Abstract
We have previously demonstrated that Cationic Arginine-Rich Peptides (CARPs) and in particular poly-arginine-18 (R18; 18-mer of arginine) exhibit potent neuroprotective properties in both in vitro and in vivo neuronal injury models. Based on the current literature, there is a consensus that arginine residues by virtue of their positive charge and guanidinium head group is the critical element for imparting CARP neuroprotective properties and their ability to traverse cell membranes. This study examined the importance of guanidinium head groups in R18 for peptide cellular uptake, localization, and neuroprotection. This was achieved by using poly-ornithine-18 (O18; 18-mer of ornithine) as a control, which is structurally identical to R18, but possesses amino head groups rather than guanidino head groups. Epifluorescence and confocal fluorescence microscopy was used to examine the cellular uptake and localization of the FITC-conjugated R18 and O18 in primary rat cortical neurons and SH-SY5Y human neuroblastoma cell cultures. An in vitro cortical neuronal glutamic acid excitotoxicity model was used to compare the effectiveness of R18 and O18 to inhibit cell death and intracellular calcium influx, as well as caspase and calpain activation. Fluorescence imaging studies revealed cellular uptake of both FITC-R18 and FITC-O18 in neuronal and SH-SY5Y cells; however, intracellular localization of the peptides differed in neurons. Following glutamic acid excitotoxicity, only R18 was neuroprotective, prevented caspases and calpain activation, and was more effective at reducing neuronal intracellular calcium influx. Overall, this study demonstrated that for long chain cationic poly-arginine peptides, the guanidinium head groups provided by arginine residues are an essential requirement for neuroprotection but are not required for entry into neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cationic arginine-rich peptides (CARPs) have gained attention in recent years due to their inherent neuroprotective properties, as demonstrated in preclinical rodent studies of acute CNS injuries such as stroke [1,2,3,4], traumatic brain injury (TBI) [5, 6], and hypoxic-ischaemic encephalopathy (HIE) [7], as well as chronic neurodegenerative diseases such as Alzheimer’s disease (AD) [8]. This class of peptide also possesses the capacity to traverse cell membranes and enter cells, and for this reason they are also known as cell-penetrating peptides (CPPs). Due to their membrane-traversing properties and ability to cross the blood–brain barrier and blood–spinal cord barrier, CARPs are highly desirable therapeutics for targeting the CNS [9,10,11]. Neuroprotective CARPs typically range from 4 to 30 amino acids, with arginine residues and an overall positive charge provided by arginine and other cationic amino acids (e.g. lysine, histidine) known to be critical for neuroprotection, as well as cell penetration [12, 13].
Our laboratory has demonstrated that CARPs consisting of long chained poly-arginine peptides, and in particular R18 (18-mer of arginine, net charge +18), provide high-level neuroprotection in both in vitro glutamic acid excitotoxic and oxygen–glucose deprivation models, as well as in rat models of stroke, HIE, and TBI [1, 2, 5,6,7, 14,15,16,17]. Furthermore, we have demonstrated that poly-arginine peptides attenuate neuronal excitotoxic calcium influx and reduce the neuronal cell surface levels of the NMDA NR2B receptor subunit [2, 12, 18]. In addition to these potential neuroprotective mechanisms, CARPs prevent cell death pathways by preserving mitochondrial function [19], and protecting mitochondrial architecture [20]. CARPs also inhibit proteolytic enzymes that activate matrix metalloproteinases (MMPs) [21], and can reduce oxidative stress [22] and pro-inflammatory responses [23]. While the exact structural chemical features of CARPs that impart neuroprotective properties have not been fully elucidated, it has been hypothesized that the guanidinium head group unique to the amino acid arginine is a critical requirement for neuroprotection [2, 12, 13, 18].
The guanidinium head group present in arginine residues and peptide charge are also considered to be important chemical elements that enable CARPs to efficiently traverse cellular membranes via endocytic energy-dependent and non-endocytic energy-independent pathways [24]. Furthermore, the cell-penetrating efficacy of CARPs is dependent on the peptide arginine content and positive charge, as demonstrated for poly-arginine peptides, which display increased cellular uptake with increasing polymer length and charge [12, 25]. The ability of CARPs to traverse cell membranes is believed to be due in part to the guanidinium head groups present in arginine residues forming bidentate hydrogen electrostatic interactions with sulphate, phosphate, and carboxylate anionic moieties present on cell surface structures, including heparin sulphate proteoglycans [26, 27], phospholipid head groups and gangliosides [28], and protein receptors [29, 30]. Moreover, anionic phospholipid head groups present in organelle membranes, including mitochondria, the endoplasmic reticulum, and the nucleus, are believed to contribute to the ability of CARPs to localize within different intracellular organelles [31,32,33,34,35]. Interestingly, features that enhance CARP cellular uptake efficiency also appear to enhance neuroprotective efficacy [1, 12, 13]. For example, increasing poly-arginine chain length and the incorporation of tryptophan residing in CARPs increases peptide cellular uptake [36], as well as neuroprotective potency [2, 12].
Despite the observation that the neuroprotective potency of CARPs appears to be correlated with cellular uptake, critical features of the peptide believed to be necessary for neuroprotection, namely guanidinium moieties, have not been thoroughly investigated. In addition, for our lead neuroprotective peptide, poly-arginine-18 (R18), we had not previously confirmed cell entry and intracellular localization. As such, this study examined the importance of guanidinium head groups in R18 for cellular uptake, intracellular localization, and neuroprotection. In order to elucidate the importance of guanidinium moieties for peptide cellular uptake and neuroprotection, we used a poly-ornithine-18 peptide (O18; 18-mer of ornithine, net charge +18) as a control. Ornithine is a cationic non-proteinogenic amino acid, but is structurally identical to arginine except that it possesses an amino group rather than a guanidino group attached to the δ-carbon (Suppl. Fig. 1A). While it is known that short-chained poly-ornithine molecules (i.e. 9-mer) do not possess high-level cell-penetrating properties [36], to our knowledge the cell entry and neuroprotective properties of longer chained ornithine polymers has not been examined. In this study, we investigated cellular uptake of R18 and O18 in SH-SY5Y cells and cortical neurons, and neuroprotective efficacy in a neuronal glutamic acid excitotoxicity model.
Experimental procedures
Peptides used in this study
R18 (H-RRRRRRRRRRRRRRRRRR-OH) and O18 (H-OOOOOOOOOOOOOOOOOO-18) peptides, and fluorescein isothiocyanate (FITC) N-terminal conjugated peptides, FITC-R18 and FITC-O18 were synthesized by Mimotopes (Melbourne, Australia) and high performance liquid chromatography purified to > 95%. Peptides were prepared as 500 μM stocks in water for irrigation (Baxter, Australia) and stored at − 20 °C before use.
Cell culture
Rat primary cortical neurons were isolated from E18 Sprague–Dawley rats and cultured in Neurobasal/2% B27 supplement (NB/2% B27; Life Technologies, Australia) as previously described [18]. Neurons were seeded into clear 96-well plastic plates (Nunc, Australia), black clear flat bottom 96-well plastic plates (Corning, Australia), or 35 mm/14 mm coverslip dishes (MatTek Corporation), and maintained in the CO2 incubator until use on in vitro day 10 or 11. Under these conditions, cultures routinely consist of > 97% neurons and 1–3% astrocytes. Approval to obtain embryonic rat cortical tissue for in vitro cultivation was obtained by the University of Western Australia Animal Ethics Committee (RA/3/100/1432) and adhered to the Animal Welfare Act 2002 (Western Australia) and the Australian Code for the Care and Use of Animals for Scientific Purposes (8th Ed. 2013).
Human SH-SY5Y neuroblastoma cells were used to confirm that R18 cellular uptake was not limited to a specific cell type (i.e. cortical neurons), which is an important consideration for a potential neuroprotective therapeutic. In addition, SH-SY5Y cells exhibit distinct morphological differences to neurons, particularly the lack of neuronal processes. SH-SY5Y cells were sourced from the European Collection of Authenticated Cell Cultures (ECACC) via CellBank Australia (ECCAC Catalogue No. 94030304).
SH-SY5Y cells were cultured and maintained in Dulbecco’s modified Eagle medium (DMEM; Life Technologies, Australia) supplemented with 5% foetal bovine serum (FBS) and maintained in a CO2 incubator at 37 °C. Cultures were seeded into 35 mm diameter dishes with a centrally located 14 mm diameter glass cover slip bottom (MatTek Corporation, MA, USA) at 300,000 cells per dish and used for peptide uptake studies 24 h after seeding.
FITC-R18 and FITC-O18 cell uptake studies using epifluorescence and confocal fluorescence microscopy
SH-SY5Y and neuronal cells growing on glass cover slips were treated with FITC-R18 or FITC-O18 (1 or 5 µM in 1 mL: DMEM/5% FBS for SH-SY5Y cells and MEM/2% B27 for neurons) for 10 min in the CO2 incubator. Following incubation, cells were washed with 1 mL ice-cold phenol red free Hanks Balanced Salt Solution (HBSS; Thermo Fisher) and fixed with 1 mL of methanol and acetone (50%/50% v/v) for 15 min at room temperature. After removing fixation solution, dishes were left to air dry for 30 min, and cells were subsequently stained with DAPI (2 µg/mL in HBSS) for 10 min. Epifluorescence microscopy was performed using the Nikon Ti2-E inverted microscope (Nikon, Melville, NY, USA) equipped with a DS-Qi2 monochrome microscope camera (Nikon) and Pre-centred Fibre Illuminator (INTENSILIGHT; C-HGFIE, Nikon). Images were captured using NIS Elements AR software (Version 5.01.00, Nikon). Confocal microscopy was performed using a Nikon C2 mounted Ti-E inverted motorized microscope and A1Si spectral detector confocal system (Nikon) controlled by NIS-C Elements software (Version 4.3, Nikon).
Neuronal glutamic acid excitotoxicity model
Cortical neurons cultured in 96-well plates were treated with R18 or O18 (0.5–5 μM in 50 µL MEM/2% B27) for 10 min in a CO2 incubator at 37 °C. To induce excitotoxicity, 50 µL of glutamic acid (200 µM; final concentration 100 µM) was added to the culture wells and incubated for 5 min in a CO2 incubator at 37 °C. After a 5-min exposure, media in wells was replaced with 100 µL of MEM/2% B27 and cultures incubated in a CO2 incubator at 37 °C for a further 24 h.
MTS cell viability assay
Cell viability was determined using the MTS assay (Cell-Titer 96 aqueous non-radioactive cell proliferation assay; Promega, Australia) 24 h post glutamic acid exposure. The assay was performed by adding 18 µL of MTS solution to each well containing 80 μL of media and incubating for 1–4 h in a CO2 incubator at 37 °C, before measuring absorbance at 490 nm. Absorbance data were converted to reflect proportional cell viability relative to both the untreated control (100% viability) and glutamic acid treated control (5% viability).
LDH cell death assay
Cell death was determined using the lactate dehydrogenase (LDH) release assay (CytoTox 96® non-radioactive cytotoxic assay; Promega) 24 h post glutamic acid exposure. The assay was performed by transferring 20 µL of culture well supernatant to a 96-plate well, adding 20 µL of LDH substrate solution, and incubating for 30 min at room temperature, before measuring absorbance at 490 nm.
Intracellular calcium kinetics
Intracellular calcium influx kinetics was performed using the fluorescent Ca2+-indicator Fura-2 AM dye (Sigma Aldrich), as previously described [2]. Briefly, neurons cultured in black clear-bottom 96-well plates were loaded with Fura-2 AM in MEM/2%B27 (5 µM in 100 µL) for 20 min in a CO2 incubator at 37 °C. After the incubation period, cells were washed with HBSS and treated with either R18 or O18 in MEM/2% B27 (5 µM in 50 µL) for 10 min in a CO2 incubator at 37 °C. After the incubation period, media was replaced with HBSS (50 µL), and fluorescent recording measurement commenced using a Cytation5 plate reader (Gen5 Version 3.05.11; BioTek) every 30 s for 1 min before and for 5 min after glutamic acid addition (50 µL in HBBS: final concentration 100 µM). Fold change in fluorescence ratio (ΔF: 340/380 nm) measurements relative to non-glutamic acid control neuronal culture was used to compare changes in the intracellular calcium influx before and after glutamic acid addition in untreated and peptide treated neuronal cultures.
Assessment of caspase and calpain activity
Caspase and calpain activation was measured using the Caspase-3, -8, and -9 Multiplex Assays (Abcam; ab219915) and Calpain Activity Assay (Abcam; ab65308), respectively according to the manufacturer's protocols. Cortical neurons cultured in 96-well plates were treated with R18 or O18 (5 μM) and glutamic acid (100 µM final concentration) as described above. At 6 or 24 h post glutamic acid exposure, media in wells was replaced with a caspase substrate solution (DEVD-ProRed™, IETD-R110, and LEHD-AMC, for caspases-3, -8, and -9, respectively) or calpain substrate solution (Ac-LLY-AFC) and incubated for 1 h in the CO2 incubator. The 6-hour time point was chosen as previous studies have demonstrated that caspase and calpain activity is triggered in neuronal cultures 4–6 h after excitotoxicity [37, 38]. The 24-hour timepoint was chosen to determine whether the amelioration of caspase and calpain activation was maintained up to 24 h post-excitotoxicity, and aligns with the preservation of cell viability at 24 h (as demonstrated by MTS assay). Cleavage of caspase- and calpain-specific substrates were measured using the Cytation5 plate reader (Ex/Em = 535/620 nm; Ex/Em = 490/525 nm; Ex/Em = 370/450 nm for caspases-3, -8, and -9, respectively and Ex/Em = 400/505 nm for calpain). Fluorescent measurements were subtracted from background signal (media and substrate solution) and are represented as fold change of the non-glutamic acid control neuronal cultures.
Statistical analysis
Statistical analysis was performed using Prism statistical software (Version 8.02, USA). For data relating to viability and biochemical assays, experimental group differences were analysed using a one-way analysis of variance (ANOVA) and subsequent Tukey post hoc tests. Data are presented as mean ± SEM, with p < 0.05 considered statistically significant. Experiments were repeated at least three times independently, with a minimum of eight replicates (n = 8) for cell viability and cell death assay, or a minimum of four replicates (n = 4) per all other biochemical assays.
Results
FITC-R18 and FITC-O18 uptake in SH-SY5Y cells and cortical neurons
Based on epifluorescence microscopy and FITC staining, FITC-R18 and FITC-O18 uptake into SH-SY5Y cells were highly efficient at the 1, 2, and 5 µM concentrations and localizing throughout the cell cytoplasm (Suppl. Fig. 2). Using a 5 µM concentration, confocal fluorescent microscopy and FITC staining in different cell layers in the XY and YZ orthogonal scans confirmed the presence of FITC-R18 and FITC-O18 within the cytoplasm, with little to no nuclear staining (Fig. 1a, b).
FITC-labelled R18 and O18 peptides exhibit similar intracellular localization in SH-SY5Y neuroblastoma cells. Representative confocal microscopy of SH-SY5Y cells incubated with FITC-conjugated a R18 and b O18 peptides (5 µM) for 10 min at 37 °C. Cells were washed 3 times with HBSS and counterstained with DAPI for 10 min. Intracellular localization of peptides was examined with confocal laser scanning microscopy (Nikon TiE; × 60 magnification). Green indicates FITC signal in the cells. (Color figure online)
Based on epifluorescence microscopy and FITC staining, FITC-R18 and FITC-O18 uptake into neurons was also highly efficient at the 1, 2, and 5 µM concentrations (Suppl. Fig. 3), with FITC-R18 localizing primarily within the cell body and neuronal processes (Fig. 2a), and FITC-O18 localizing primarily within the cell body and nucleus (Fig. 2b). When using a 5 µM concentration, confocal fluorescent microscopy and FITC staining in different cell layers in the XY and YZ orthogonal scans confirmed FITC-R18 distribution within the cell body and neuronal processes, and FITC-O18 was distributed in the cell body and nucleus (Fig. 2a, b).
FITC-labelled R18 and O18 peptides exhibit different cellular localization in primary cortical neurons. Representative confocal microscopy of cortical neuronal cultures incubated with FITC-conjugated a R18 and b O18 peptides (5 µM) for 10 min at 37 °C. Cells were washed 3 times with HBSS and counterstained with DAPI for 10 min. Intracellular localization of peptides was examined with confocal laser scanning microscopy (Nikon TiE; × 60 magnification). Green indicates FITC signal in the cells. (Color figure online)
Effects of R18 and O18 on neuronal survival and calcium influx after exposure to glutamic acid
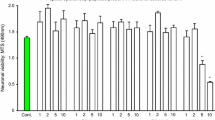
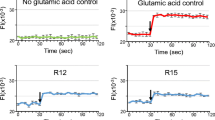
As previously demonstrated [1], R18 displayed high-level neuroprotection at the 1, 2 and 5 μM concentrations following exposure to glutamic acid, as shown by increased cell survival and reduced LDH release (Fig. 3a, b). In contrast, O18 treatment did not display any significant neuroprotection through preserved cell viability or reduced cell death (Fig. 3a, b). In addition, R18 (5 µM) pre-treatment significantly reduced neuronal intracellular calcium levels after glutamic acid exposure, whereas O18 resulted in a non-significant trend in reducing glutamic acid-induced intracellular calcium levels (Fig. 3c).
R18, but not O18, provides neuroprotection against glutamic acid excitotoxicity in cortical neurons. Neuronal viability was determined by a MTS, and b LDH assays at 24 h post glutamic acid exposure following a 10-min peptide pre-treatment (0.5, 1, 2, and 5 μM). Absorbance values were adjusted to represent cell viability (untreated control as 100%, and glutamic acid control as 5% viability, respectively). Values are mean ± SEM; n = 8; *p < 0.05. c Intracellular calcium influx was measured following glutamic acid insult with the fluorogenic Fura-2 AM dye. Neurons were pre-loaded with Fur-2 AM (5 µM) for 20 min prior to peptide incubation (5 µM R18 or O18; 10 min). Basal intracellular calcium concentrations were measured with the Cytation5 fluorescent plate reader (BioTek) every 30 s for 1.5 min. Glutamic acid (100 µM final concentration) was then added, and changes to intracellular calcium concentrations were measured every 30 s thereafter for a further 5 min
Effects of R18 and O18 on neuronal caspase and calpain activation after exposure to glutamic acid
Following glutamic acid exposure, neuronal caspase-3, -8 and -9 and calpain activity were significantly elevated at 6 and 24 h post glutamic acid exposure (Figs. 4a–c and 5a, b). R18 at 1, 2, and 5 μM prevented caspase-3, -8, and -9 and calpain activation at both the 6- and 24-hour time points. In contrast, O18 at 5 μM (only the 5 µM concentration was assessed for O18) did not prevent the activation of caspases or calpain.
R18, but not O18, attenuates activation of caspases 3, 8, and 9 at both 6 and 24 h post glutamic acid excitotoxicity in cortical neurons. Neuronal cultures received a 10-min pre-treatment with R18 (1, 2, or 5 µM) or O18 (5 µM) and subsequent 5-min glutamic acid exposure (100 µM). Culture media was then replaced, and activity of a caspase 3, b caspase 8, and c caspase 9 were measured with the use of specific fluorometric substrates at 6 h and 24 h. Background fluorescence was subtracted, and values were adjusted to relative fold change compared to untreated control. Values are mean ± SEM; n = 4; *p < 0.05
R18, but not O18, attenuates calpain activation at both 6 and 24 h post glutamic acid excitotoxicity in cortical neurons. Neuronal cultures received a 10-min pre-treatment with R18 (1, 2, or 5 µM) or O18 (5 µM) and subsequent 5-min glutamic acid exposure (100 µM). Culture media was then replaced, and calpain was measured with the use of fluorometric calpain substrate, Ac-LLY-AFC, at a 6 h and b 24 h. Background fluorescence was subtracted, and values were adjusted to relative fold change compared to untreated control. Values are mean ± SEM; n = 4; *p < 0.05
Discussion
R18 and O18 cellular uptake
There is an increasing body of research that has demonstrated that cationic arginine-rich peptides (CARPs) have innate neuroprotective and cell-penetrating properties, however the key peptide structural features that underpin these properties have yet to be fully elucidated. In this in vitro study, we examined cellular uptake of the neuroprotective CARP, poly-arginine-18 (R18), and the potential importance of the guanidinium moiety present on arginine residues for both cell penetration and neuroprotection. Importantly, and as expected based on other poly-arginine peptide cellular uptake studies [36, 39,40,41], we confirmed that R18 has the capacity to enter both cortical neuronal cells and neuroblastoma cells (SH-SY5Y), and demonstrated that the guanidinium head group is essential for neuroprotection after excitotoxic injury.
We also demonstrated that O18 displays high-level cell-penetrating properties in both SH-SY5Y and cortical neuronal cells. This was unexpected based on a previous study showing that O9 (9-mer of ornithine) displays a poor capacity to enter Jurkat cells [36]; however, it is important to note that studies examining poly-ornithine peptide cellular uptake are limited, and these results may be specific to this cell type and O9. These results suggest that for long chain cationic amino acid polymers (i.e. 18-mer of arginine, lysine, ornithine), positive charge alone is sufficient for cellular uptake of the peptide. This is not entirely unexpected, as increasing cationic amino acid polymer length also increases overall peptide positive charge, which would have the effect of strengthening peptide electrostatic interactions with anionic structures on the outer plasma membrane that are critical for cellular uptake. It also provides an explanation as to why short poly-arginine peptides (e.g. R8 and R9), but not short poly-lysine and poly-ornithine peptides (e.g. 9-mer) have high cell-penetrating properties (Suppl. Fig. 1A). The superior cell-penetrating properties of arginine polymers is likely due to the ability of the guanidinium head group on arginine residues to form bidentate hydrogen electrostatic interactions with sulphate, phosphate, and carboxylate anionic moieties on cell surface structures, while the amide head group on lysine and ornithine can only form weaker monodentate hydrogen electrostatic interactions [31,32,33, 35, 42] (Suppl. Fig. 1B). However, it appears that the weaker electrostatic peptide cell surface interactions associated with the short chained ornithine and lysine polymers, compared to arginine polymers, can be overcome by increasing polymer charge and length, and in doing so improve cell uptake efficiency. At present, the minimum peptide charge and chain length of ornithine and lysine polymers that result in a significant improvement in peptide cell entry still remains to be determined.
R18 and O18 intracellular localization
Following entry into SH-SY5Y cells, R18 and O18 exhibited a similar cytoplasmic localization; while following entry into neurons, R18 localized mainly within the cell body and neuronal processes, whereas O18 localized mainly to the cell body and nucleus. The reasons for these differences in intracellular localization of the two peptides is not known; however, there are a number of possibilities. SH-SY5Y cells lack neuronal processes, and thus have a more uniform outer surface plasma membrane structure. It is therefore conceivable that anionic interactions between R18 and O18 peptides and the outer cell surface, and subsequent cell entry mechanisms, induce a consistent and uniform uptake of both peptides into the cytoplasm. In contrast, primary neurons possess highly specialized outer membrane structures including axonal and dendritic processes, synapses and cell body, as well as possessing a high resting transmembrane potential. Furthermore, the composition of the plasma membrane within the different membrane structures is likely to differ, which together may influence the differential uptake of FITC-R18 and FITC-O18 within different cytoplasmic domains (i.e. neuronal processes). For example, the availability of anionic moieties in neuronal processes may be sufficient for R18 entry due to the stronger electrostatic attractions provided by guanidinium head groups, while the amino head groups of O18 may not be sufficient to induce uptake within these structures. More intriguing was the inability of FITC-R18 and FITC-O18 to localize within SH-SY5Y cell nuclei, while FITC-O18 was exclusively able to localize to neuronal nuclei. This observation may be due to differences in the properties of the nuclear envelope in the two cell types and its differential permeability to the R18 and O18 peptides.
R18 and O18 neuroprotection: importance of guanidinium head groups
While both FITC-R18 and FITC-O18 were able to enter neurons, although at different cellular locations, only the R18 peptide reduced cell death in the glutamic acid excitotoxicity model. The lack of neuroprotective properties for O18 is in line with our previous study demonstrating that poly-lysine-10 (K10; 10-mer of lysine; net charge +10), which has a similar structure to arginine and ornithine, but possesses an amino group attached to the ε-carbon, is also ineffective at reducing neuronal death in the glutamic acid excitotoxicity model [12] (Suppl. Fig. 1A). Together, the positive neuroprotective result for R18 (and other poly-arginine peptides and CARPs) and negative neuroprotective findings for O18 and K10 indicate that the guanidinium head group in arginine residues is an essential structural element for neuroprotection in poly-arginine peptides, and mostly likely also in other neuroprotective CARPs.
The high neuroprotective potency of R18 observed in the excitotoxicity model is likely to be mediated in part by the capacity of the peptide to inhibit glutamic acid-induced neuronal intracellular calcium influx. It is noteworthy that O18 also reduced neuronal intracellular calcium influx, but not to a level to elicit a neuroprotective effect in the excitotoxicity model. This finding indicates that while guanidinium head groups in poly-arginine peptides provide a high capacity for the polymer to block glutamic acid-induced intracellular calcium influx, positively charged amino groups on ornithine and most likely lysine polymers, also have some capacity to block calcium influx. One mechanism whereby R18 can reduce glutamic acid-induced intracellular calcium influx may involve reducing the surface expression of calcium-permeable glutamate receptors. To this end, we have previously shown that poly-arginine-12 (R12) is able to rapidly reduce neuronal NMDA NR2B receptor surface levels [18] which is an important subunit for intracellular calcium influx during excitotoxicity [43]. Related CARPs have also been shown to modulate surface expression of receptors including NMDARs [44, 45] and voltage-gated calcium channels (VGCCs) [46,47,48], as well as TNF receptors [49]. It is hypothesized that the ability of cationic cell-penetrating peptides to modulate surface levels of cell surface receptors may be due to their ability to induce endocytosis during peptide uptake, causing receptor internalization [13, 49]. Furthermore, given the differences in neuroprotective potency and intracellular localization of R18 and O18 following treatment of neurons, these findings suggest that the uptake mechanisms for the two peptides and effects on surface ion channel receptors may also differ.
Additionally, R18 may antagonize ion channel receptor function directly through electrostatic interactions mediated by its positively charged guanidino moieties. Guanidinium-containing agents, such as agmatine [50], amiloride [51, 52], diarylguanidines [53], 2-guanidinobenzimidazole [54, 55], and tetrodotoxin [56, 57] are known to inhibit the function of various ligand gated and voltage-gated ion channel receptors by interacting with anionic moieties within the ion channel pore or channel regulatory structures [58]. Similarly, poly-amino-containing agents, such as putrescine, spermidine and spermine, also have the capacity to block different ion channel receptors by interacting at or near the channel pore [59,60,61]. Taken together, it is feasible to conclude that guanidino and amino moieties within the R18 and O18 peptides, respectively, also antagonize calcium influx during excitotoxicity by interacting with ion channel function. Furthermore, it appears that guanidino moieties are more potent inhibitors of ion channel calcium influx than amino moieties.
R18 neuroprotection: potential intracellular neuroprotective mechanisms
The observation that although R18 did not completely block glutamic acid-induced neuronal calcium influx, it still provided complete neuroprotection suggests that additional intracellular neuroprotective mechanisms may also be at play. The multi-modal action of CARPs has been well documented, including the ability to target mitochondria and preserve mitochondrial membrane potential (ΔΨm) and stabilization of ATP production [40], maintenance of cytochrome c integrity [62], attenuation of ROS production [22], and inhibition of calcium influx into the organelle [63]. Moreover, since mitochondria can act as central mediators of cell death events, such as caspase and calpain activation and the release of pro-cell death molecules [64, 65], R18 may also be having positive effects on these pathways after injury. To this end, arginine, ornithine and lysine tetramers (4-mer) have been shown to exert beneficial effects on isolated mitochondria when challenged with high calcium concentrations by inhibiting calcium-induced mitochondrial permeability transition, transmembrane potential disruption, mitochondrial swelling and glutathione release, with poly-arginine showing the greatest efficacy, followed by poly-ornithine and poly-lysine [63]. Furthermore, CARPs can activate pro-cell survival signalling and possess antioxidant properties [45, 66,67,68], which would also be beneficial to neurons following excitotoxic stress.
Given that R18 was highly neuroprotective in the glutamic acid excitotoxicity model and significantly attenuated the calcium influx, we also confirmed that treatment of neuronal cultures with the peptide blocked the activation calcium-dependent proteolytic enzymes involved in cell death pathways, which are known to be activated following excitotoxicity [69, 70]. R18 blocked activation of both the initiator (caspase 8 and 9) and effector caspases (caspase 3), whereas O18 did not have any impact on the activation of the proteolytic enzymes. These findings are supported by a recent study that demonstrated the ability of R18 to significantly attenuate the activation of caspases-3, -8 and -9 in the brain in a controlled cortical impact TBI model in the rat [71]. Similarly, a study by Marshall et al. demonstrated that the poly-arginine-7 (R7) peptide successfully attenuated NMDA-induced activation of caspases-3, as well as caspases-1, -4, and -7 in a murine model of excitotoxic retinal neuronal death [40]. In addition, R18 also resulted in a marked reduction in glutamic acid-induced activation of calpains, which are calcium-dependent proteases that exert their neurotoxic actions through proteolytic degradation of cytoskeletal and structural cellular components [38, 72], as well as subsequent apoptotic signalling via activation of caspase-9 [73]. The ability of other CARPs such as TAT-CBD3 (YGRKKRRQRRR-ARSRLAELRGVPRGL; net charge +11) and R9-CBD3 (RRRRRRRRR-ARSRLAELRGVPRGL; net charge +12) to reduce calpain activity following glutamic acid excitotoxicity have also been demonstrated [74].
Limitations of study
While the ability of CARPs to inhibit glutamic acid-induced neuronal intracellular calcium influx is well established, further studies are required to explore other intracellular mechanisms of action that underlie their neuroprotective effects. For example, future studies should attempt to further distinguish neuroprotective mechanisms that may be occurring at the cell surface and within the cell, such as mitochondrial-targeted mito-protection, ROS mitigation and proteolytic enzyme inactivation. This could be achieved by direct intracellular administration of the peptide using whole cell patch-clamp delivery and subsequent intracellular biochemical and functional assays. In addition, studies should examine peptide cell entry at lower concentrations and perform a quantitative assessment of peptide uptake using fluorescent densitometry or flow cytometry. Future studies should also examine CARP uptake and potential cytoprotective mechanisms in glial cells that also play a pivotal role in acute and chronic brain injury and neuroprotection, such as astrocytes, microglia and brain endothelial cells.
Conclusion
In this study, we have demonstrated that for long chain cationic peptide polymers (e.g. 18-mers), peptide positive charge provided by either guanidino or amino moieties is sufficient for cellular uptake; however, subsequent intracellular localization of the peptides may differ in different cell types. In addition, the study confirms that the guanidinium head groups of arginine residues in neuroprotective poly-arginine peptides, and most likely other CARPs, are an essential requirement for neuroprotection.
Abbreviations
- CPP:
-
Cell-penetrating peptide
- CARP:
-
Cationic arginine-rich peptide
- HIE:
-
Hypoxic-ischaemic encephalopathy
- MMP:
-
Matrix metalloproteinases
- TAT:
-
‘Trans-activator of transcription’ HIV-1 protein
- TBI:
-
Traumatic brain injury
- R18:
-
Poly-arginine-18 peptide
- O18:
-
Poly-ornithine-18 peptide
References
Meloni BP, Milani D, Edwards AB et al (2015) Neuroprotective peptides fused to arginine-rich cell penetrating peptides: neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol Ther 153:36–54. https://doi.org/10.1016/j.pharmthera.2015.06.002
Meloni BP, Milani D, Cross JL et al (2017) Assessment of the neuroprotective effects of arginine-rich protamine peptides, poly-arginine peptides (R12-Cyclic, R22) and arginine–tryptophan-containing peptides following in vitro excitotoxicity and/or permanent middle cerebral artery occlusion in rats. NeuroMolecular Med 19:271–285. https://doi.org/10.1007/s12017-017-8441-2
Milani D, Cross JL, Anderton RS et al (2017) Neuroprotective efficacy of poly-arginine R18 and NA-1 (TAT-NR2B9c) peptides following transient middle cerebral artery occlusion in the rat. Neurosci Res 114:9–15. https://doi.org/10.1016/j.neures.2016.09.002
Edwards A, Feindel K, Cross J et al (2017) Neuroprotective efficacy of poly-arginine-18 (R18) peptides using an in vivo model of perinatal hypoxic ischaemic encephalopathy (HIE). J Cereb Blood Flow Metab 37:18–19
Chiu LS, Anderton RS, Cross JL et al (2019) Poly-arginine peptide R18D reduces neuroinflammation and functional deficits following traumatic brain injury in the long-evans rat. Int J Pept Res Ther. https://doi.org/10.1007/s10989-018-09799-8
Chiu LS, Anderton RS, Cross JL et al (2017) Assessment of R18, COG1410, and APP96-110 in excitotoxicity and traumatic brain injury. Transl Neurosci 8:147–157. https://doi.org/10.1515/tnsci-2017-0021
Edwards AB, Anderton RS, Knuckey NW, Meloni BP (2018) Assessment of therapeutic window for poly-arginine-18D (R18D) in a P7 rat model of perinatal hypoxic-ischaemic encephalopathy. J Neurosci Res. https://doi.org/10.1002/jnr.24315
Fonar G, Polis B, Meirson T et al (2018) Subcutaneous sustained-release of poly-arginine ameliorates cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Adv Alzheimer’s Dis 7:153–182. https://doi.org/10.4236/aad.2018.74011
Jiang N, Frenzel D, Schartmann E et al (2016) Blood-brain barrier penetration of an Aβ-targeted, arginine-rich, d-enantiomeric peptide. Biochim Biophys Acta Biomembr 1858:2717–2724. https://doi.org/10.1016/j.bbamem.2016.07.002
Davoli E, Sclip A, Cecchi M et al (2014) Determination of tissue levels of a neuroprotectant drug: the cell permeable JNK inhibitor peptide. J Pharmacol Toxicol Methods 70:55–61. https://doi.org/10.1016/j.vascn.2014.04.001
Tang M, Waring AJ, Hong M (2007) Phosphate-mediated arginine insertion into lipid membranes and pore formation by a cationic membrane peptide from solid-state NMR. J Am Chem Soc 129:11438–11446. https://doi.org/10.1021/ja072511s
Meloni BP, Brookes LM, Clark VW et al (2015) Poly-arginine and arginine-rich peptides are neuroprotective in stroke models. J Cereb Blood Flow Metab 35:993–1004. https://doi.org/10.1038/jcbfm.2015.11
Meloni BP, Craig AJ, Milech N et al (2014) The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cell Mol Neurobiol 34:173–181. https://doi.org/10.1007/s10571-013-9999-3
MacDougall G, Anderton RS, Mastaglia FL et al (2018) Mitochondria and neuroprotection in stroke: cationic arginine-rich peptides (CARPs) as a novel class of mitochondria-targeted neuroprotective therapeutics. Neurobiol Dis 121:17–33. https://doi.org/10.1016/j.nbd.2018.09.010
Edwards AB, Cross JL, Anderton RS et al (2018) Poly-arginine R18 and R18D (d-enantiomer) peptides reduce infarct volume and improves behavioural outcomes following perinatal hypoxic-ischaemic encephalopathy in the P7 rat. Mol Brain 11:1–12. https://doi.org/10.1186/s13041-018-0352-0
Milani D, Clark VW, Cross JL et al (2016) Poly-arginine peptides reduce infarct volume in a permanent middle cerebral artery rat stroke model. BMC Neurosci 17:19. https://doi.org/10.1186/s12868-016-0253-z
Milani D, Cross JL, Anderton RS et al (2017) Delayed 2-h post-stroke administration of R18 and NA-1 (TAT-NR2B9c) peptides after permanent and/or transient middle cerebral artery occlusion in the rat. Brain Res Bull 135:62–68. https://doi.org/10.1016/j.brainresbull.2017.09.012
MacDougall G, Anderton RS, Edwards AB et al (2017) The neuroprotective peptide poly-arginine-12 (R12) reduces cell surface levels of NMDA NR2B receptor subunit in cortical neurons; Investigation into the involvement of endocytic mechanisms. J Mol Neurosci 61:235–246. https://doi.org/10.1007/s12031-016-0861-1
Szeto HH, Liu S, Soong Y et al (2011) Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22:1041–1052. https://doi.org/10.1681/ASN.2010080808
Birk AV, Chao WM, Liu S et al (2015) Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim Biophys Acta 1847:1075–1084. https://doi.org/10.1016/j.bbabio.2015.06.006
Cameron A, Appel J, Houghten RA, Lindberg I (2000) Polyarginines are potent furin inhibitors. J Biol Chem 275:36741–36749. https://doi.org/10.1074/jbc.M003848200
Cerrato CP, Pirisinu M, Vlachos EN, Langel Ü (2015) Novel cell-penetrating peptide targeting mitochondria. FASEB J 29:4589–4599. https://doi.org/10.1096/fj.14-269225
Hilchie AL, Wuerth K, Hancock REW (2013) Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9:761–768. https://doi.org/10.1038/nchembio.1393
Herce HD, Garcia AE, Cardoso MC (2014) Fundamental molecular mechanism for the cellular uptake of guanidinium-rich molecules. J Am Chem Soc. https://doi.org/10.1021/ja507790z
Åmand HL, Rydberg HA, Fornander LH et al (2012) Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim Biophys Acta Biomembr 1818:2669–2678. https://doi.org/10.1016/j.bbamem.2012.06.006
Gonçalves E, Kitas E, Seelig J (2005) Binding of oligoarginine to membrane lipids and heparan sulfate: structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry 44:2692–2702. https://doi.org/10.1021/bi048046i
Console S, Marty C, García-Echeverría C et al (2003) Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem 278:35109–35114. https://doi.org/10.1074/jbc.M301726200
Ziegler A, Li Blatter X, Seelig A, Seelig J (2003) Protein transduction domains of HIV-1 and SIV TAT interact with charged lipid vesicles. Binding mechanism and thermodynamic analysis. Biochemistry 42:9185–9190. https://doi.org/10.1021/bi0346805
Schug KA, Lindner W (2005) Noncovalent binding between guanidinium and anionic groups: focus on biological- and synthetic-based arginine/guanidinium interactions with phosph[on]ate and sulf[on]ate residues. Chem Rev 105:67–114. https://doi.org/10.1021/cr040603j
Kawaguchi Y, Takeuchi T, Kuwata K, Chiba J, Hatanaka Y, Nakase I, Futaki S (2016) Syndecan-4 is a receptor for clathrin-mediated endocytosis of arginine-rich cell-penetrating peptides. Bioconjug Chem 27:1119–1130. https://doi.org/10.1021/acs.bioconjchem.6b00082
Bogacheva M, Egorova A, Slita A et al (2017) Arginine-rich cross-linking peptides with different SV40 nuclear localization signal content as vectors for intranuclear DNA delivery. Bioorg Med Chem Lett 27:4781–4785. https://doi.org/10.1016/j.bmcl.2017.10.001
Fischer R, Köhler K, Fotin-Mleczek M, Brock R (2004) A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J Biol Chem 279:12625–12635. https://doi.org/10.1074/jbc.M311461200
Pantos A, Tsiourvas D, Sideratou Z et al (2004) Interactions of complementary PEGylated liposomes and characterization of the resulting aggregates. Langmuir 20:6165–6172. https://doi.org/10.1021/la040026u
Rothbard JB, Jessop TC, Lewis RS et al (2004) Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc 126:9506–9507. https://doi.org/10.1021/ja0482536
Vivès E, Brodin P, Lebleu B (1997) A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272:16010–16017. https://doi.org/10.1074/jbc.272.25.16010
Mitchell DJ, Steinman L, Kim DT et al (2000) Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res 56:318–325. https://doi.org/10.1034/j.1399-3011.2000.00723.x
Brorson JR, Marcuccilli CJ, Miller RJ (1995) Delayed antagonism of calpain reduces excitotoxicity in cultured neurons. Stroke 26:1259–1266. https://doi.org/10.1161/01.str.26.7.1259
Volbracht C, Chua BT, Ng CP et al (2005) The critical role of calpain versus caspase activation in excitotoxic injury induced by nitric oxide. J Neurochem 93:1280–1292. https://doi.org/10.1111/j.1471-4159.2005.03122.x
Jones SW, Christison R, Bundell K et al (2005) Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol 145:1093–1102. https://doi.org/10.1038/sj.bjp.0706279
Marshall J, Wong KY, Rupasinghe CN et al (2015) Inhibition of N-Methyl-d-aspartate-induced retinal neuronal death by polyarginine peptides is linked to the attenuation of stress-induced hyperpolarization of the inner mitochondrial membrane potential. J Biol Chem 290:22030–22048. https://doi.org/10.1074/jbc.M115.662791
Oh D, Nasrolahi Shirazi A, Northup K et al (2014) Enhanced cellular uptake of short polyarginine peptides through fatty acylation and cyclization. Mol Pharm 11:2845–2854. https://doi.org/10.1021/mp500203e
Mishra A, Lai GH, Schmidt NW et al (2011) Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc Natl Acad Sci USA 108:16883–16888. https://doi.org/10.1073/pnas.1108795108
Li V, Wang YT (2016) Molecular mechanisms of NMDA receptor-mediated excitotoxicity: implications for neuroprotective therapeutics for stroke. Neural Regen Res 11:1752–1753. https://doi.org/10.4103/1673-5374.194713
Sinai L, Duffy S, Roder JC (2010) Src inhibition reduces NR2B surface expression and synaptic plasticity in the amygdala. Learn Mem 26:364–371. https://doi.org/10.1101/lm.1765710
Cook DR, Gleichman AJ, Cross SA et al (2011) NMDA receptor modulation by the neuropeptide apelin: implications for excitotoxic injury. J Neurochem 118:1113–1123. https://doi.org/10.1111/j.1471-4159.2011.07383.x
Brittain JM, Piekarz AD, Wang Y et al (2009) An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem 284:31375–31390. https://doi.org/10.1074/jbc.M109.009951
Chi XX, Schmutzler BS, Brittain JM et al (2009) Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci 122:4351–4362. https://doi.org/10.1242/jcs.053280
Wang Y, Brittain JM, Wilson SM, Khanna R (2010) Emerging roles of collapsin response mediator proteins (CRMPs) as regulators of voltage-gated calcium channels and synaptic transmission. Commun Integr Biol 3:172–175. https://doi.org/10.4161/cib.3.2.10620
Fotin-Mleczek M (2005) Cationic cell-penetrating peptides interfere with TNF signalling by induction of TNF receptor internalization. J Cell Sci 118:3339–3351. https://doi.org/10.1242/jcs.02460
Weng X-C, Gai X-D, Zheng J-Q, Li J (2003) Agmatine blocked voltage-gated calcium channel in cultured rat hippocampal neurons. Acta Pharmacol Sin 24:746–750
Murphy E, Perlman M, London RE, Steenbergen C (1991) Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res 68:1250–1258. https://doi.org/10.1161/01.RES.68.5.1250
Garcia ML, King VF, Shevell JL et al (1990) Amiloride analogs inhibit l-type calcium channels and display calcium entry blocker activity. J Biol Chem 265:3763–3771
Keana JF, McBurney RN, Scherz MW et al (1989) Synthesis and characterization of a series of diarylguanidines that are noncompetitive N-methyl-d-aspartate receptor antagonists with neuroprotective properties. Proc Natl Acad Sci USA 86:5631–5635. https://doi.org/10.1073/pnas.86.14.5631
Hong L, Kim IH, Tombola F (2014) Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc Natl Acad Sci USA 11:9971–9976. https://doi.org/10.1073/pnas.1324012111
Reddy NL, Hu LY, Cotter RE et al (1994) Synthesis and structure-activity studies of N, N′-Diarylguanidine Derivatives. N-(1-Naphthyl)-N′-(3-ethylphenyl)-N′-methylguanidine: a new, selective noncompetitive NMDA receptor antagonist. J Med Chem 37:260–267. https://doi.org/10.1021/jm00028a009
Jang S, Park SH (2018) Antidiabetic drug metformin protects neuronal cells against quinolinic acid-induced excitotoxicity by decreasing intracellular calcium. Chonnam Med J 54:24–30. https://doi.org/10.4068/cmj.2018.54.1.24
Durán-Riveroll LM, Cembella AD (2017) Guanidinium toxins and their interactions with voltage-gated sodium ion channels. Mar Drugs 15:E303. https://doi.org/10.3390/md15100303
Kalia J, Swartz KJ (2011) Elucidating the molecular basis of action of a classic drug: guanidine compounds as inhibitors of voltage-gated potassium channels. Mol Pharmacol 80:1085–1095. https://doi.org/10.1124/mol.111.074989
Bowie D (2018) Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J Biol Chem 293:18789–18802. https://doi.org/10.1074/jbc.TM118.003794
Bowie D, Lange GD, Mayer ML (2018) Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci 18:8175–8185. https://doi.org/10.1523/jneurosci.18-20-08175.1998
Williams K (1997) Modulation and block of ion channels: a new biology of polyamines. Cell Signal 9:1–13. https://doi.org/10.1016/s0898-6568(96)00089-7
Zhao K, Zhao GM, Wu D et al (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279:34682–34690. https://doi.org/10.1074/jbc.M402999200
Rigobello MP, Barzon E, Marin O, Bindoli A (1995) Effect of polycation peptides on mitochondrial permeability transition. Biochem Biophys Res Commun 217:144–149. https://doi.org/10.1006/bbrc.1995.2756
Prentice H, Modi JP, Wu J-Y (2015) Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev 2015:1–7. https://doi.org/10.1155/2015/964518
Zhang YM, Bhavnani BR (2006) Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci 15:49. https://doi.org/10.1186/1471-2202-7-49
O’Donnell LA, Agrawal A, Sabnekar P et al (2007) Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem 102:1905–1917. https://doi.org/10.1111/j.1471-4159.2007.04645.x
Yang Y, Zhang X, Cui H et al (2014) Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3 K/Akt and ERK1/2 signaling pathways. Neurosci Lett 568:44–49. https://doi.org/10.1016/j.neulet.2014.03.037
Courderot-Masuyer C, Dalloz F, Maupoil V, Rochette L (1999) Antioxidant properties of aminoguanidine. Fundam Clin Pharmacol 13:535–540. https://doi.org/10.1111/j.1472-8206.1999.tb00358.x
Velier JJ, Ellison JA, Kikly KK et al (1999) Caspase-8 and caspase-3 are expressed by different populations of cortical neurons undergoing delayed cell death after focal stroke in the rat. J Neurosci 19:5932–5941
Galluzzi L, Morselli E, Kepp O, Kroemer G (2009) Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta Bioenerg 1787:402–413. https://doi.org/10.1016/j.bbabio.2008.09.006
Batulu H, Du G, Li D et al (2019) Effect of poly-arginine R18 on neurocyte cell growth via autophagy in traumatic brain injury. Exp Ther Med 17:4109–4115. https://doi.org/10.3892/etm.2019.7423
Chimura T, Launey T, Yoshida N (2015) Calpain-mediated degradation of drebrin by excitotoxicity in vitro and in vivo. PLoS ONE 10:e0125119. https://doi.org/10.1371/journal.pone.0125119
Nath R, Raser KJ, Stafford D et al (2015) Non-erythroid α-spectrin breakdown by calpain and interleukin 1 β-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 1:683–690. https://doi.org/10.1042/bj3190683
Brittain JM, Chen L, Wilson SM et al (2011) Neuroprotection against traumatic brain Injury by a peptide derived from the Collapsin Response Mediator Protein 2 (CRMP2). J Biol Chem 286:37778–37792. https://doi.org/10.1074/jbc.M111.255455
Acknowledgements
The authors would like to acknowledge the Pierce-Armstrong Foundation and the Ian Potter Foundation for funding.
Funding
This work was supported in part by University Postgraduate Award (UPA) from the University of Notre Dame, Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B.P. Meloni and N.W. Knuckey are named inventors of several patent applications (Provisional Patents: 2013904197; 30/10/2013 and 2014902319; 17/6/2014 and PCT/AU2014/050326; 30/10/2104) regarding the use of arginine-rich peptides as neuroprotective agents. The other authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MacDougall, G., Anderton, R.S., Ouliel, E. et al. In vitro cellular uptake and neuroprotective efficacy of poly-arginine-18 (R18) and poly-ornithine-18 (O18) peptides: critical role of arginine guanidinium head groups for neuroprotection. Mol Cell Biochem 464, 27–38 (2020). https://doi.org/10.1007/s11010-019-03646-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03646-0