Abstract
The heart is a very dynamic pumping organ working perpetually to maintain a constant blood supply to the whole body to transport oxygen and nutrients. Unfortunately, it is also subjected to various stresses based on physiological or pathological conditions, particularly more vulnerable to damages caused by oxidative stress. In this study, we investigate the molecular mechanism and contribution of IGF-IIRα in endoplasmic reticulum stress induction in the heart under doxorubicin-induced cardiotoxicity. Using in vitro H9c2 cells, in vivo transgenic rat cardiac tissues, siRNAs against CHOP, chemical ER chaperone PBA, and western blot experiments, we found that IGF-IIRα overexpression enhanced ER stress markers ATF4, ATF6, IRE1α, and PERK which were further aggravated by DOX treatment. This was accompanied by a significant perturbation in stress-associated MAPKs such as p38 and JNK. Interestingly, PARKIN, a stress responsive cellular protective mediator was significantly downregulated by IGF-IIRα concomitant with decreased expression of ER chaperone GRP78. Furthermore, ER stress-associated pro-apoptotic factor CHOP was increased considerably in a dose-dependent manner followed by elevated c-caspase-12 and c-caspase-3 activities. Conversely, treatment of H9c2 cells with chemical ER chaperone PBA or siRNA against CHOP abolished the IGF-IIRα-induced ER stress responses. Altogether, these findings suggested that IGF-IIRα contributes to ER stress induction and inhibits cellular stress coping proteins while increasing pro-apoptotic factors feeding into a cardio myocyte damage program that eventually paves the way to heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoplasmic reticulum (ER) is an important hub for regulating cellular protein homeostasis during pathophysiological processes. Based on recent literatures, it is evident that ER stress is involved in the development and progression of cardiovascular diseases including cardiac hypertrophy, ischemic heart diseases, and heart failure [1]. While others have reported that chronic ER stress acts as a crucial factor in the placenta contributing to the pathophysiology of pre-eclampsia or fetal growth restriction. However, recent studies have suggested for a mutually contrasting association between ER stress and autophagy in that ER stress can either stimulate or inhibit autophagy in a context-dependent manner [2, 3]. During conditions of increased protein synthesis, alterations in the redox status, or disturbances in calcium storage, ER stress is triggered. Three transmembrane ER stress sensors, namely activating transcription factor 6 (ATF6), inositol‑requiring enzyme 1 (IRE1), and double‑stranded RNA‑activated protein kinase‑like ER kinase (PERK), are activated to restore ER homeostasis through an unfolded protein response (UPR) via triggering downstream signaling pathways [4, 5], while, if ER stress is not attenuated, the UPR switches to a pro‑apoptotic response. The resultant activation of pro-apoptotic proteins, such as C/EBP homologous protein (CHOP), also known as GADD153 (CHOP/GADD153) and caspase‑12 ultimately leads to cell death [6,7,8]. Doxorubicin (DOX) is used for treatment of various malignant cancers, but its uses have been hindered by dose-dependent and cumulative cardiotoxicity, primarily in pediatric cancer patient that surface more than a few years following culmination of treatment. Multifactorial mechanisms such as accumulation of reactive oxygen species (ROS), mitochondrial destruction, impaired autophagy, epigenetic changes, DNA damage, and transcriptome alterations have been implicated in DOX-induced cardiomyopathy [9,10,11,12,13]. However, the role of ER stress in DOX-induced cardiotoxicity has been less understood despite indications that ER stress plays a key role in DOX-induced cardiac damage and apoptosis [14, 15]. Some studies have reported diastolic calcium leakage from ER with elevated levels in cytosol and depletion of ER calcium stores as one of the early events in DOX cardiotoxicity [16, 17]. GRP78, which is a key modulator of UPR in ER stress, regulates calcium homeostasis and mobilization from the ER to mitochondria via its interaction with the Phosphoinositol-3 Receptor (IP3R) [18]. Intriguingly, DOX treatment impaired the protective ER stress response and that refurbishment of GRP78 expression ameliorated ER stress-induced cytotoxicity after DOX treatment [19]. Previous studies identified a novel stress-inducible protein IGF-IIRα (150 kDa) whose molecular and cellular characteristics were not completely defined. Besides, this alternative splicing isoform was in concordance to full-length IGF-IIR (300 kDa) except the C-terminal 15 amino acids. IGF-IIRα was indicated to involve in cardiac pathological hypertrophy changes under high salt-induced cardiac stresses [20]. However, the contribution of IGF-IIRα in cardiac ER stress conditions has not been investigated.
Our earlier studies showed that upregulation of IGF-IIRα enhanced DOX-induced cardiac oxidative stresses and led to cardiomyocyte apoptosis [21]. The increased expression of IGF-IIRα during cardiac stresses perturbed mitochondrial autophagy and cardiac apoptosis causing heart function impairment. However, it was noted that IGF-IIRα not only altered mitochondrial autophagy processes, but also impaired the expressions of PARKIN which is a key player in removal of defective mitochondria [22]. Interestingly, several others have suggested that CHOP, an ER stress-associated apoptotic factor, and PARKIN have inverse correlations during cardiac stresses. PARKIN was identified to blunt excessive CHOP levels to avert maladaptive ER stress-induced cell death and adverse cardiac ventricular remodeling changes [23]. In this study, we employed both in vitro and in vivo approaches to examine the molecular mechanisms and contributions of IGF-IIRα in the ER stress induction and ensuing cardiac damages.
Methodology
Cell culture
H9c2 cells were acquired from American-Type Culture Collection (ATCC, USA) and maintained as described before [21]. In brief, cells were grown in DMEM medium supplemented with glutamine (2 mM), streptomycin (100 mg/ml), penicillin (100 U/ml), pyruvate (1 mM), and 10% fetal bovine serum while incubated in humidified air incubator (5% CO2) at 37 °C. Overexpression plasmids, IGF-IIRα-pEGFP, as generated earlier [22] or siRNA against CHOP (Thermo Fisher Scientific, s218107) were transfected using jetPRIME reagent (Polyplus-transfection S.A., France) as per the manufacturer’s recommendations.
Animal tissues
The transgenic rat animal was maintained as per the protocol from National Laboratory Animal Center (NLAC). The protocols were approved by the Institutional Animal Care and Use Committee of China Medical University (Protocol number: 2016-065-1) and confirmed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The transgenic animals were generated and confirmed as described previously [20,21,22]. Briefly, the pronuclear microinjections were performed to insert full-length IGF-IIRα-pcDNA3.1-myc-His construct driven by α-MHC cardiac-specific promoter, and transgenic animals were confirmed by PCR genotyping and western blot. All wild-type (WT) Sprague Dawley (SD) rats and transgenic rats [SD-TG (IGF-IIRα)] were housed at a constant temperature (22 °C) on a 12-h light/dark cycle with food and tap water. The cardiac tissue samples were collected at the end of designated treatments and stored at − 80 °C [21, 22].
Chemicals and antibodies
All the chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. Doxorubicin was dissolved in dimethyl sulfoxide and aliquot stored at − 20 °C for further experiments. The following antibodies were used in this study: Parkin (H-300) (sc-30130), ATF4 (D4B8) (#11815), ATF-6α (F-7) (sc-166659), CHOP (L63F7) (#2895), IRE1α (B-12) (sc-390960), GRP78 (N-20) (sc-1050), p-eIF2α (Ser51) (#9721), HA-Tag (HA-ChIP) (Abcam, ab9110), GFP (FL) (sc-8334), cleaved caspase-3 (Asp175) (5A1) (Cell signaling, #9664), p-JNK (G-7) (sc-6254), GAPDH (sc-47724), Tubulin (sc-5286), and β-Actin (sc-8432). All the secondary antibodies (anti-rabbit, mouse and goat, HRP conjugated) were purchased from Santa Cruz Biotechnology unless otherwise noted.
Western blot
Western blot (WB) analysis was performed as described previously [24, 25] with minor modifications. Lysis buffer containing Tris-base (pH 7.4, 50 mM/L), EDTA (1 M/L), NaCl (0.5 M/L), beta-mercaptoethanol (1 mM/L), NP-40 (1%), IGEPAL CA-630 (Sigma-Aldrich), 10% glycerol, and protease inhibitor cocktail tablets (Roche) were used to harvest cellular protein extracts. Cell lysates were centrifuged at 16,000 rpm for 30 min, and protein supernatants were quantified using Bradford assay (Bio-Rad). Protein samples were resolved using SDS-PAGE (8–12%) and then blotted onto PVDF membranes (Millipore). 5% skimmed milk (Tris-buffered saline-Tween-20) powder was used for non-specific blocking of proteins for at RT for 1 h. Primary antibodies (1:1000) at 4 °C overnight incubation followed with secondary antibodies at RT for 1 h were used to probe specific proteins and detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA). Membrane stripping was performed as and when required using Restore Western Blot Stripping Buffer (Pierce, IO) and re-probed with other antibodies. Immunoblots were analyzed densitometrically using KETA C chemiluminescence system & Magic Chemi software (Taiwan) and quantified using Image J software (NIH, MD, USA).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
Apoptotic cells were detected using TUNEL Assay (In situ cell death detection kit, Roche, Mannheim, Germany) as described previously [26]. The cardiac tissue Sections (0.2 µm) were obtained as reported in our previous study [22] and deparaffinized by immersion in xylene, rehydrated in a graded series ethanol, and added with 3% H2O2 to inactivate endogenous peroxidases. This was followed by Proteinase K (20 µg/mL) (ZYMED, CA) treatment for 30 min, rinsed with PBS for 5 min twice. Permeabilization was done using sodium citrate buffer (containing 0.1% Triton X-100) for 15 min followed by PBS wash for 5 min twice. Apoptotic cells were detected using TUNEL fluorescence reagent and counterstained with DAPI (4′, 6-diamidine-2-phenylindole dihydrochloride) for nuclei and followed by fluorescence microscopy (Olympus, Tokyo, Japan).
Immunohistochemistry
The cardiac tissue samples were obtained as described in our previous study [22] and tissue section slides were subjected to immunohistochemical staining. Tissue Sections (0.2 µm) were dried at 58 °C overnight, then subjected to deparaffinization in xylene, followed by rehydration in a graded series of ethanol (95%, 90%, 80%, 70%, 60%). Endogenous peroxidase activity was deactivated using 3% H2O2 for 10 min. Next, rinsed with water for 15 min followed with microwave treatment in citrate buffer for 15 min, the non-specific blocking was done using 2.5% horse serum in PBS-T for 15 min, followed with primary antibody (1:100) incubation for 2 h, rinsing with PBS, and incubating with secondary antibody for 30 min at RT. Next, slides were rinsed with PBS and streptavidin-HRP conjugate was added for 30 min. Further, slides were rinsed with PBS as before and chromogenic substrate diaminobenzidine (DAB) was added and incubated for 1–3 min at RT. Finally, slides were rinsed with PBS, air dried, mounted using VectaMount™ (H-5000), and imaged using microscope (Olympus, Tokyo, Japan).
Statistical analysis
All experimental data were expressed as the mean ± SD from at least three independent experiments. Pairwise statistical comparisons were performed using one-way ANOVA using SPSS 16 software. Differences were considered statistically significant when P < 0.05.
Results
IGF-IIRα leads to ER stress induction in H9c2 cells
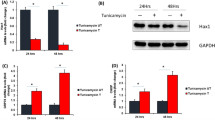
To begin to delineate the role of IGF-IIRα in ER stress induction in heart, we found an increased expression of ER stress markers PERK, ATF4, ATF6, and IRE1 concomitant with IGF-IIRα overexpression. However, DOX treatment enhanced this genetic perturbation resulting in synergistic upregulation of ATF4 and PERK, while interestingly, ATF6 and IRE1 did not exhibit a combinatorial effect (Fig. 1A). Besides, protein translation initiation factor p-eIF2α (Ser51) exhibited a positive modulation, while ER chaperone GRP78 was downregulated by IGF-IIRα. There was dose-dependent increase in the expression of ER stress-associated pro-apoptotic factor CHOP, while PARKIN, an E3 ubiquitin ligase, prominently linked with removal of damaged mitochondria, showed an opposite trend to CHOP expression (Fig. 1B). These results suggested that IGF-IIRα contributes to ER stress induction in H9c2 cells.
IGF-IIRα leads to ER stress induction in H9c2 cells. A H9c2 cells were transfected with IGF-IIRα-pEGFP plasmid construct for 24 h and then subjected to DOX (1 µM) treatment for another 24 h. At the end of 48 h, cellular protein extracts were analyzed by WB technique. Representative images showing protein expression of ER stress-associated markers PERK, ATF4 (D4B8), ATF6α (F-7), IRE1α (B-12), eIF2α (pSer51), GRP78, and their respective quantifications. Comparison groups were as follows: DOX alone treatment vs no treatment; IGF-IIRα + DOX treatment vs DOX alone, respectively; n = 3; *p < 0.05; **p < 0.01; B Increasing concentrations of IGF-IIRα-pEGFP plasmids (2.5 µg, 5.0 µg, 10.0 µg) were transfected into H9c2 cells for 24 h. Cellular protein lysates were harvested and subjected to WB analysis. Protein expression of CHOP and PARKIN showed opposite correlations in a dose-dependent manner
IGF-IIRα-induced ER stress contributed to cardiac myocytes apoptosis
The above observations suggested the involvement of IGF-IIRα in ER stress generation in H9c2 cells, so we were tempted to elucidate its downstream cellular impact. We found that IGF-IIRα leads to increased expression of ER stress-associated pro-apoptosis factors. ER resident apoptotic caspase-12 together with c-caspase-3 activity were upregulated. Besides, CHOP that acts to decrease the Bcl2/ Bax ratio and induce apoptosis under cellular stress conditions was also prominently enhanced (Fig. 2A). Interestingly, we found that IGF-IIRα has a synergistic consequence to DOX-induced cardiomyocyte damage that was indicated by enhanced apoptotic effects. Besides, we also determined the expression pattern of cellular stress-associated MAPKs such as p-p38 and p-JNK that exhibited similar results as above, while PARKIN underwent a suppression (Fig. 2B). Above results suggested that IGF-IIRα contributed to cardiomyocyte ER stress induction and leads to more severe effects in conjunction with DOX treatments.
IGF-IIRα-induced ER stress contributed to cardiac myocytes apoptosis. A IGF-IIRα-pEGFP plasmid was transfected into H9c2 cells for 24 h and then treated with DOX (1 µM) for another 24 h. WB analysis showing protein expression of ER stress-associated pro-apoptotic markers Caspase-12, cleaved Caspase-3, and CHOP. Representative blot images have been presented. n = 3; *p < 0.05; **p < 0.01; B Protein expression of cardiac stress-associated MAPKs p-JNK, p-p38, and PARKIN analyzed using WB from above experiment. They exhibited synergistic modulations in their expressions under combined effect of IGF-IIRα plus DOX treatments
In vivo validation of IGF-IIRα-induced ER stress in cardiac tissues
In order to corroborate the findings from in vitro studies, cardiac tissue samples from DOX-treated IGF-IIRα overexpressing transgenic rats were assessed for ER stress markers. In line with in vitro results, IGF-IIRα overexpression in cardiac tissues upregulated PERK and ATF4 expressions that were exacerbated upon DOX treatment. ATF6 and IRE1 were also increased, but they did not show combinatorial effects as above (Fig. 3A). Pro-apoptotic agent caspase-12, caspase-3, and CHOP were significantly upregulated, while ER chaperone GRP78 was suppressed by IGF-IIRα and exhibited synergistic effects with DOX treatments (Fig. 3B). This was accompanied by increased apoptotic nuclei in cardiac tissues induced by IGF-IIRα (Fig. 3C). Next, immunohistochemical analysis on cardiac tissue samples revealed an increased expression of CHOP in IGF-IIRα overexpressing compared to WT animals, while treatment of TG animals with DOX resulted in conspicuous accumulation of CHOP compared to IGF-IIRα alone or DOX only or WT animals alone (Fig. 3D). Further, we also determined the expression levels of stress-associated MAPKs p-p38 and p-JNK that were increased by IGF-IIRα while PARKIN was inhibited (Fig. 3E). Importantly, our results showed that IGF-IIRα has a synergistic contribution to DOX-induced cardiomyocyte damage that was indicated by enhanced cardiac apoptotic effects. Above results suggested that IGF-IIRα played a significant role in cardiac ER stress induction and leads to irreparable cardiac damages triggering an apoptosis cascade.
In vivo validation of IGF-IIRα-induced ER stress in transgenic animal cardiac tissues. A WB analysis showing protein expression of PERK, ATF4 (D4B8), ATF6α (F-7), and IRE1α (B-12) in cardiac tissues from WT and transgenic IGF-IIRα-HA-tagged rats (TG) treated with/without DOX and their respective quantifications. Representative blot images have been shown. Comparison groups were as follows: wild type (WT) alone vs WT + DOX treatment; TG + DOX treatment vs WT + DOX treatment, respectively. n = 3; *p < 0.05, **p < 0.01; B ER stress-associated pro-apoptotic markers analyzed using WB analysis. Panel showing protein expressions of Caspase-12, cleaved Caspase-3, CHOP, and GRP78. A combinatorial effect was observed in TG + DOX treatment group vs WT + DOX treatment group, respectively. n = 3; *p < 0.05; **p < 0.01; C TUNEL assay showing apoptotic nuclei in TG animals vs WT treated with/without DOX; D Immunohistochemistry staining showing pro-apoptotic CHOP expression in cardiac tissue sections obtained from WT and TG animals after DOX treatment. Protein expressions of CHOP were higher in TG animals that were upregulated further upon DOX treatment. E Cardiac tissue samples exhibited synergistic alterations in expressions of stress-associated MAPKs p-JNK, p-p38, and E3 ubiquitin ligase PARKIN under combined effect of IGF-IIRα and DOX treatments in TG animal vs WT animals
CHOP ablation or chemical ER chaperone reversed the ER stress response induced by IGF-IIRα
CHOP being an important transcription factor regulating Bcl2/Bax ratio and apoptosis during ER stress induction, we ascertained whether silencing of CHOP could alleviate ER stress responses by IGF-IIRα in H9c2 cells. As expected, DOX treatment increased CHOP and caspase-12 levels which were further enhanced by IGF-IIRα overexpression. A similar trend was observed for GADD34, a downstream apoptosis effector of CHOP during ER stress. Conversely, targeting CHOP using siRNA, the ER stress-associated apoptosis crusaders were attenuated in a dose-dependent manner (Fig. 4A). These results implicated that IGF-IIRα contributed to ER stress-induced apoptosis via modulations of CHOP activity. To further confirm the role IGF-IIRα in cardiac ER stress induction and ensuing apoptosis, we asked whether treatment with 4-phenylbutyrate (PBA), a chemical ER chaperone to mimic the function of GRP78, could ameliorate resultant apoptosis induction. In line with previous results, there was synergistic impairment in the levels of CHOP, caspase-12, GADD34, and GRP78 upon IGF-IIRα overexpression plus DOX treatment compared to other groups; however, interestingly, after treatment with PBA, these effects were ameliorated. It was found that PBA could significantly reduce the combined incremental changes by IGF-IIRα plus DOX treatment and diminished the levels of above pro-apoptosis agents (Fig. 4B). We also ascertained whether this reduction in pro-apoptosis agents is due to modulations in ER stress-associated factors induced by IGF-IIRα. Interestingly, PBA treatment not only reverted the impaired levels of ATF4 and PERK by IGF-IIRα plus DOX treatment to control levels, but also stress responsive PARKIN and ER chaperone GRP78 expressions were restored (Fig. 4B, C). Together, these findings suggested that IGF-IIRα significantly contributed to ER stress induction and that treatment with ER stress chaperone could mitigate damaging effects incurred by IGF-IIRα.
CHOP depletion or chemical ER chaperone 4-PBA mitigated the ER stress responses by IGF-IIRα. A Co-transfection with siRNA (5 nM, 10 nM, 20 nM)-targeting CHOP and IGF-IIRα-pEGFP plasmid (5 µM) was performed into H9c2 cells using jetPRIME reagent (Polyplus-transfection S.A., France) and incubated for 24 h. Later, media was replaced with fresh media and DOX treatment (1 µM) was done for another 24 h. At the end of 48 h, cellular protein extracts were subjected to WB analysis for detection of CHOP, GADD34, and Caspase12 protein levels. B, C H9c2 cells were treated with 4-PBA (5 mM) and incubated for 12 h. Then, they were rinsed with PBS and replaced with fresh medium. IGF-IIRα-pEGFP plasmid (5 µM) was transfected as earlier and incubated for 24 h. Subsequently, media was replaced with fresh medium containing DOX (1 µM) and incubated for another 12 h. At the end of 48 h, protein was harvested and WB was performed to measure CHOP, GADD34, Caspase12, GRP78, ATF4, PERK, and PARKIN protein expressions. n = 3; *p < 0.05; **p < 0.01
Discussion
In this study, we aimed to investigate the role of IGF-IIRα in modulation of ER stress under DOX-induced cardiac damage. Previous studies have shown that DOX treatment causes ER stress induction and leads to cardiac apoptosis via upregulation of pro-apoptotic CHOP activity [14]. Another study in a hypertension-induced PARKIN knockout mice model reported that PARKIN regulation of CHOP modulates susceptibility to cardiac ER stress [23]. Previously, we showed that IGF-IIRα leads to reduction in PARKIN expression and mitophagy impairment in DOX-induced cardiotoxicity [22]. This study showed that increased IGF-IIRα expression leads to ER stress induction that was severely aggravated under DOX-induced stresses. While ER stress markers ATF4 and PERK exhibited synergistic upregulation, ATF6 and IRE1 were also enhanced, but did not show combinatorial increments. On the other hand, there was increased expression of MAPKs such as p-p38 and p-JNK that were frequently associated with cardiac damage during stresses [27]. These observations were in concordance with several other studies that demonstrated the effect of DOX in the induction of cardiac damage [28]. Interestingly, PARKIN, a stress responsive protein, was markedly downregulated upon IGF-IIRα overexpression. This reduction in PARKIN level has also been shown by several other studies, in a context-dependent manner, leading to catastrophic demise of cells [23]. Our results showed that increased IGF-IIRα expression leads to inhibition of PARKIN, while the expression of ER stress-associated pro-apoptosis transcription factor CHOP was enhanced in a dose-dependent manner. Besides, eukaryotic translation initiation factor p-eIF2α (ser51) was only slightly elevated upon IGF-IIRα overexpression and/ or DOX treatment reflecting an un-halted global protein translation while concomitantly, ER chaperone GRP78 was significantly downregulated potentially contributing to severe accumulation of unfolded or misfolded or damaged cellular proteins. Several studies have shown that phosphorylation of eIF2α (ser51) is important for attenuation of global protein translation allowing the synthesis of only ER stress ameliorating protein molecules [29]. ER chaperone GRP78 and ER resident apoptosis inducer caspase12 along with executioner caspase-3 displayed increased expression implicating the involvement of IGF-IIRα in ER stress induction and apoptosis. Studies by several others have also shown the involvement of GRP78 and caspase12 in ER stress-induced cardiac apoptosis [30]. The protein expression of pro-apoptotic transcription factor CHOP was markedly increased in cardiac tissues derived from transgenic animals. These results suggested the interplay of CHOP in IGF-IIRα-induced ER stress induction and cardiac apoptosis. Therefore, we questioned whether suppression of CHOP or supplementing with ER chaperone mimic could mitigate the ER stress in the current scenario. To delineate this answer, when CHOP was silenced, it resulted in reduced expression of caspase-12 and GADD34 compared to IGF-IIRα plus DOX or DOX alone treated cardiomyocytes. While treatment with chemical ER chaperone 4-PBA resulted in downregulation of CHOP, and caspase-3 compared to un-treated controls. Not only that, ER stress markers ATF4, PERK, ATF 6, and IRE1 were salvaged to control levels, and PARKIN was also restored to normal levels. All these observations suggest for the critical contribution of ER stress induction in the IGF-IIRα-induced cardiac damages.
Conclusion
In summary (Fig. 5), we found that IGF-IIRα significantly contributed to ER stress induction in heart and ensuing cardiac damage under stress conditions. This effect was mitigated in part upon suppression of CHOP or treatment with a chemical ER chaperone 4-PBA which indicated that reducing the ER stress per se or inhibiting the ER stress-associated pro-apoptotic transcription factor could ameliorate the ER impairment caused by IGF-IIRα. However, several questions remain to be investigated such as the role of PARKIN and its molecular mechanism in context of ER stress induction by IGF-IIRα. Future studies are warranted to unravel the answers to above questions.
Data availability
All the data are contained in the manuscript file and will be made available from the corresponding author upon reasonable request.
References
Wang S, Binder P, Fang Q et al (2018) Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol 175:1293–1304. https://doi.org/10.1111/bph.13888
Nakashima A, Cheng SB, Kusabiraki T et al (2019) Endoplasmic reticulum stress disrupts lysosomal homeostasis and induces blockade of autophagic flux in human trophoblasts. Sci Rep 9:11466. https://doi.org/10.1038/s41598-019-47607-5
Rashid HO, Yadav RK, Kim HR et al (2015) ER stress: autophagy induction, inhibition and selection. Autophagy 11:1956–1977. https://doi.org/10.1080/15548627.2015.1091141
Ji C (2015) Advances and new concepts in alcohol-induced organelle stress, unfolded protein responses and organ damage. Biomolecules 5:1099–1121. https://doi.org/10.3390/biom5021099
Vo DH, Hartig R, Weinert S et al (2019) G-protein-coupled estrogen receptor (GPER)-specific agonist G1 induces ER stress leading to cell death in MCF-7 cells. Biomolecules. https://doi.org/10.3390/biom9090503
Minamino T, Kitakaze M (2010) ER stress in cardiovascular disease. J Mol Cell Cardiol 48:1105–1110. https://doi.org/10.1016/j.yjmcc.2009.10.026
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190. https://doi.org/10.1038/ncb0311-184
Gaballah HH, Zakaria SS, Elbatsh MM et al (2016) Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem Biol Interact 251:10–16. https://doi.org/10.1016/j.cbi.2016.03.023
Li DL, Wang ZV, Ding G et al (2016) Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation 133:1668–1687. https://doi.org/10.1161/CIRCULATIONAHA.115.017443
Octavia Y, Tocchetti CG, Gabrielson KL et al (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225. https://doi.org/10.1016/j.yjmcc.2012.03.006
Sishi BJ, Loos B, van Rooyen J et al (2013) Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol 85:124–134. https://doi.org/10.1016/j.bcp.2012.10.005
Takemura G, Fujiwara H (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352. https://doi.org/10.1016/j.pcad.2006.10.002
Hanf A, Oelze M, Manea A et al (2019) The anti-cancer drug doxorubicin induces substantial epigenetic changes in cultured cardiomyocytes. Chem Biol Interact 313:108834. https://doi.org/10.1016/j.cbi.2019.108834
Fu HY, Sanada S, Matsuzaki T et al (2016) Chemical endoplasmic reticulum chaperone alleviates doxorubicin-induced cardiac dysfunction. Circ Res 118:798–809. https://doi.org/10.1161/CIRCRESAHA.115.307604
Lou Y, Wang Z, Xu Y et al (2015) Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int J Mol Med 36:873–880. https://doi.org/10.3892/ijmm.2015.2291
Wang YX, Korth M (1995) Effects of doxorubicin on excitation-contraction coupling in guinea pig ventricular myocardium. Circ Res 76:645–653. https://doi.org/10.1161/01.res.76.4.645
Sag CM, Kohler AC, Anderson ME et al (2011) CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J Mol Cell Cardiol 51:749–759. https://doi.org/10.1016/j.yjmcc.2011.07.016
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610. https://doi.org/10.1016/j.cell.2007.08.036
Arola OJ, Saraste A, Pulkki K et al (2000) Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 60:1789–1792
Chang RL, Nithiyanantham S, Huang CY et al (2019) Synergistic cardiac pathological hypertrophy induced by high-salt diet in IGF-IIRalpha cardiac-specific transgenic rats. PLoS ONE 14:e0216285. https://doi.org/10.1371/journal.pone.0216285
Pandey S, Kuo WW, Ho TJ et al (2019) Upregulation of IGF-IIRalpha intensifies doxorubicin-induced cardiac damage. J Cell Biochem 120:16956–16966. https://doi.org/10.1002/jcb.28957
Pandey S, Kuo WW, Shen CY et al (2019) Insulin-like growth factor II receptor-alpha is a novel stress-inducible contributor to cardiac damage underpinning doxorubicin-induced oxidative stress and perturbed mitochondrial autophagy. Am J Physiol Cell Physiol 317:C235–C243. https://doi.org/10.1152/ajpcell.00079.2019
Han K, Hassanzadeh S, Singh K et al (2017) Parkin regulation of CHOP modulates susceptibility to cardiac endoplasmic reticulum stress. Sci Rep 7:2093. https://doi.org/10.1038/s41598-017-02339-2
Wu KM, Hsu YM, Ying MC et al (2019) High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr Metab (Lond) 16:36. https://doi.org/10.1186/s12986-019-0356-5
Lee CF, Chiang NN, Lu YH et al (2018) Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. Biomedicine (Taipei) 8:15. https://doi.org/10.1051/bmdcn/2018080315
Liu SP, Shibu MA, Tsai FJ et al (2020) Tetramethylpyrazine reverses high-glucose induced hypoxic effects by negatively regulating HIF-1alpha induced BNIP3 expression to ameliorate H9c2 cardiomyoblast apoptosis. Nutr Metab (Lond) 17:12. https://doi.org/10.1186/s12986-020-0432-x
Darling NJ, Cook SJ (2014) The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta 1843:2150–2163. https://doi.org/10.1016/j.bbamcr.2014.01.009
Tscheschner H, Meinhardt E, Schlegel P et al (2019) CaMKII activation participates in doxorubicin cardiotoxicity and is attenuated by moderate GRP78 overexpression. PLoS ONE 14:e0215992. https://doi.org/10.1371/journal.pone.0215992
Fu HY, Mukai M, Awata N et al (2017) Protein quality control dysfunction in cardiovascular complications induced by anti-cancer drugs. Cardiovasc Drugs Ther 31:109–117. https://doi.org/10.1007/s10557-016-6709-7
Hu J, Wu Q, Wang Z et al (2019) Inhibition of CACNA1H attenuates doxorubicin-induced acute cardiotoxicity by affecting endoplasmic reticulum stress. Biomed Pharmacother 120:109475. https://doi.org/10.1016/j.biopha.2019.109475
Funding
This study was supported by Grants from the China Medical University and Asia University, Taiwan. Grant numbers: [CMU107-ASIA-10]; [ASIA-106-CMUH-03].
Author information
Authors and Affiliations
Contributions
Conceptualization: SP; Data curation: YLY, WWK, and RJC; Formal analysis: CHK, YLY, and RJC; Funding acquisition: CYH; Investigation: SP; Methodology: SP; Project administration: TJH and CYH; Resources: TJH and CYH; Supervision: TJH and CYH; Validation: CYH; Writing—original draft: SP; Writing—review & editing: WSTC, CHD, and PYP.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pandey, S., Kuo, CH., Chen, W.ST. et al. Perturbed ER homeostasis by IGF-IIRα promotes cardiac damage under stresses. Mol Cell Biochem 477, 143–152 (2022). https://doi.org/10.1007/s11010-021-04261-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04261-8