-137 was decreased in pancreatic cancer cells (AsPC-1 and PANC-1 cell) after the induction of anoikis in time-dependent. Overexpression of miR-137 reduced the resistance to anoikis in pancreatic cancer cells in vitro and in vivo by negatively modulating paxillin (PXN), which resulted in the activation of the AKT signaling pathways [35]. MiR-133a-3p and miR-133b were frequently low expression in various types of cancers and were reported to be tumor suppressors [36, 37]. In prostate cancer, upregulation of miR-133a-3p suppressed cancer stem cell-like phenotypes and attenuated anoikis resistance of prostate cancer cells by directly targeting multiple cytokine receptors, including EGFR, FGFR1, IGF1R, and MET, which further inhibited PI3K/AKT signaling [38]. Also, in esophageal squamous cell carcinoma, miR-133b negatively modulated anoikis resistance and anchorage-independent growth through the regulation of EGFR-mediated ITGB4/FAK/Grb2, AKT, and ERK signaling [39]. Zhang and collaborators verified that decreased in gastric cancer specimens, miR-204 reduced cell invasion and anoikis resistance in gastric cancer cells via modulating the SIRT1-LKB1 pathway [40]. Additionally, the miR-204 levels were significantly down-regulated in an anoikis pattern of epithelial ovarian cancer cells. Furthermore, restored expression of miR-204 enabled cells to acquire more sensitivity to anoikis through the inhibition of BDNF, contributing to the inactivation of the PI3K/AKT signaling pathway [41]. Besides, miR-296 was involved in the inhibitory effects of epigallocatechin gallate (EGCG), the most active and abundant polyphenol in green tea, on anoikis-resistant nasopharyngeal carcinoma cells through the downregulation of STAT3 activation [42]. MiR-200c, an important member of the miR-200 family that emerged as a potent regulator of epithelial to mesenchymal transition, has been implicated in the resistance to anoikis in various cancers. Howe et al. identified, using a microarray profiling, several direct targets of miR-200c, including the genes encoding fibronectin 1 (FN1), moesin (MSN), neurotrophic tyrosine receptor kinase type 2 (NTRK2 or TrkB), leptin receptor (LEPR), and Rho GTPase activating protein 19 (ARHGAP19) [43]. Among these targets, TrkB was a tyrosine kinase receptor that contributed to the ability of miR-200c to suppress anoikis resistance [43]. In ovarian cancer, restoration of miR-200c resulted in decreasing the resistance to anoikis and adherence to biologic substrates in ovarian cancer cells by targeting TrkB, a tyrosine kinase receptor [44]. Moreover, miR-200c overexpression enhanced anoikis sensitivity through the regulation of an NF-κB up-regulated TrkB/NTF3 autocrine signaling loop in triple-negative breast cancer [45]. All these data indicated that miRNAs as tumor suppressor genes and regulate the cancer-associated signaling axis, leading to abnormal anoikis of tumor cells.
As transmembrane receptors, integrins are well known as adhesion molecules, which mediate cell-ECM interaction and exert important roles in regulating anoikis resistance [46, 47]. The study by Sa et al. showed that overexpression of miR-124 attenuated the anoikis resistance in colorectal cancer cells by targeting integrin alpha 3 (ITGA3), a member of integrins [48]. In addition, miR-363-3p was found to suppress anoikis resistance of papillary thyroid carcinoma cells (B-CPAP cells) by negatively modulating its target gene integrin alpha 6 (ITGA6)[49]. Moreover, decreased in hepatocellular carcinoma tissues, miR-424-5p suppressed anoikis resistance in anchorage-independent hepatocellular carcinoma cells by targeting ICAT/CTNNBIP1, a potent b-catenin inhibitor [50]. MiR-10a, inversely correlated with distant metastasis and invasion depth of colorectal cancer, decreased cell adhesion and anoikis resistance activities by targeting matrix metalloproteinase 14 (MMP14) and actin gamma 1 (ACTG1). Furthermore, MMP14 is an ECM remodeling protein; while ACTG1 is involved in muscle contraction, cell motility, cell adhesion, and cell shape maintenance [51].
Autophagy, an evolutionarily conserved process, has been reported to be involved in the modulation of anoikis resistance in hepatocellular carcinoma [52, 53]. MiR-30a, decreased in hepatocellular carcinoma and cell lines, was proved to inhibit Beclin 1 and Atg5-dependent autophagy, and further suppress autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma cells [54].
Reactive oxygen species (ROS) is verified to participate in the anoikis resistance [55, 56]. N-acetyltransferase 1 (NAT1), a xenobiotic-metabolizing enzyme, has been unraveled to inhibit anoikis by suppressing ROS. Moreover, NAT1-mediated inhibitory effects on anoikis resistance were abolished by miR-6744-5p in both luminal A and triple-negative breast cancer cell lines [57].
Other mechanisms including cell cycle protein engaged in anoikis resistance of cancer cells. Zhang et al. reported that the suppression of miR-26a contributed to the anoikis resistance acquisition of esophageal adenocarcinoma cells. Also, the authors found that Rb1 was the direct target of miR-26a, and revealed that the reduction of miR-26a expression leads to increased Rb1 protein level and thus inhibits the function of E2F1, by which it influences the phenotypes of cell cycle and anoikis [58]. Guo and collaborators showed that the levels of miR-1827 were decreased in non-small cell lung cancer tumor tissues and cells, and were associated with tumor grade and lymph node metastasis. The upregulation of miR-1827 suppressed anchorage-independent growth and anoikis resistance of lung adenocarcinoma A549 cells through negatively regulating the expression of caveolin-1 (CAV-1)[59], an important regulator in anoikis resistance of cancer cells [60].
Impact of lncRNAs in anoikis resistance
LncRNAs are a type of ncRNA with > 200 nucleotides in length, which had not the ability of protein-encoding [12, 61, 62]. It has been shown that lncRNAs function as important tumor modulators and participate in cancer pathogenesis, such as cell growth, metastasis, stemness, and drug resistance [63,64,65]. An increasing body of research has suggested that dysregulation of lncRNAs played crucial roles in the modulation of anoikis resistance in various cancers [1].
LncRNAs act as positive regulators of anoikis resistance
Accumulating evidence documents that lncRNAs can also modulate anoikis resistance of cancer cells via regulating pathways, apoptosis-associated proteins, or other molecules (Table 3, Fig. 2).
The lncRNA HOTAIR, the HOX transcript antisense intergenic RNA, is upregulated and has been associated with poor prognosis, invasiveness, and aggressiveness of various cancer types [66, 67]. Small interfering RNA (siRNA)-mediated knockdown of HOTAIR expression markedly reduced the abilities of anoikis resistance, migration, and invasion in the ovarian cancer cells under the suspension condition. Moreover, HOTAIR enhanced the anoikis resistance and spheroid forming ability of ovarian cancer cells by recruiting EZH2 and influencing H3K27 methylation [68]. In hepatocellular carcinoma, HOTAIR was also reported to be able to promote the escape from anoikis through downregulating c-Met signaling [69]. In addition, HOTAIR silencing markedly decreased the anoikis resistance of gastric cancer cells [70]. Zhang and collaborators showed that lncRNA ANRIL was positively correlated with glioma grade. Silencing lncRNA ANRIL obviously attenuated anoikis resistance and induced cell cycle arrest in G0/G1 phase, while regulating the activity of caspase-3/8/9 and the AKT signaling pathway in glioma cells. Moreover, overexpression of miR-203a could partially reverse these functions [71].
The study by Lu et al. indicated that lncRNA APOC1P1-3 was upregulated in malignant cell lines of breast cancer and was negatively associated with the survival rate of patients with breast cancer. Also, lncRNA APOC1P1-3 significantly enhanced the capacity of anoikis resistance of breast cancer cells by specifically binding to miR-188-3p to block the inhibition of Bcl-2[72]. High lncRNA MALAT1 was shown to be associated with increased stage, recurrence, and reduced survival in ovarian cancer. The expression of lncRNA MALAT1 was markedly increased in multiple anoikis-resistant ovarian cancer cell lines. Moreover, lncRNA MALAT1 suppression resulted in decreased proliferation, invasion, anchorage-independent growth, and increased anoikis by regulating RBFOX2-mediated alternative splicing of the pro-apoptotic isoform of KIF1B [73]. These data suggest that lncRNA plays a key role in anoikis resistance by affecting the expression of apoptosis-associated proteins.
Interestingly, lncRNA VAL induced by AKT signaling was found to promote cell adhesion, invasion, and anoikis resistance in lung adenocarcinoma through directly binding to Vimentin and competitively abrogating Trim16-dependent Vimentin polyubiquitination and degradation [74]. LncRNA FOXD2-AS1, upregulated in thyroid carcinoma tissues and cells, promoted cancer stem cell-like phenotypes and enhanced the anoikis resistance in vitro by sponging miR-7-5p and up-regulating the expression of telomerase reverse transcriptase (TERT) [75]. Lastly, LINC00958 was identified to be significantly upregulated in bladder tumor samples compared with normal samples. In addition, LINC00958 knockdown significantly attenuated anoikis resistance of bladder cancer cells [76].
LncRNA acts as negative regulators of anoikis resistance
As a crucial signaling pathway in cancers, inactivation of Hippo signaling is found to contributes to tumor progression and metastasis [77, 78]. LncRNA-MAPK8IP1P2, decreased in the thyroid cancer tissues with lymphatic metastasis, inhibited anoikis resistance in vitro and lymphatic metastasis of thyroid cancer cells in vivo through the activation of Hippo signaling by sponging miR-146b-3p [79].
The role of circRNAs in anoikis resistance
CircRNAs are a kind of endogenous ncRNA that are single-stranded closed-loop RNA molecules lacking terminal 5' caps and 3' poly(A) tails [80, 81]. Emerging studies showed the key roles of circRNAs in regulating tumor progression [82,83,84], which also are involved in the regulation of anoikis resistance (Table 3, Fig. 2). CircSIPA1L1 was highly expressed in osteosarcoma tissue samples and cell lines. Knockdown circSIPA1L1 impaired the capacities of invasion, migration, proliferation, and survival of osteosarcoma cells by regulating the RAB9A signaling pathway via sponging miR-411-5p [85]. Yin et al. found that the expression of circUBAP2 was higher in lung cancer tissue samples than that in normal tissue samples. Moreover, circUBAP2 silencing obviously reduced the anoikis resistance of lung cancer cells [86].
Conclusions
Given that anoikis resistance is the hallmark of invasiveness, metastasis, therapy resistance, and relapse of cancer cells [7], anoikis resistance provides an attractive target for cancer therapeutic benefit. It has been reported that aberrant ncRNA expression is associated with anoikis resistance in several types of human cancers [1], suggesting that targeting ncRNA-based therapeutic strategies may obviously suppress anoikis resistance of tumor cells and further reduce the incidence of metastasis and recurrence. Anoikis resistance has been identified to be modulated by several factors including signaling pathways, cell adhesion, growth/apoptosis protein, oxidative stress, stemness, autophagy, and metabolic reprogramming [7]. Although ncRNAs exert the regulatory effects on anoikis resistance via multiple mechanisms, the role and mechanisms of ncRNA in the modulation of anoikis resistance remain to be resolved. For example, the Warburg effect, more specifically, diminished glucose oxidation, promoted anoikis resistance and metastasis in cancers [7, 87], but which and how ncRNA is involved in anoikis resistance by regulating the Warburg effect remains unclear. Hence, it is of great importance to in-depth investigate the role and mechanisms of ncRNA in anoikis resistance at the current stage. Based on our knowledge from the available literature, the role of miRNAs in anoikis resistance has been relatively demonstrated, but the effect of lncRNA and circRNA in the mechanism of anoikis resistance still largely stay in the early stage. For instance, only two circRNAs, circSIPA1L1 and circUBAP2, were verified to participate in the regulation of anoikis resistance in osteosarcoma or lung cancer [85, 86]. Therefore, further investigation is warranted to determine how specific lncRNA or circRNA influence the role of anoikis resistance in cancers. Although some problems remain for the relationship between ncRNAs and anoikis resistance, verify the detailed function and mechanism of ncRNA on anoikis resistance, which may contribute to a better understanding of cancer metastasis and provide new insights into the treatment of this disease.
Data availability
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
References
Lee HY, Son SW, Moeng S, Choi SY, Park JK (2021) The role of noncoding RNAs in the regulation of anoikis and anchorage-independent growth in cancer. Int J Mol Sci 22(2):627. https://doi.org/10.3390/ijms22020627
Tajbakhsh A, Rivandi M, Abedini S, Pasdar A, Sahebkar A (2019) Regulators and mechanisms of anoikis in triple-negative breast cancer (TNBC): a review. Crit Rev Oncol Hematol 140:17–27. https://doi.org/10.1016/j.critrevonc.2019.05.009
Sakamoto S, Kyprianou N (2010) Targeting anoikis resistance in prostate cancer metastasis. Mol Aspects Med 31:205–214. https://doi.org/10.1016/j.mam.2010.02.001
Simpson C, Anyiwe K, Schimmer A (2008) Anoikis resistance and tumor metastasis. Cancer Lett 272:177–185. https://doi.org/10.1016/j.canlet.2008.05.029
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochem Biophys Acta 1833:3481–3498. https://doi.org/10.1016/j.bbamcr.2013.06.026
Coates JM, Galante JM, Bold RJ (2010) Cancer therapy beyond apoptosis: autophagy and anoikis as mechanisms of cell death. J Surg Res 164:301–308. https://doi.org/10.1016/j.jss.2009.07.011
Adeshakin FO, Adeshakin AO, Afolabi LO, Yan D, Zhang G, Wan X (2021) Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Front Oncol 11:626577. https://doi.org/10.3389/fonc.2021.626577
Guha D, Saha T, Bose S, Chakraborty S, Dhar S, Khan P, Adhikary A, Das T, Sa G (2019) Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis 24:958–971. https://doi.org/10.1007/s10495-019-01573-5
Satyavarapu EM, Das R, Mandal C, Mukhopadhyay A, Mandal C (2018) Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis 9:934. https://doi.org/10.1038/s41419-018-0989-8
Wang YN, Zeng ZL, Lu J, Wang Y, Liu ZX, He MM, Zhao Q, Wang ZX, Li T, Lu YX, Wu QN, Yu K, Wang F, Pu HY, Li B, Jia WH, Shi M, Xie D, Kang TB, Huang P, Ju HQ, Xu RH (2018) CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 37:6025–6040. https://doi.org/10.1038/s41388-018-0384-z
Mattick JS, Makunin IV (2006) Non-coding RNA. Hum Mol Genet 15(Suppl 1):R17–R29. https://doi.org/10.1093/hmg/ddl046
Panni S, Lovering RC, Porras P, Orchard S (2020) Non-coding RNA regulatory networks. Biochim Biophys Acta Gene Regul Mech 1863:194417. https://doi.org/10.1016/j.bbagrm.2019.194417
Zhang P, Wu W, Chen Q, Chen M (2019) Non-coding RNAs and their integrated networks. J Integr Bioinform 16(3):20190027. https://doi.org/10.1515/jib-2019-0027
Anastasiadou E, Jacob LS, Slack FJ (2018) Non-coding RNA networks in cancer. Nat Rev Cancer 18:5–18. https://doi.org/10.1038/nrc.2017.99
Hausser J, Zavolan M (2014) Identification and consequences of miRNA-target interactions–beyond repression of gene expression. Nat Rev Genet 15:599–612. https://doi.org/10.1038/nrg3765
Rupaimoole R, Calin G, Lopez-Berestein G, Sood A (2016) miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov 6:235–246. https://doi.org/10.1158/2159-8290.cd-15-0893
O’Connell R, Rao D, Chaudhuri A, Baltimore D (2010) Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10:111–122. https://doi.org/10.1038/nri2708
Redis R, Calin S, Yang Y, You M, Calin G (2012) Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther 136:169–174. https://doi.org/10.1016/j.pharmthera.2012.08.003
Marengo B, Pulliero A, Izzotti A, Domenicotti C (2020) miRNA regulation of glutathione homeostasis in cancer initiation, progression and therapy resistance. MicroRNA (Shariqah, United Arab Emirates) 9:187–197. https://doi.org/10.2174/2211536609666191218103220
Altschuler J, Stockert JA, Kyprianou N (2021) Non-coding RNAs set a new phenotypic frontier in prostate cancer metastasis and resistance. Int J Mol Sci 22(4):2100. https://doi.org/10.3390/ijms22042100
Liu X, Fu Y, Zhang G, Zhang D, Liang N, Li F, Li C, Sui C, Jiang J, Lu H, Zhao Z, Dionigi G, Sun H (2019) miR-424-5p promotes anoikis resistance and lung metastasis by inactivating hippo signaling in thyroid cancer. Mol Ther Oncolytics 15:248–260. https://doi.org/10.1016/j.omto.2019.10.008
Yu SJ, Hu JY, Kuang XY, Luo JM, Hou YF, Di GH, Wu J, Shen ZZ, Song HY, Shao ZM (2013) MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin Cancer Res 19:1389–1399. https://doi.org/10.1158/1078-0432.CCR-12-1959
Mak CS, Yung MM, Hui LM, Leung LL, Liang R, Chen K, Liu SS, Qin Y, Leung TH, Lee KF, Chan KK, Ngan HY, Chan DW (2017) MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol Cancer 16:11. https://doi.org/10.1186/s12943-017-0582-2
Sun Q, Yang Z, Li P, Wang X, Sun L, Wang S, Liu M, Tang H (2019) A novel miRNA identified in GRSF1 complex drives the metastasis via the PIK3R3/AKT/NF-kappaB and TIMP3/MMP9 pathways in cervical cancer cells. Cell Death Dis 10:636. https://doi.org/10.1038/s41419-019-1841-5
Hida K, Kawamoto T, Maishi N, Morimoto M, Akiyama K, Ohga N, Shindoh M, Shinohara N, Hida Y (2017) miR-145 promoted anoikis resistance in tumor endothelial cells. J Biochem 162:81–84. https://doi.org/10.1093/jb/mvx033
Zhong X, Rescorla F (2012) Cell surface adhesion molecules and adhesion-initiated signaling: understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell Signal 24:393–401. https://doi.org/10.1016/j.cellsig.2011.10.005
Nagaprashantha L, Vatsyayan R, Lelsani P, Awasthi S, Singhal S (2011) The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int J Cancer 128:743–752. https://doi.org/10.1002/ijc.25725
Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De Pitta C, Pinatel E, Stadler MB, Provero P, Bernengo MG, Osman I, Taverna D (2011) microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J 30:1990–2007. https://doi.org/10.1038/emboj.2011.102
Derouet MF, Liu G, Darling GE (2014) MiR-145 expression accelerates esophageal adenocarcinoma progression by enhancing cell invasion and anoikis resistance. PLoS ONE 9:e115589. https://doi.org/10.1371/journal.pone.0115589
Derouet MF, Dakpo E, Wu L, Zehong G, Conner J, Keshavjee S, de Perrot M, Waddell T, Elimova E, Yeung J, Darling GE (2018) miR-145 expression enhances integrin expression in SK-GT-4 cell line by down-regulating c-Myc expression. Oncotarget 9:15198–15207. https://doi.org/10.18632/oncotarget.24613
Yang J, Zheng Z, Yan X, Li X, Liu Z, Ma Z (2013) Integration of autophagy and anoikis resistance in solid tumors. Anat Rec 296:1501–1508. https://doi.org/10.1002/ar.22769
Taylor MA, Sossey-Alaoui K, Thompson CL, Danielpour D, Schiemann WP (2013) TGF-beta upregulates miR-181a expression to promote breast cancer metastasis. J Clin Invest 123:150–163. https://doi.org/10.1172/JCI64946
Zhao MY, Wang LM, Liu J, Huang X, Liu J, Zhang YF (2018) MiR-21 Suppresses anoikis through targeting PDCD4 and PTEN in human esophageal adenocarcinoma. Curr Med Sci 38:245–251. https://doi.org/10.1007/s11596-018-1872-7
Chen M, Liu LX (2020) MiR-525-5p repressed metastasis and anoikis resistance in cervical cancer via blocking UBE2C/ZEB1/2 signal axis. Dig Dis Sci 65:2442–2451. https://doi.org/10.1007/s10620-019-05916-9
Li L, He Z, Zhu C, Chen S, Yang Z, Xu J, Bi N, Yu C, Sun C (2020) MiR-137 promotes anoikis through modulating the AKT signaling pathways in pancreatic cancer. J Cancer 11:6277–6285. https://doi.org/10.7150/jca.44037
Zhang X, Li Z, Xuan Z, Xu P, Wang W, Chen Z, Wang S, Sun G, Xu J, Xu Z (2018) Novel role of miR-133a-3p in repressing gastric cancer growth and metastasis via blocking autophagy-mediated glutaminolysis. J Exp Clin Cancer Res 37:320. https://doi.org/10.1186/s13046-018-0993-y
Gao G, Tian Z, Zhu H, Ouyang X (2018) miRNA-133b targets FGFR1 and presents multiple tumor suppressor activities in osteosarcoma. Cancer Cell Int 18:210. https://doi.org/10.1186/s12935-018-0696-7
Tang Y, Pan J, Huang S, Peng X, Zou X, Luo Y, Ren D, Zhang X, Li R, He P, Wa Q (2018) Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J Exp Clin Cancer Res 37:160. https://doi.org/10.1186/s13046-018-0813-4
Zhu JF, Liu Y, Huang H, Shan L, Han ZG, Liu JY, Li YL, Dong X, Zeng W (2018) MicroRNA-133b/EGFR axis regulates esophageal squamous cell carcinoma metastases by suppressing anoikis resistance and anchorage-independent growth. Cancer Cell Int 18:193. https://doi.org/10.1186/s12935-018-0684-y
Zhang L, Wang X, Chen P (2013) MiR-204 down regulates SIRT1 and reverts SIRT1-induced epithelial-mesenchymal transition, anoikis resistance and invasion in gastric cancer cells. BMC Cancer 13:290. https://doi.org/10.1186/1471-2407-13-290
Yan H, Wu W, Ge H, Li P, Wang Z (2015) Up-regulation of miR-204 enhances anoikis sensitivity in epithelial ovarian cancer cell line via brain-derived neurotrophic factor pathway in vitro. Int J Gynecol Cancer 25:944–952. https://doi.org/10.1097/IGC.0000000000000456
Lin CH, Wang HH, Chen TH, Chiang MC, Hung PH, Chen YJ (2020) Involvement of microRNA-296 in the inhibitory effect of epigallocatechin gallate against the migratory properties of anoikis-resistant nasopharyngeal carcinoma cells. Cancers (Basel) 12(4):973. https://doi.org/10.3390/cancers12040973
Howe EN, Cochrane DR, Richer JK (2011) Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res 13:R45. https://doi.org/10.1186/bcr2867
Cittelly DM, Dimitrova I, Howe EN, Cochrane DR, Jean A, Spoelstra NS, Post MD, Lu X, Broaddus RR, Spillman MA, Richer JK (2012) Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol Cancer Ther 11:2556–2565. https://doi.org/10.1158/1535-7163.MCT-12-0463
Howe EN, Cochrane DR, Cittelly DM, Richer JK (2012) miR-200c targets a NF-kappaB up-regulated TrkB/NTF3 autocrine signaling loop to enhance anoikis sensitivity in triple negative breast cancer. PLoS ONE 7:e49987. https://doi.org/10.1371/journal.pone.0049987
Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, Goud B, Ivaska J (2015) Integrin endosomal signalling suppresses anoikis. Nat Cell Biol 17:1412–1421. https://doi.org/10.1038/ncb3250
Toricelli M, Melo F, Peres G, Silva D, Jasiulionis M (2013) Timp1 interacts with beta-1 integrin and CD63 along melanoma genesis and confers anoikis resistance by activating PI3-K signaling pathway independently of Akt phosphorylation. Mol Cancer 12:22. https://doi.org/10.1186/1476-4598-12-22
Sa KD, Zhang X, Li XF, Gu ZP, Yang AG, Zhang R, Li JP, Sun JY (2018) A miR-124/ITGA3 axis contributes to colorectal cancer metastasis by regulating anoikis susceptibility. Biochem Biophys Res Commun 501:758–764. https://doi.org/10.1016/j.bbrc.2018.05.062
Pan Y, Zhu X, Wang K, Chen Y (2019) MicroRNA-363-3p suppresses anoikis resistance in human papillary thyroid carcinoma via targeting integrin alpha 6. Acta Biochim Biophys Sin (Shanghai) 51:807–813. https://doi.org/10.1093/abbs/gmz066
Zhang Y, Li T, Guo P, Kang J, Wei Q, Jia X, Zhao W, Huai W, Qiu Y, Sun L, Han L (2014) MiR-424-5p reversed epithelial-mesenchymal transition of anchorage-independent HCC cells by directly targeting ICAT and suppressed HCC progression. Sci Rep 4:6248. https://doi.org/10.1038/srep06248
Liu Y, Zhang Y, Wu H, Li Y, Zhang Y, Liu M, Li X, Tang H (2017) miR-10a suppresses colorectal cancer metastasis by modulating the epithelial-to-mesenchymal transition and anoikis. Cell Death Dis 8:e2739. https://doi.org/10.1038/cddis.2017.61
Talukdar S, Pradhan A, Bhoopathi P, Shen X, August L, Windle J, Sarkar D, Furnari F, Cavenee W, Das S, Emdad L, Fisher P (2018) MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc Natl Acad Sci USA 115:5768–5773. https://doi.org/10.1073/pnas.1721650115
Peng Y, Shi Y, Ding Z, Ke A, Gu C, Hui B, Zhou J, Qiu S, Dai Z, Fan J (2013) Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy 9:2056–2068. https://doi.org/10.4161/auto.26398
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, Shi GM, Gao Q, Wang XY, Song K, Fan J, Ding ZB (2018) MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett 412:108–117. https://doi.org/10.1016/j.canlet.2017.10.012
Monteiro H, Rodrigues E, Amorim Reis A, Longo L, Ogata F, Moretti A, da Costa P, Teodoro A, Toledo M, Stern A (2019) Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: a redox signaling perspective. Nitric Oxide Biol Chem 89:1–13. https://doi.org/10.1016/j.niox.2019.04.009
Du S, Miao J, Zhu Z, Xu E, Shi L, Ai S, Wang F, Kang X, Chen H, Lu X, Guan W, Xia X (2018) NADPH oxidase 4 regulates anoikis resistance of gastric cancer cells through the generation of reactive oxygen species and the induction of EGFR. Cell Death Dis 9:948. https://doi.org/10.1038/s41419-018-0953-7
Malagobadan S, Ho CS, Nagoor NH (2020) MicroRNA-6744-5p promotes anoikis in breast cancer and directly targets NAT1 enzyme. Cancer Biol Med 17:101–111. https://doi.org/10.20892/j.issn.2095-3941.2019.0010
Zhang YF, Zhang AR, Zhang BC, Rao ZG, Gao JF, Lv MH, Wu YY, Wang SM, Wang RQ, Fang DC (2013) MiR-26a regulates cell cycle and anoikis of human esophageal adenocarcinoma cells through Rb1-E2F1 signaling pathway. Mol Biol Rep 40:1711–1720. https://doi.org/10.1007/s11033-012-2222-7
Guo X, Wang Z, Sun Q, Sun C, Hua H, Huang Q (2020) The inhibitory effect of microRNA-1827 on anoikis resistance in lung adenocarcinoma A549 cells via targeting caveolin-1. Acta Biochim Biophys Sin (Shanghai) 52:1148–1155. https://doi.org/10.1093/abbs/gmaa102
Chunhacha P, Chanvorachote P (2012) Roles of caveolin-1 on anoikis resistance in non small cell lung cancer. Int J Physiol Pathophysiol Pharmacol 4:149–155
Chi Y, Wang D, Wang J, Yu W, Yang J (2019) Long non-coding RNA in the pathogenesis of cancers. Cells 8:1015. https://doi.org/10.3390/cells8091015
Peng WX, Koirala P, Mo YY (2017) LncRNA-mediated regulation of cell signaling in cancer. Oncogene 36:5661–5667. https://doi.org/10.1038/onc.2017.184
Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T (2018) Emerging roles of long non-coding RNA in cancer. Cancer Sci 109:2093–2100. https://doi.org/10.1111/cas.13642
Prabhu K, Raza A, Karedath T, Raza S, Fathima H, Ahmed E, Kuttikrishnan S, Therachiyil L, Kulinski M, Dermime S, Junejo K, Steinhoff M, Uddin S (2020) Non-coding RNAs as regulators and markers for targeting of breast cancer and cancer stem cells. Cancers 12(2):351. https://doi.org/10.3390/cancers12020351
Yue J, Wu Y, Qiu L, Zhao R, Jiang M, Zhang H (2021) LncRNAs link cancer stemness to therapy resistance. Am J Cancer Res 11:1051–1068
Liu X, Liu Z, Sun M, Liu J, Wang Z, De W (2013) The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 13:464. https://doi.org/10.1186/1471-2407-13-464
Tan S, Pastori C, Penas C, Komotar R, Ivan M, Wahlestedt C, Ayad N (2018) Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer 17:74. https://doi.org/10.1186/s12943-018-0822-0
Dai ZY, Jin SM, Luo HQ, Leng HL, Fang JD (2021) LncRNA HOTAIR regulates anoikis-resistance capacity and spheroid formation of ovarian cancer cells by recruiting EZH2 and influencing H3K27 methylation. Neoplasma. https://doi.org/10.4149/neo_2021_201112N1212
Topel H, Bagirsakci E, Comez D, Bagci G, Cakan-Akdogan G, Atabey N (2020) lncRNA HOTAIR overexpression induced downregulation of c-Met signaling promotes hybrid epithelial/mesenchymal phenotype in hepatocellular carcinoma cells. Cell Commun Signal 18:110. https://doi.org/10.1186/s12964-020-00602-0
Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, Goel A (2014) Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis 35:2731–2739. https://doi.org/10.1093/carcin/bgu200
Dai W, Tian C, Jin S (2018) Effect of lncRNA ANRIL silencing on anoikis and cell cycle in human glioma via microRNA-203a. Onco Targets Ther 11:5103–5109. https://doi.org/10.2147/OTT.S169809
Lu Q, Wang L, Gao Y, Zhu P, Li L, Wang X, Jin Y, Zhi X, Yu J, Li X, Qin X, Zhou P (2021) lncRNA APOC1P1-3 promoting anoikis-resistance of breast cancer cells. Cancer Cell Int 21:232. https://doi.org/10.1186/s12935-021-01916-w
Gordon MA, Babbs B, Cochrane DR, Bitler BG, Richer JK (2019) The long non-coding RNA MALAT1 promotes ovarian cancer progression by regulating RBFOX2-mediated alternative splicing. Mol Carcinog 58:196–205. https://doi.org/10.1002/mc.22919
Tian H, Lian R, Li Y, Liu C, Liang S, Li W, Tao T, Wu X, Ye Y, Yang X, Han J, Chen X, Li J, He Y, Li M, Wu J, Cai J (2020) AKT-induced lncRNA VAL promotes EMT-independent metastasis through diminishing Trim16-dependent Vimentin degradation. Nat Commun 11:5127. https://doi.org/10.1038/s41467-020-18929-0
Liu X, Fu Q, Li S, Liang N, Li F, Li C, Sui C, Dionigi G, Sun H (2019) LncRNA FOXD2-AS1 functions as a competing endogenous RNA to regulate TERT expression by sponging miR-7-5p in thyroid cancer. Front Endocrinol (Lausanne) 10:207. https://doi.org/10.3389/fendo.2019.00207
Seitz AK, Christensen LL, Christensen E, Faarkrog K, Ostenfeld MS, Hedegaard J, Nordentoft I, Nielsen MM, Palmfeldt J, Thomson M, Jensen MT, Nawroth R, Maurer T, Orntoft TF, Jensen JB, Damgaard CK, Dyrskjot L (2017) Profiling of long non-coding RNAs identifies LINC00958 and LINC01296 as candidate oncogenes in bladder cancer. Sci Rep 7:395. https://doi.org/10.1038/s41598-017-00327-0
Dey A, Varelas X, Guan K (2020) Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discovery 19:480–494. https://doi.org/10.1038/s41573-020-0070-z
Calses P, Crawford J, Lill J, Dey A (2019) Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends in cancer 5:297–307. https://doi.org/10.1016/j.trecan.2019.04.001
Liu X, Fu Q, Bian X, Fu Y, Xin J, Liang N, Li S, Zhao Y, Fang L, Li C, Zhang J, Dionigi G, Sun H (2020) Long non-coding RNA MAPK8IP1P2 inhibits lymphatic metastasis of thyroid cancer by activating hippo signaling via sponging miR-146b-3p. Front Oncol 10:600927. https://doi.org/10.3389/fonc.2020.600927
Zhang C, Ding R, Sun Y, Huo ST, He A, Wen C, Chen H, Du WW, Lai W, Wang H (2021) Circular RNA in tumor metastasis. Mol Therap—Nucl Acids 23:1243–1257. https://doi.org/10.1016/j.omtn.2021.01.032
Yang X, Ye T, Liu H, Lv P, Duan C, Wu X, Jiang K, Lu H, Xia D, Peng E, Chen Z, Tang K, Ye Z (2021) Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer. Mol Cancer 20:4. https://doi.org/10.1186/s12943-020-01300-8
Chen S, Chen C, Hu Y, Song G, Shen X (2021) The diverse roles of circular RNAs in pancreatic cancer. Pharmacol Ther 226:107869. https://doi.org/10.1016/j.pharmthera.2021.107869
Long F, Lin Z, Li L, Ma M, Lu Z, Jing L, Li X, Lin C (2021) Comprehensive landscape and future perspectives of circular RNAs in colorectal cancer. Mol Cancer 20:26. https://doi.org/10.1186/s12943-021-01318-6
Ma S, Kong S, Wang F, Ju S (2020) CircRNAs: biogenesis, functions, and role in drug-resistant tumours. Mol Cancer 19:119. https://doi.org/10.1186/s12943-020-01231-4
Xu Y, Yao T, Ni H, Zheng R, Huang K, Huang Y, Gao J, Qiao D, Shen S, Ma J (2021) Circular RNA circSIPA1L1 contributes to osteosarcoma progression through the miR-411-5p/RAB9A signaling pathway. Front Cell Dev Biol 9:642605. https://doi.org/10.3389/fcell.2021.642605
Yin Y, Gao H, Guo J, Gao Y (2017) Effect of circular RNA UBAP2 silencing on proliferation and invasion of human lung cancer A549 cells and its mechanism. Zhongguo Fei Ai Za Zhi 20:800–807. https://doi.org/10.3779/j.issn.1009-3419.2017.12.02
Lu J, Tan M, Cai Q (2015) The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 356:156–164. https://doi.org/10.1016/j.canlet.2014.04.001
Penna E, Orso F, Cimino D, Vercellino I, Grassi E, Quaglino E, Turco E, Taverna D (2013) miR-214 coordinates melanoma progression by upregulating ALCAM through TFAP2 and miR-148b downmodulation. Cancer Res 73:4098–4111. https://doi.org/10.1158/0008-5472.CAN-12-3686
Acknowledgements
This research was funded by Suzhou Science & Technology plan project, Grant Number SYS2019035 and Wujiang Science & education revitalizing health project, Grant Number WWK201921.
Funding
This research was funded by Suzhou Science & Technology Plan Project, Grant Number SYS2019035 and Wujiang Science & Education Revitalizing Health Project, Grant Number WWK201921.
Author information
Authors and Affiliations
Contributions
TS, CZ, and SX performed and wrote articles, TS and CZ modified the language, TS and SX designed the articles.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, T., Zhang, C. & Xia, S. The potential roles and mechanisms of non-coding RNAs in cancer anoikis resistance. Mol Cell Biochem 477, 1371–1380 (2022). https://doi.org/10.1007/s11010-022-04384-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04384-6