Abstract
To investigate the expression status of FAM98A and its potential involvement in endometrial carcinoma, the relative expression of FAM98A in clinical endometrial carcinoma tissues was analyzed by immunohistochemistry and real-time polymerase chain reaction. Endogenous FAM98A protein was determined by Western blotting. The overall survival was calculated by the Kaplan–Meier’s analysis. Cell growth/viability/proliferation was evaluated by cell counting, 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide assay, and clonogenic assay, respectively. Cell apoptosis was determined by the Annexin V/7-AAD double-staining methods followed by flow cytometry analysis. The regulatory effect of miR-142-3p on FAM98A was interrogated by luciferase reporter assay. Aberrant overexpression of FAM98A was found in endometrial carcinoma both in vitro and in vivo. Furthermore, high level of FMA98A was associated with poor prognosis. FAM98A deficiency in Ishikawa and RL95-2 cells significantly inhibited cell growth, cell viability, and cell proliferation. In addition, FAM98A-knockdown stimulated remarkable cell apoptosis, which might be mediated by down-regulation of BCL2 and up-regulation of BAX. Mechanistically, it was demonstrated that miR-142-3p directly targeted FAM98A, and modulated its expression. In conclusion, we unraveled the oncogenic properties of FAM98A in endometrial carcinoma and highlighted the miR-142-3p-FAM98A signaling in this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endometrial carcinoma is one of the most common gynecological malignancies derived from the endometrium [1]. In 2012, 320,000 new cases were diagnosed and 76,000 cancer-related deaths were claimed by this disease [2]. Clinically, approximately 40% of incidence of endometrial carcinoma is related to obesity [3]. The other risk factors include excessive estrogen exposure, hypertension, and diabetes [4]. In addition, the hereditary genetic abnormalities account for 2 to 5% of morbidity of endometrial carcinoma [5]. Histologically, endometrial cancer is roughly categorized into endometrioid carcinoma, adenocarcinoma, and serous carcinoma [6]. Endometrial carcinoma is commonly diagnosed by endometrial biopsy or by obtaining samples during the procedure such as dilation and curettage. Clinically, the primary treatment for this disease is abdominal hysterectomy, along with the surgical removal of fallopian tubes and ovaries. Radiotherapy, chemotherapy, and hormone therapy either individually or in combination are applicable for the advanced patients [7]. The overall 5-year survival rate is relatively favorable with intervention at the early stage.
Family with sequence similarity 98 members A (FAM98A) was identified with a divergent N-terminal calponin homology (CH)-like domain adjoined to an array of C-terminal heptad repeats to form a coiled-coil structure [8]. The ensuing genome-wide association study identified SNP (rs1900780) of FAM98A might contribute to the type 2 diabetes (T2D) via interaction with insulin secretion loci [9]. In the osteopetrosis mice model, FAM98A was characterized to associate with nuclear distribution protein nudE-like 1 (NDEL1) and pleckstrin homology domain-containing family M member 1 (PLEKHM1) which consequently connected lysosomes to microtubules, whereas DEF8 interacted with PLEKHM1 to promote its binding to RAB7 [10]. In this context, PKHKEM1, DEF8, FAM98A, and NDEL1 constituted a molecular complex that regulated lysosome positioning and secretion through RAB7. Ozeki et al. demonstrated that FAM98A associated with stress granule-localized proteins such as DDX1, ATXN2, ATXN2L, and NUFIP2 and played a partial role in organization of stress granules [11]. More recently, the emerging lines of evidence indicated that FAM98A might involve in the tumor biology as well. Akter et al. for the first time showed that FAM98A was arginine-methylated by PRMT1 and widely expressed in numerous ovarian cancer cell lines and important for the malignant characteristics [12]. Further investigation found that two structural homologs FMA98A and FAM98B included in a novel complex with DDX1 and C14orf166 were required for PRMT1 expression and colorectal cancer progression [13]. However, the relative expression status of FAM98A in endometrial carcinoma is still elusive currently.
Here we set out to address this issue in our endometrial carcinoma sample panel at both transcript and protein level. We hypothesize that FAM98A participates in cancer progression in endometrial carcinoma.
Materials and methods
Patient samples
All human-related study was approved by the Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University Medical College. In total, 20 adjacent non-tumor endometrial tissues and 69 endometrial carcinoma tissues were collected from cancer patients with written informed consents.
Cell culture
The immortalized normal human endometrial cell line NEEC and carcinoma cell lines Ishikawa, RL95-2, HEC-1A, HEC-1B, and AN3CA were ordered from the Shanghai Cell Bank (Shanghai, China) and authenticated to be mycoplasma free. The exponential cells were maintained in RPMI-modified medium (HyClone, MO, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cells were cultured in the humidified CO2 (5%, 37 °C) incubator. For cell counting, the indicated cells were detached by trypsin digestion and prepared into single-cell suspension. Cell counting was performed under light microscope with hemacytometer.
Transfection
The indicated plasmids were transfected into host cells using the lipofectamine 2000 (Invitrogen, MA, USA) as guided by the manufacturer. To obtain higher efficiency, we adopted suspension transfection routine in this study. The single-cell suspension was prepared in complete culture medium in 6-well plate. 2 μg plasmids were pre-packaged with 10 μL lipofectamine 2000 and incubated at ambient temperature for 5 min. The mixture was then added into cell suspension dropwise and dispersed well by cross wobbling.
Immunohistochemistry
The paraffin-embedded tissue sections (5 μM, formalin-fixed) were dewaxed and rehydrated. Antigen unmasking was performed with sodium citrate retrieval buffer (pH6). Sections were stained with anti-FAM98A (1 μg/mL, Thermo Fisher, PA5-57584) or isotype control antibody overnight at 4 °C. The secondary antibody (Vector Laboratories, CA, USA) was incubated at room temperature for 1 h. The signal was detected with DAB method followed by hematoxylin staining.
Real-time polymerase chain reaction (PCR)
The TRIzol reagent (Invitrogen, MA, USA) was used to extract intracellular RNA following the manufacturer’s guide. RNA was precipitated by absolute isopropanol and washed with 75% ethanol. RNA concentration was quantified using the Nanodrop 2000 (Thermo Fisher, Waltham, MA USA). The integrity of RNA was quality checked by agarose electrophoresis. Reverse transcription for total mRNA was performed with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher, Waltham, MA USA), for miRNA was performed with the miScript II RT Kit (Qiagen, Valencia, CA, USA) according to the provider’s manual. For quantitative purpose, the SYBR Green Real-Time Master Mix was used and assay was performed on the CFX96 Touch (Bio-Rad, Hercules, CA, USA). The 2−ΔΔCt method was adopted to calculate the relative expression. The primers used in this study were as follows: FAM98A (F: GTGCCTGACAGAGGTGGTAGAC, R: CCTCCGTATGAGGAATGTTCATAG). BAX BCL2: (F: GACATGTTTTCTGACGGCAAC, R: GACATCAGTCGCTTCAGTGAC). (F:CTGAGAAGGTGAGATAAGCCCTG, R: CAATTCTGTTGACGTGGAAATG). GAPDH: (F: TGTCATCAATGGAAATCCCATC, R: CAGTGGACTCCACGACGTACTC).
Western blotting
The cell lysates were prepared in radioimmunoprecipitation lysis buffer on ice and followed by high-speed refrigerated centrifugation to completely remove cell debris. The protein quantification was conducted with BCA Protein Quantification Kit (ab102536, Abcam, Cambridge, MA, USA). The protein samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane. The non-specific background was eliminated by skim milk (5% in tris-buffered saline and Tween-20 buffer) blocking at room temperature for one hour. The target bands were blotted with specific primary antibody overnight at 4 °C and hybridized with corresponding HRP-conjugated secondary antibodies. The commercially available enhanced electrochemiluminescence kit (ECL, Millipore, Billerica, MA, USA) was used for visualization.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay
Cell viability was determined by the commercial available MTT Assay Kit (ab211091, Abcam, Cambridge, MA, USA). Briefly, 10,000 cells were seeded into each well in 96-well plate and cultured for 24 h. The culture medium was completely aspirated and replaced by the mixture of serum-free medium and MTT reagent (100 uL, 1:1). After incubation at 37 °C for 3 h, 150 uL of MTT solvent was added into each well and subjected to vigorous shaking for 15 min. The absorption at 590 nm was read on the PowerWave XS2 Microplate Spectrophotometer (BioTek, VT, USA).
Clonogenic assay
The exponential Ishikawa or RL95-2 cells were prepared into single-cell suspension via trypsin digestion. 1000 cells were seeded into each well of 6-well plate in triplicate and allowed consecutive culture for 2 weeks. After the evident colonies were visible, the culture medium was removed and replaced with phosphate-buffered saline for wash 3 times. Cells were then fixed with 4% paraformaldehyde at room temperature for 15 min and stained with crystal violet (0.25% in 20% methanol) for 10 min. The representative images were acquired under optical microscope.
Cell apoptosis
The log phase cells were cultured in 60 mm petri dish with serum-free medium for 48 h. The single-cell suspension (1 × 106 cells/ml in staining buffer) was prepared and stained with Annexin V (5 μL for 100 uL cell suspension, BioLegend, San Diego, CA, USA) and 7-AAD (5 μL for 100 μL cell suspension) at room temperature for 15 min in the dark. After adding 400 μL of Annexin V binding buffer, the apoptosis was analyzed with the BD FACSCelesta (BD BioSciences, Franklin Lakes, NJ, USA).
Luciferase reporter assay
The 3′UTR sequence was subcloned into the dual-luciferase reporter vector psiCHECK2 and co-transfected with either scramble control or miRNA mimic into Ishikawa and RL95-2 cells. The relative luciferase activity was determined using Luciferase Assay System (Promega, Madison, WI, USA) on the SpectraMax iD3 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
The results were obtained from three independent repeats unless indicated. Data were analyzed and processed using the PRISM 6.0 software. The one- or two-way ANOVA followed by a Tukey post hoc test was employed for statistical comparison. P value was calculated and P < 0.05 was considered as significantly different.
Results
FAM98A is upregulated in endometrial carcinoma and increased FAM98A expression predicts poor prognosis in endometrial carcinoma patients
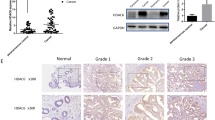
We first evaluated the relative expression status of FAM98A protein in endometrial carcinoma patients. As shown in Fig. 1a, the immunohistochemistry (IHC) results indicated the remarkably high level of FAM98A in comparison with the adjacent non-tumor control. The expression of FAM98A transcripts in more clinical endometrial carcinoma samples was determined by real-time PCR. We totally compared 69 cancer cases with 20 adjacent non-tumor controls for this end. The significant increase of FAM98A mRNA was observed in the malignant ones (3.2 vs. 1.3, P < 0.01) compared to the adjacent non-tumor group (Fig. 1b). Furthermore, we compared FAM98A protein in our endometrial carcinoma cell line panel with the immortalized endometrial epithelial cell. As shown in Fig. 1c, the relative high expression of FAM98A was observed in all our collected endometrial carcinoma cell lines including Ishikawa, RL95-2, HEC-1A, HEC-1B, and AN3CA. In addition, we analyzed the potential prognostic relevance of aberrant expression of FAM98A in our clinical patient pool. Our results unambiguously demonstrated that high FAM98A was significantly associated with unfavorable outcome (Fig. 1d, P < 0.05). Our study firstly characterized aberrant overexpression of FAM98A in endometrial carcinoma, which predicted the poor prognosis of this disease.
FAM98A is upregulated in endometrial carcinoma and increased FAM98A expression predicts poor prognosis in endometrial carcinoma patients. a IHC analysis of FAM98A expression in endometrial carcinoma tissues. Representative 400X pictures were presented. Scale bars, 50 μm. b Quantitative RT-PCR analysis of the level of FAM98A RNA in endometrial carcinoma tissues. GAPDH served as loading controls. c WB detection of FAM98A protein in endometrial carcinoma cells lines. β-actin served as loading controls. d Kaplan–Meier’s analysis of the correlation between FAM98A expression and overall survival of endometrial carcinoma patients. Data were presented as mean ± SD of three independent experiments. **P < 0.01
FAM98A-deficiency inhibits endometrial carcinoma cells proliferation
Our previous results implicated the oncogenic property of FAM98A in endometrial carcinoma both in vitro and in vivo. Next, we attempted to silence the FAM98A expression in Ishikawa and RL95-2 cells and investigate its impact on the cell proliferative behavior. The success in establishment of stable FAM98A-deficient cell lines is shown in Fig. 2a. To exclude the potential off-target effect of individual shRNA, here we employed two independent shRNA to knockdown FAM98A. FAM98A-deficiency significantly suppressed cell growth in both Ishikawa and RL95-2 cells in comparison with scramble control (Fig. 2b, c). Likewise, our MTT assay results demonstrated that knockdown of FAM98A compromised the cell viability as well (Fig. 2d, e). We further evaluated the clonogenic capacity in response to FAM98A knockdown in endometrial carcinoma cells. The visible colonies were remarkably decreased in FAM98A-deficient Ishikawa and RL95-2 cells (Fig. 2f, g). Therefore, our results highlighted the critical role of FAM98A in maintenance of cell growth in endometrial carcinoma.
FAM98A inhibits endometrial carcinoma cells proliferation. a Western blotting assay for the levels of FAM98A in Ishikawa and RL95-2 cells stably expressing shCtrl or shFAM98A. β-Actin served as loading controls. b Ishikawa c RL95-2 cells were subjected to cell number assay every 24 h. Cell proliferation assay of d Ishikawa and e RL95-2 cells were measured at 96 h by MTT. f Ishikawa g RL95-2 cells were subjected to cell colony formation assay. Data were presented as mean ± SD of three independent experiments. *P < 0.05; **P < 0.01
Knockdown of FAM98A induces cell apoptosis in endometrial carcinoma cell lines
Next, we sought to clarify whether FAM98A inhibited spontaneous apoptosis in endometrial carcinoma cells. The Ishikawa and RL95-2 cells were deprived of serum in the culture medium for 48 h. The cell apoptosis was measured with the Annexin V/7-AAD method. As shown in Fig. 3a, the FAM98A-deficiency stimulated remarkable apoptosis in response to serum starvation in Ishikawa cells. Both early and late apoptosis were increased in FAM98A-knockdown cells. Consistently, the comparable effects were observed in RL95-2 cells (Fig. 3b). In addition to the pro-proliferative effects, here we demonstrated that knockdown of FAM98A induced significant cell apoptosis in endometrial carcinoma cells.
Cellular expression of apoptosis-related gene was analyzed in endometrial carcinoma cells
Next, we pursued to investigate the alterations in apoptotic signaling in the FAM98A-deficient cells upon serum restriction. The transcripts of BAX and BCL2 were quantified by real-time PCR. FAM98A-silencing significantly increased BAX transcription and decreased BCL2 transcription in both Ishikawa and RL95-2 cells (Fig. 4a). Likewise, the protein level of BAX and BCL2 was regulated in the same manner in response to FAM98A manipulation (Fig. 4b). Therefore, in agreement with previously observed apoptotic phenotype in FAM98A-deficient cell while deprived of serum, here we further demonstrated the fundamental changes in the apoptotic molecular events.
Cellular expression of apoptosis-related gene was analyzed in endometrial carcinoma cells. a BAX and BCL2 levels were measured via qRT-PCR and normalized to the level of GAPDH. b Western blot analysis of BCL2 and BAX expression. β-actin serves as an internal control. Data are mean ± S.D. of three independent experiment and each measured in triplicate (*P < 0.05)
FAM98A is a direct target of miR-142-3p
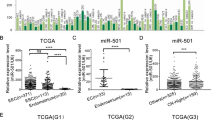
Our previous results uncovered the aberrant overexpression of FAM98A in endometrial carcinoma and its oncogenic properties. However, the upstream signaling in mediating the up-regulation of FAM98A is still elusive in this context. Here, we focused on the potential involvement of microRNA in the regulatory actions of FAM98A. With the aid of online bioinformatics algorithm (http://www.targetscan.org/), we identified a putative binding site of miR-142-3p in the 3′UTR region (Fig. 5a) and constructed the scramble control luciferase reporter plasmid. Co-transfection with miR-142-3p mimic significantly inhibited the luciferase activity in comparison with negative control. However, the mutation introduced into the putative sequence completely abolished this inhibitory effect (Fig. 5b). The endogenous FAM98A protein was remarkably decreased in response to ectopic expression of miR-142-3p mimic in both Ishikawa and RL95-2 cells (Fig. 5c). In view of the potential regulatory action of miR-142-3p on FAM98A in endometrial carcinoma, we further analyzed the expression status of miR-142-3p in our clinical endometrial carcinoma sample panel. As shown in Fig. 5d, the relative expression of miR-142-3p in malignancies was significantly lower than in the normal counterparts. Comparison of FAM98A and miR-142-3p identified the reverse correlation in this disease (Fig. 5e). Therefore, we demonstrated that miR-142-3p directly modulated FAM98A expression in endometrial carcinoma.
FAM98A is a direct target of miR-142-3p. a Sequences of miRNA and the potential miRNA binding sites at the 3′UTR of FAM98A. b Relative luciferase activity of Ishikawa and RL95-2 cells after co-transfected with wild-type or mutant FAM98A 3′UTR reporter genes and miR-ctrl or miR-142-3p mimic. c Overexpression of miR-142-3p reduced the protein expression of FAM98A in Ishikawa and RL95-2 cells. d miR-142-3p levels in cancer tissues and adjacent non-tumor tissues were detected via qRT-PCR and normalized to the level of U6. e The correlation between miR-142-3p and FAM98A in endometrial carcinoma tissues was analyzed. Data are mean ± S.D. of three independent experiment and each measured in triplicate (**P < 0.01)
Discussion
The previous investigations unraveled the intimate association between aberrant expression of FAM98A and incidence of some human malignancies such as ovarian carcinoma and colorectal cancer [12, 13]. However, the relative expression status of FAM98A in endometrial carcinoma and its potential mechanistic involvement in this disease was still elusive. Here, we for the first time characterized the aberrant overexpression of FAM98A at both protein and mRNA levels in our clinical endometrial carcinoma panel. The comparable results were consolidated in the cell culture. Furthermore, the Kaplan–Meier’s survival curve implicated the evident unfavorable prognostic value of high FAM98A. Therefore, our data for the first time uncovered the oncogenic properties of FMA98A in endometrial carcinoma. Through gene manipulation with specific shRNAs, we demonstrated that FAM98A-deficiency in endometrial carcinoma cells tremendously impaired cell proliferation and cell viability. Notably, we detected spontaneous cell apoptosis in the FMA98A-deficient Ishikawa and RL95-2 cells in sharp contrast to the parental control upon serum deprivation. In addition to the pro-proliferative effects, here we disclosed the anti-apoptotic properties of FAM98A in endometrial carcinoma. In this regard, decreased BCL2 and increased BAX was observed in FAM98A-knockdown cells under the serum-free culture conditions, which suggested that these key factors played critical role in mediating cell apoptosis in this context. Mechanistically, we predicted and identified that miR-142-3p directly modulated FAM98A expression via post-transcriptional mode. Ectopic introduction of miR-142-3p remarkably inhibited endogenous FAM98A. Most importantly, we detected the concomitant down-regulation of miR-142-3p in FAM98A-proficient endometrial carcinoma cells and the reverse correlation between these two genes. The fundamental role of the miR-142-3p-FAM98A signaling underlying the malignant behaviors of endometrial carcinoma was highlighted in our investigation.
Assembling lines of evidence suggested the indispensable roles of miR-142-3p in variety of human cancers. For instance, Jia et al. characterized aberrant expression of miR-142-3p in breast cancer and identified the target genes HMGA1 and FZD7, through which miR-142-3p might serve as tumor suppressor and potential diagnostic marker and therapeutic target [14]. Gao et al. demonstrated that miR-142-3p inhibited cell proliferation and chemoresistance in ovarian cancer via targeting sirtuin-1 [15]. Wang et al. showed the down-regulation of miR-142-3p and its tumor suppressor role in gastric cancer [16]. Godfrey et al. provided evidence that miR-142-3p was downregulated in aggressive p53 mutant mouse models of pancreatic ductal adenocarcinoma by hypermethylation of its locus [17]. Wan et al. proposed that miR-142 regulated CD4 + T cells in human non-small cell lung cancer through PD-L1 expression via the PTEN pathway [18]. Trissal et al. identified miR-142 loss-of-function mutations derepressed ASH1L to increase HOXA gene expression and promoted leukemogenesis [19]. Lee et al. unraveled miR-142-3p was involved in the regulation of MGMT expression glioblastoma cells [20]. In hepatocellular carcinoma, Hua et al. demonstrated that miR-142-3p inhibited aerobic glycolysis and cell proliferation via targeting LDHA [21]. Wang et al. found that miR-142 suppressed tumorigenesis of non-small-cell lung cancer by targeting PIK3CA [22]. On the contrary, the oncogenic properties of miR-142 were proposed in a number of reports. Islam et al. suggested that high miR-142 expression was associated with the biological aggressiveness of cancer [23]. Bai et al. demonstrated that miR-142 induced cancer stem cell-like properties of cutaneous squamous cell carcinoma via inhibiting PTEN [24]. Liu et al. showed that miR-142 promoted cell growth and migration in renal cell carcinoma by targeting BTG3 [25]. In agreement with the tumor suppressor function of miR-142-3p, here we characterized the down-regulation of miR-142-3p in endometrial carcinoma and implicated in FAM98A regulation, which consequently contributed to the tumorigenesis of endometrial carcinoma.
With advance in understanding the fundamental functions of microRNAs in human cancers, a number of microRNAs have been identified to play kaleidoscopic roles in endometrial carcinoma. MiR-302 family members including 302b-3p, 302c-3p, and 302d-3p inhibited epithelial–mesenchymal transition and promoted apoptosis [26]. MiR-101 was shown to inhibit angiogenesis via COX-2 in endometrial carcinoma [27]. Ma et al. demonstrated that miR-302a-5p/367-3p-HMGA2 axis regulated malignant processes during endometrial cancer development [28]. Lu et al. reported miR-424/E2F6 feedback loop modulated cell invasion, migration, and epithelial-to-mesenchymal transition [29]. Devor et al. uncovered that dysregulation of miR-181c expression influenced the recurrence of endometrial endometrioid adenocarcinoma by modulating NOTCH2 expression [30]. Chen et al. showed that miR-29b inhibited angiogenesis by targeting vascular endothelial growth factor through the MAPK/ERK and PI3 K/AKT signaling pathway [31]. Here, we for the first time characterized suppressive expression of miR-142-3p in endometrial carcinoma, which negatively regulated FAM98A expression post-transcriptionally and consequently contributed to the malignant behaviors.
Conclusion
In summary, we unraveled the aberrant overexpression of FAM98A in endometrial carcinoma and highlighted the critical role of miR-142-3p-FAM98A signaling in this disease.
References
Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S (2011) Endometrial cancer. BMJ 343:d3954. https://doi.org/10.1136/bmj.d3954
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30. https://doi.org/10.3322/caac.21166
Fader AN, Arriba LN, Frasure HE, von Gruenigen VE (2009) Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol 114(1):121–127. https://doi.org/10.1016/j.ygyno.2009.03.039
Galaal K, Al Moundhri M, Bryant A, Lopes AD, Lawrie TA (2014) Adjuvant chemotherapy for advanced endometrial cancer. Cochrane Database Syst Rev 5:CD010681. https://doi.org/10.1002/14651858.cd010681.pub2
Zhou JY, Zhang L, Wei LH, Wang JL (2016) Endometrial carcinoma-related genetic factors: application to research and clinical practice in China. BJOG 123(Suppl 3):90–96. https://doi.org/10.1111/1471-0528.14007
Murali R, Soslow RA, Weigelt B (2014) Classification of endometrial carcinoma: more than two types. Lancet Oncol 15(7):e268–278. https://doi.org/10.1016/S1470-2045(13)70591-6
Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C, Group EGW (2011) Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):35–39. https://doi.org/10.1093/annonc/mdr374
Schou KB, Andersen JS, Pedersen LB (2014) A divergent calponin homology (NN-CH) domain defines a novel family: implications for evolution of ciliary IFT complex B proteins. Bioinformatics 30(7):899–902. https://doi.org/10.1093/bioinformatics/btt661
Keaton JM, Hellwege JN, Ng MCY, Palmer ND, Pankow JS, Fornage M, Wilson JG, Correa A, Rasmussen-Torvik LJ, Rotter JI, Chen YI, Taylor KD, Rich SS, Wagenknecht LE, Freedman BI, Bowden DW (2017) Genome-wide interaction with selected type 2 diabetes loci reveals novel loci for type 2 diabetes in African Americans. Pac Symp Biocomput 22:242–253. https://doi.org/10.1142/9789813207813_0024
Fujiwara T, Ye S, Castro-Gomes T, Winchell CG, Andrews NW, Voth DE, Varughese KI, Mackintosh SG, Feng Y, Pavlos N, Nakamura T, Manolagas SC, Zhao H (2016) PLEKHM1/DEF8/RAB7 complex regulates lysosome positioning and bone homeostasis. JCI Insight 1(17):e86330. https://doi.org/10.1172/jci.insight.86330
Ozeki K, Sugiyama M, Akter KA, Nishiwaki K, Asano-Inami E, Senga T (2018) FAM98A is localized to stress granules and associates with multiple stress granule-localized proteins. Mol Cell Biochem 2:3. https://doi.org/10.1007/s11010-018-3397-6
Akter KA, Mansour MA, Hyodo T, Ito S, Hamaguchi M, Senga T (2016) Erratum to: FAM98A is a novel substrate of PRMT1 required for tumor cell migration, invasion and colony formation. Tumour Biol 37(5):7001. https://doi.org/10.1007/s13277-016-4833-4
Akter KA, Mansour MA, Hyodo T, Senga T (2017) FAM98A associates with DDX1-C14orf166-FAM98B in a novel complex involved in colorectal cancer progression. Int J Biochem Cell Biol 84:1–13. https://doi.org/10.1016/j.biocel.2016.12.013
Jia XP, Meng LL, Fang JC, Wang HW, Chen J, Zhou J, Wang CN, Jiang WF (2018) Aberrant expression of miR-142-3p and its target gene HMGA1 and FZD7 in breast cancer and its clinical significance. Clin Lab 64(6):915–921. https://doi.org/10.7754/Clin.Lab.2017.171114
Gao J, Wu N, Liu X, Xia Y, Chen Y, Li S, Deng Z (2018) MicroRNA-142-3p inhibits cell proliferation and chemoresistance in ovarian cancer via targeting sirtuin 1. Exp Ther Med 15(6):5205–5214. https://doi.org/10.3892/etm.2018.6107
Wang Y, Cao Z, Wang L, Liu S, Cai J (2018) Downregulation of microRNA-142-3p and its tumor suppressor role in gastric cancer. Oncol Lett 15(5):8172–8180. https://doi.org/10.3892/ol.2018.8330
Godfrey JD, Morton JP, Wilczynska A, Sansom OJ, Bushell MD (2018) MiR-142-3p is downregulated in aggressive p53 mutant mouse models of pancreatic ductal adenocarcinoma by hypermethylation of its locus. Cell Death Dis 9(6):644. https://doi.org/10.1038/s41419-018-0628-4
Cai Y, Wang W, Guo H, Li H, Xiao Y, Zhang Y (2018) miR-9-5p, miR-124-3p, and miR-132-3p regulate BCL2L11 in tuberous sclerosis complex angiomyolipoma. Lab Invest. https://doi.org/10.1038/s41374-018-0051-6
Trissal MC, Wong TN, Yao JC, Ramaswamy R, Kuo I, Baty J, Sun Y, Jih G, Parikh N, Berrien-Elliott MM, Fehniger TA, Ley TJ, Maillard I, Reddy PR, Link DC (2018) MIR142 loss-of-function mutations derepress ASH1L to increase HOXA gene expression and promote leukemogenesis. Cancer Res 78(13):3510–3521. https://doi.org/10.1158/0008-5472.CAN-17-3592
Lee YY, Yarmishyn AA, Wang ML, Chen HY, Chiou SH, Yang YP, Lin CF, Huang PI, Chen YW, Ma HI, Chen MT (2018) MicroRNA-142-3p is involved in regulation of MGMT expression in glioblastoma cells. Cancer Manag Res 10:775–785. https://doi.org/10.2147/CMAR.S157261
Hua S, Liu C, Liu L, Wu D (2018) miR-142-3p inhibits aerobic glycolysis and cell proliferation in hepatocellular carcinoma via targeting LDHA. Biochem Biophys Res Commun 496(3):947–954. https://doi.org/10.1016/j.bbrc.2018.01.112
Wang Z, Liu Z, Fang X, Yang H (2017) MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in non-small cell lung cancer. Cell Physiol Biochem 43(6):2505–2515. https://doi.org/10.1159/000484459
Islam F, Gopalan V, Vider J, Lu CT, Lam AK (2018) MiR-142-5p act as an oncogenic microRNA in colorectal cancer: clinicopathological and functional insights. Exp Mol Pathol 104(1):98–107. https://doi.org/10.1016/j.yexmp.2018.01.006
Bai X, Zhou Y, Chen P, Yang M, Xu J (2018) MicroRNA-142-5p induces cancer stem cell-like properties of cutaneous squamous cell carcinoma via inhibiting PTEN. J Cell Biochem 119(2):2179–2188. https://doi.org/10.1002/jcb.26379
Liu L, Liu S, Duan Q, Chen L, Wu T, Qian H, Yang S, Xin D, He Z, Guo Y (2017) MicroRNA-142-5p promotes cell growth and migration in renal cell carcinoma by targeting BTG3. Am J Transl Res 9(5):2394–2402
Li Y, Huo J, Pan X, Wang C, Ma X (2018) MicroRNA 302b-3p/302c-3p/302d-3p inhibits epithelial-mesenchymal transition and promotes apoptosis in human endometrial carcinoma cells. Onco Targets Ther 11:1275–1284. https://doi.org/10.2147/OTT.S154517
Liu Y, Li H, Zhao C, Jia H (2018) MicroRNA-101 inhibits angiogenesis via COX-2 in endometrial carcinoma. Mol Cell Biochem. https://doi.org/10.1007/s11010-018-3313-0
Ma J, Li D, Kong FF, Yang D, Yang H, Ma XX (2018) miR-302a-5p/367-3p-HMGA2 axis regulates malignant processes during endometrial cancer development. J Exp Clin Cancer Res 37(1):19. https://doi.org/10.1186/s13046-018-0686-6
Lu Z, Nian Z, Jingjing Z, Tao L, Quan L (2017) MicroRNA-424/E2F6 feedback loop modulates cell invasion, migration and EMT in endometrial carcinoma. Oncotarget 8(69):114281–114291. https://doi.org/10.18632/oncotarget.23218
Devor EJ, Miecznikowski J, Schickling BM, Gonzalez-Bosquet J, Lankes HA, Thaker P, Argenta PA, Pearl ML, Zweizig SL, Mannel RS, Brown A, Ramirez NC, Ioffe OB, Park KJ, Creasman WT, Birrer MJ, Mutch D, Leslie KK (2017) Dysregulation of miR-181c expression influences recurrence of endometrial endometrioid adenocarcinoma by modulating NOTCH2 expression: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 147(3):648–653. https://doi.org/10.1016/j.ygyno.2017.09.025
Chen HX, Xu XX, Tan BZ, Zhang Z, Zhou XD (2017) MicroRNA-29b inhibits angiogenesis by targeting VEGFA through the MAPK/ERK and PI3 K/Akt signaling pathways in endometrial carcinoma. Cell Physiol Biochem 41(3):933–946. https://doi.org/10.1159/000460510
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All patients have written informed consents.
Research involving human participants and/or animals
This study had been approved by the Affiliated Yantai Yuhuangding Hospital of Qingdao University Medical College.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Li, N., Sun, X. et al. FAM98A promotes cancer progression in endometrial carcinoma. Mol Cell Biochem 459, 131–139 (2019). https://doi.org/10.1007/s11010-019-03556-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03556-1