Abstract
Stress granules are evolutionally conserved ribonucleoprotein structures that are formed in response to various stress stimuli. Recent studies have demonstrated that proteins with low complexity (LC) regions play a critical role for the formation of stress granules. In this study, we report that FAM98A, whose biological functions are unknown, is a novel component of stress granules. FAM98A is localized to stress granules, but not to P-bodies, after various stress stimuli. Analysis with deletion mutants revealed that C-terminal region that contains LC region was essential for FAM98A accumulation to stress granules. Depletion of FAM98A using two different siRNAs decreased the number of stress granules formed per cell. Finally, we show that FAM98A associates with stress granule-localized proteins, such as DDX1, ATXN2, ATXN2L, and NUFIP2. Our results show a partial role of FAM98A for the organization of stress granules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress granules are non-membranous ribonucleoprotein structures that are induced by various stress stimuli, such as heat, osmotic shock and oxidation [1]. One of the critical events for stress granule formation is phosphorylation of serine 51 of eIF2α (eukaryotic initiation factor 2 alpha), which is mediated by one or more of the four protein kinases, HRI (heme-regulated inhibitor), PERK (protein kinase R-like endoplasmic reticulum kinase), PKR (protein kinase R), GCN2 (general control non-derepressible 2) [2]. The phosphorylation inhibits formation of eIF2/tRNAiMet/GTP ternary complex that is essential for the initiation of translation [3]. In the absence of the ternary complex, ribosomes already in translation “run-off” polysomes, promoting accumulation of messenger ribonucleoprotein particles (mRNPs) that leads to the assembly of stress granule. In addition to translational suppression, post-translational modifications, such as phosphorylation, ubiquitination, and arginine methylations, are important for either assembly or disassembly of stress granules [4, 5].
Stress granule formation has been demonstrated to be critical for the survival of cells in response to stresses. For example, RACK1 (receptor for activated C kinase 1) is a scaffolding protein that promotes activation of p38/JNK (c-Jun N-terminal kinase) signaling cascade to initiate apoptosis [6]. Upon stresses, such as hypoxia, RACK1 is sequestered in stress granules so that the activation of apoptotic pathway is inhibited [7]. Another protein that is trapped in stress granules for cell survival is ROCK1 (rho associated coiled-coil containing kinase 1), which phosphorylates JIP-3 (JNK interacting protein 3) for the activation of JNK pathway for the induction of apoptosis in response to stress stimuli [8]. Stress granules are also known to promote cell survival by protecting mRNAs coding factors essential for the activation of survival pathways. Once stress granules are disassembled by the removal of stress, proteins necessary for cell survival are translated from mRNAs protected in stress granules [3].
Accumulating studies have identified a number of proteins that are associated with stress granule organization [9]. For instance, G3BP1 (Ras GTPase activating protein binding 1) is one of the critical constituents of stress granules and its depletion inhibits stress granule organization [10,11,12,13]. G3BP1 has multiple binding proteins and functions to recruit various proteins and RNAs to stress granules [2]. Recent proteomics studies have reported important functions of proteins with low complexity (LC) regions for stress granule formation [14, 15]. LC regions contain little diversity in amino acid sequence, but play important roles for protein–protein and protein–RNA interactions [16, 17]. In vitro studies have shown that proteins with LC regions can assemble hydrogels through a process called liquid–liquid phase separation (LLPS), which allows proteins to reversibly concentrate in discrete foci in cells [18]. It is speculated that LLPS mediated by proteins with LC regions regulates the organization of ribonucleoprotein (RNP) granules, including stress granules.
We previously reported that FAM98A (family with sequence similarity 98 member A) is a novel substrate of PRMT1 (protein arginine methyltransferase 1) and is required for the malignancy of ovarian cancer [19]. FAM98A has LC regions rich in glycine in the C-terminus. A previous study reported that FAM98A was identified by mass spectrometry analysis using purified core parts of stress granules [9]. In this report, we show that FAM98A is a stress granule-localized protein and form a complex with stress granule-localized proteins, such as DDX1 (DEAD box helicase 1), ATXN2 (ataxin 2), ATXNL2 (ataxin 2 like), and NUFIP2 (FMR1 interacting protein 2).
Materials and methods
Cells, antibodies, and chemicals
HeLa cells that were obtained from RIKEN BioResource Center (Tsukuba, Japan) were cultured in DMEM (Wako, Osaka, Japan) supplemented with 10% FBS and antibiotics at 37 °C. Antibodies were obtained from the following companies: anti-FAM98A antibody (ARP44265-P050), Aviva Systems Biology (San Diego, CA, USA); anti-G3BP1 antibody (611,126), BD Biosciences (San Jose, CA, USA); anti-eIF4E antibody (sc-9976), Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-DCP1A antibody (H00055802-M06), Abnova (Taipei,Taiwan); anti-Flag antibody (014-22383), Wako; anti-ATXN2 (A301-118A-T), anti-ATXN2L (A301-371A-T), anti-NUFIP2 (A301-599A-T), and anti-DDX1 (A300-521A-T) antibodies, Bethyl laboratories (Montogomery,TX, USA). Adenosine-2′ 3′-dialdehyde (Adox) was purchased from Wako.
siRNA transfection
The sequences of the siRNAs used to suppress FAM98A expression were 5′-CCAAACCUCCAGCCAAUAUTT-3′ (FAM98A siRNA#1), 5′-CCGAAACGUUCAGUCUUAUTT-3′ (FAM98A siRNA#2). HeLa cells were transfected with 50 nM siRNA using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA).
Generation of stable cell lines
To produce HeLa cells that constitutively expressed Flag tagged full length, C-terminus, or N-terminus FAM98A, each cDNA was cloned into pQCXIP retroviral vector (Clontech, Mountain View, CA, USA). 293T cells were transfected with the pQCXIP vector encoding each FAM98A cDNA together with pVPack-GP and pVPack-Ampho vectors (Stratagene, Tokyo, Japan). The culture supernatant was collected 48 h later and applied to HeLa cells with 2 µg ⁄ mL of polybrene (Sigma-Aldrich, St. Louis, MO, USA). After 24 h, 1 µg ⁄ mL of puromycin (Sigma-Aldrich) was added to select for infected cells.
Immunoblot analysis
Cells were lysed with Laemmli sample buffer (20% glycerol, 135 mM Tris–HCl, pH 6.8, 4% SDS, 10% 2-mercaptoethanol, 0.003% BPB) and denatured by boiling for 5 min. The protein concentrations of the lysates were measured using the RC-DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Equal protein quantities of the lysates were electrophoresed on SDS–polyacrylamide gels and transferred to PVDF membranes (Millipore, Darmstadt, Germany). The membrane was blocked with 0.5% non-fat skim milk for 1 h, incubated with primary antibody for 1 h, washed with TBS-T for 15 min, incubated with HRP-conjugated secondary antibody for 1 h. and then signals were detected using ECL system (Nacalai Tokyo Japan).
Immunofluorescence analysis
Cells cultured on fibronectin-coated glass cover slips were transfected with siRNAs and then stimulated with 0.5 mM arsenite for 30 min, 300 mM sorbitol for 30 min, 1 mM hydrogen peroxide for 1.5 h, or heat (44 °C) for 30 min. Cells were fixed with 4% paraform aldehyde for 20 min and blocked with phosphate-buffered saline (PBS) containing 10% FBS for 30 min. The cells were incubated with primary antibodies for 1 h, washed with PBS, and incubated with Alexa Fluor 488- or Alexa Fluor 594-labeled secondary antibodies (Invitrogen, Carlsbad, CA, USA) for 1 h. Images were acquired using an FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Immunoprecipitation
Cells were lysed in lysis buffer (35 mM Tris–HCl (pH 7.4), 150 mM NaCl, and 1% NP-40) for 15 min on ice and centrifuged at 15,000 rpm for 20 min. The supernatants were incubated with anti-Flag antibody beads (Wako, Osaka, Japan) at 4 °C for 1 h. The beads were washed three times with lysis buffer and suspended in sample buffer.
Statistical analysis
Values are expressed as the mean ± SD. Comparisons between groups were performed with a one-way or two-way ANOVA using GraphPad Prism software version 7.0.
Results
FAM98A localizes to stress granules
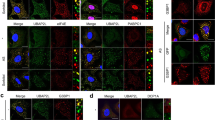
To determine whether FAM98A is a component of stress granules, HeLa cells were treated with different concentrations of arsenite for 30 min and then immunostained with anti-FAM98A and anti-G3BP1 antibodies. Formation of stress granules, which was judged by the accumulation of G3BP1, was observed with concentrations of arsenite higher than 0.1 mM (Fig. 1a). FAM98A was clearly co-localized with G3BP1 in cells treated with 0.1 and 0.5 mM of arsenite (Fig. 1a). Stress granules are formed by other stresses, such as heat, osmotic shock, and hydrogen peroxide. FAM98A was also co-localized with G3BP1 in cells treated with these stresses (Fig. 1b). Cycloheximide, which inhibits elongation of ribosome for protein synthesis, is known to disrupt formation of stress granules [20]. HeLa cells were stimulated with arsenite in the absence or presence of cycloheximide and immunostained for FAM98A and eIF4E, which is one of the stress granule markers [21]. As shown in Fig. 1c, both FAM98A and eIF4E localized to the cytoplasmic dots, and the organization of these dots were disrupted in the presence of cycloheximide. These results clearly show that FAM98A is a novel component of stress granules.
FAM98A is localized to stress granules. a HeLa cells were treated with different concentrations of arsenite (AS) for 30 min and immunostained for FAM98A and G3BP1 (scale bar = 10 μm). b HeLa cells were treated with 0.5 mM of arsenite (AS) for 30 min, 0.3 M of sorbitol for 30 min, heat shocked (44 °C) for 30 min, or 1 mM of hydrogen peroxide (H2O2) for 1.5 h. The cells were immunostained for G3BP1 and FAM98A (scale bar = 10 μm). c Cells were treated with 0.5 mM of arsenite for 30 min in the presence or absence of cycloheximide (CHX) and then immunostained for eIF4E and FAM98A (scale bar = 10 μm). d Cells treated with arsenite were immunostained for DCP1A and FAM98A (scale bar = 10 μm)
Processing body (P-body) is a cytoplasmic foci that is composed of various enzymes for mRNA turnover [1]. Some proteins are known to localize to both stress granules and P-bodies. We tested if FAM98A is also localized to P-body by immunofluorescent analysis. HeLa cells treated or non-treated with arsenite were immunostained for FAM98A and DCP1A, which is a specific marker for P-bodies [1]. As shown in Fig. 1d, FAM98A did not co-localize with DCP1A, indicating that FAM98A specifically localizes to stress granules.
C-terminal region is responsible for FAM98A localization to stress granules
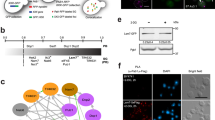
We next tested which region of FAM98A was required for the localization. FAM98A has a LC region that is rich in glycine in the C-terminus, but has no specific domain in the N-terminus. HeLa cells that constitutively expressed Flag tagged either full length (FL), C-terminus (C) or N-terminus (N) FAM98A was generated and immunostained for Flag and G3BP1 after arsenite treatment. Although full length and C-terminus clearly localized to stress granules, N-terminus did not show any specific localization (Fig. 2a). The specific localization of the C-terminus was observed in cells treated with 44 °C for 30 min as well (Fig. 2a). These results show that the C-terminal region that contains LC region is critical for the localization of FAM98A to stress granules.
C-terminus of FAM98A is responsible for the localization to stress granules. a Cells that constitutively expressed Flag tagged full length (FL), N-terminus (N), or C-terminus (C) FAM98A were treated with 0.5 mM of arsenite for 30 min or heat shocked at 44 °C for 30 min. The cells were immunostained Flag and G3BP1 (scale bar = 10 μm). b Full length FAM98A-expressing cells were cultured in the presence or absence of Adox for 24 h and then treated with 0.5 mM of arsenite for 30 min. Cells were immunostained for FAM98A and G3BP1 (scale bar = 10 μm)
We previously reported that the C-terminal region of FAM98A was arginine methylated by PRMT1 [19]. To test if arginine methylation was required for FAM98A localization to the stress granules, cells were treated with arsenite in the presence or absence of Adox, an inhibitor for arginine methylation. Addition of 100 uM of Adox, which is sufficient for the inhibition of arginine methylation of FAM98A [19], did not affect FAM98A accumulation to stress granules (Fig. 2b). The result indicates that arginine methylation is dispensable for the FAM98A localization to stress granules.
Depletion of FAM98A reduces the number of stress granules per cell
We next examined whether FAM98A was required for the organization of stress granules. Treatment of HeLa cells with FAM98A siRNAs efficiently reduced FAM98A expression (Fig. 3a). Cells treated with FAM98A siRNAs were treated with arsenite and immunostained for G3BP1 and FAM98A. Formation of stress granules was observed in cells depleted of FAM98A (Fig. 3b); however, the number of stress granules per cell appeared to be smaller than that of control siRNA-transfected cells. As shown in Fig. 3c, the number of stress granules in FAM98A-depleted cells was around 70% of control siRNA-transfected cells. The similar results were also observed when cells were treated at 44 °C (Fig. 3d). These results indicate that FAM98A is partially required for organization of stress granules.
Depletion of FAM98A reduces the number of stress granules per cell. a HeLa cells were transfected with control or FAM98A siRNAs and 72 h later, cells were lysed and expression of FAM98A was examined by immunoblot. b Cells transfected with siRNAs were treated with 0.5 mM of arsenite for 30 min and immunostained with anti-FAM98A and anti-G3BP1 antibodies (scale bar = 10 μm). c, d The number of stress granules per cell under each condition is presented in the graph. 50 cells were evaluated for the number of stress granules per cell (*P < 0.05). Image J software was used to count stress granules
FAM98A associates with multiple stress granule-localized proteins
To gain more insight for the function of FAM98A, we previously performed mass spectrometry analysis [22]. In this analysis, we found that some stress granule-localized proteins, such as DDX1, NUFIP2, ATXN2, and ATXNL2 [23,24,25,26], were in complex with FAM98A. To confirm the association of these proteins with FAM98A, Flag-FAM98A, Flag-FAM98A-N, or Flag-FAM98A-C was expressed in 293T cells and then Flag-FAM98A and associated proteins were immunoprecipitated with anti-Flag antibody. The immunoprecipitates were subjected to immunoblot analysis with anti-DDX1, anti-NUFIP2, anti-ATX2 or anti-ATXNL2 antibody. As shown in Fig. 4a, NUFIP2, ATX2, and ATXNL2 were co-precipitated with Flag-FAM98A and Flag-FAM98A-N. Interestingly, DDX1 was co-precipitated with both N and C-terminus of FAM98A. We tested if localization of these proteins to stress granules was regulated by FAM98A. HeLa cells transfected with FAM98A siRNA were treated with arsenite and immunostained for G3BP1 and FAM98A-associating proteins. Although ATXN2, ATXNL2, and NUFIP2 were localized to stress granules in the absence FAM98A, localization of DDX1 was clearly diminished (Fig. 4b). These results show that FAM98A is essential for the localization of DDX1 to stress granules, but dispensable for the accumulation of ATXN2, ATXNL2, and NUFIP2 to stress granules.
FAM98A associates stress granule-localized proteins. (A) HeLa cells that constitutively expressed Flag tagged full length (FL), N-terminus (N), or C-terminus (C) FAM98A were lysed and cell lysates were immunoprecipitated with anti-Flag antibody. The immunoprecipitates were subjected to immunoblot analysis with the indicated antibodies. (B) Cells transfected control or FAM98A siRNAs were treated with 0.5 mM of arsenite for 30 min and then immunostained for the indicated proteins (scale bar = 10 μm)
Discussion
In this report, we showed that FAM98A is a novel component of stress granules. Immunofluorescence analysis using specific antibody demonstrated accumulation of endogenous FAM98A to stress granules, but not P-bodies, under various stress stimuli, including arsenite, hydrogen peroxide, heat and osmotic shock. In addition, exogenously expressed Flag tagged FAM98A showed clear localization to the stress granule. FAM98A has LC regions that are rich in glycine and arginine. Expression of Flag tagged deletion mutants demonstrated that C-terminus with LC regions, but not N-terminus, was essential for stress granule localization. LC regions of FAM98A contained multiple arginine-glycine-glycine (RGG) motifs whose arginines are often methylated by PRMT1 [27]. Although previous study showed that LC region of FAM98A was arginine methylated [19], our result showed that the arginine methylation was dispensable for the FAM98A accumulation to stress granules. Similarly previous study reported that localization of ATXN2L to the stress granules was independent of arginine methylation of the protein [24]. These results show that FAM98A localization to stress granules is mediated by LC regions in the C-terminus but independent of arginine methylation.
Mass spectrometry and immunoprecipitation analysis for FAM98A-interacting proteins revealed that FAM98A can form a protein complex with proteins, such as DDX1, ATXN2, ATX2L, and NUFIP2. DDX1 is a RNA helicase that regulates micro RNA maturation or virus replication [28, 29]. ATXN2 and ATXN2L are members of the spinocerebellar ataxia family which are associated with neurodegenerative disorders [30]. NUFIP2 is a protein of unknown function that interacts with FMR1 (fragile mental retardation 1) [31]. All these proteins have been reported to localize to the stress granules, but how these proteins are recruited to stress granules are unknown. We tested if FAM98A was essential for these proteins to accumulate to the stress granules. Interestingly, only DDX1 was no longer accumulated to stress granules in the absence of FAM98A. These results show that although FAM98A associates with multiple proteins, FAM98A is essential for localization of DDX1, but not of ATXN2, ATXNL2, and NUFIP, to stress granules.
FAM98A has a homolog called FAM98B, which has LC regions in the C-terminus as well. Recent studies showed that FAM98B is a member of large protein complex that contain RNA ligase, RTCB (RNA 2′,3′-cyclic phosphate and 5′-OH ligase) [32, 33]. The FAM98B-containing complex was shown to be essential for RNA ligation during the process of tRNA splicing [34]. In addition, the complex promoted ligation of XBP1 (X-box binding protein 1) mRNA that is cleaved by IRE1 (inositol requiring enzyme 1) activated by unfolded protein response [35,36,37,38]. These results have shown important functions of FAM98B complex for the ligation of cleaved RNA for RNA processing. Our previous mass spectrometry analysis [22] demonstrated that FAM98A was in complex with RTCB and other proteins that are involved in RNA ligation, such as DDX1 and C14orf166, indicating that FAM98A is involved in ligation of RNAs. Although further detailed analysis is needed, stress granule-localized FAM98A complex may promote processing of RNAs for the survival of cells under stressed conditions.
In conclusion, we have shown that FAM98A is localized to the stress granule after various stress stimuli. In addition, FAM98A associated with multiple stress granule-localized proteins that are associated with neurodegenerative diseases. Our previous studies have shown that FAM98A was associated with progression of colon and ovarian cancers [19, 22]. Further investigation will reveal interesting feature of FAM98A for stress pathway, cancer progression and neurodegenerative pathogenesis.
References
Erickson SL, Lykke-Andersen J (2011) Cytoplasmic mRNP granules at a glance. J Cell Sci 124(Pt 3):293–297. https://doi.org/10.1242/jcs.072140
Anderson P, Kedersha N, Ivanov P (2015) Stress granules, P-bodies and cancer. Biochim Biophys Acta 1849(7):861–870
Kedersha N, Ivanov P, Anderson P (2013) Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci 38(10):494–506
Nostramo R, Herman PK (2016) Deubiquitination and the regulation of stress granule assembly. Curr Genet 62(3):503–506
Tsai WC, Gayatri S, Reineke LC, Sbardella G, Bedford MT, Lloyd RE (2016) Arginine demethylation of G3BP1 promotes stress granule assembly. J Biol Chem 291(43):22671–22685
López-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z (2005) RACK1 mediates activation of JNK by protein kinase C. Mol Cell 19(3):309–320
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M (2008) Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol 10(11):1324–1332
Tsai NP, Wei LN (2010) RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cell Signal 22(4):668–675
Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R (2016) ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164(3):487–498
Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M (2013) Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 18(2):135–146
Valiente-Echeverría F, Melnychuk L, Vyboh K, Ajamian L, Gallouzi IE, Bernard N, Mouland AJ (2014) eEF2 and Ras-GAP SH3 domain-binding protein (G3BP1) modulate stress granule assembly during HIV-1 infection. Nat Commun 5:4819
Somasekharan SP, El-Naggar A, Leprivier G, Cheng H, Hajee S, Grunewald TG, Zhang F, Ng T, Delattre O, Evdokimova V, Wang Y, Gleave M, Sorensen PH (2015) YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J Cell Biol 208(7):913–929
Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P, Anderson P (2016) G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol 212(7):845–860
Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL (2012) Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149(4):768–779
Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, McKnight SL (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149(4):753–767
Protter DS, Parker R (2016 Sep) Principles and properties of stress granules. Trends Cell Biol 26(9):668–679
Panas MD, Ivanov P, Anderson P (2016) Mechanistic insights into mammalian stress granule dynamics. J Cell Biol 215(3):313–323
Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP (2015 Sep) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163(1):123–133
Akter KA, Mansour MA, Hyodo T, Ito S, Hamaguchi M, Senga T (2016) FAM98A is a novel substrate of PRMT1 required for tumor cell migration, invasion, and colony formation. Tumour Biol 37(4):4531–4539
Lui J, Castelli LM, Pizzinga M, Simpson CE, Hoyle NP, Bailey KL, Campbell SG, Ashe MP (2014) Granules harboring translationally active mRNAs provide a platform for P-body formation following stress. Cell Rep 9(3):944–954
Low WK, Dang Y, Schneider-Poetsch T, Shi Z, Choi NS, Merrick WC, Romo D, Liu JO (2005) Inhibition of eukaryotic translation initiation by the marine natural product pateamine A. Mol Cell 20(5):709–722
Akter KA, Mansour MA, Hyodo T, Senga T (2016) FAM98A associates with DDX1-C14orf166-FAM98B in a novel complex involved in colorectal cancer progression. Int J Biochem Cell Biol 84:1–13
Bish R, Cuevas-Polo N, Cheng Z, Hambardzumyan D, Munschauer M, Landthaler M, Vogel C (2015) Comprehensive protein interactome analysis of a key RNA helicase: detection of novel stress granule proteins. Biomolecules 5(3):1441–1466
Kaehler C, Guenther A, Uhlich A, Krobitsch S (2015) PRMT1-mediated arginine methylation controls ATXN2L localization. Exp Cell Res 334(1):114–125
Lastres-Becker I, Nonis D, Eich F, Klinkenberg M, Gorospe M, Kötter P, Klein FA, Kedersha N, Auburger G (2016) Mammalian ataxin-2 modulates translation control at the pre-initiation complex via PI3K/mTOR and is induced by starvation. Biochim Biophys Acta 1862(9):1558–1569
Yasuda-Inoue M, Kuroki M, Ariumi Y (2013) DDX3 RNA helicase is required for HIV-1 Tat function. Biochem Biophys Res Commun 441(3):607–611
Bedford MT, Richard S (2005) Arginine methylation an emerging regulator of protein function. Mol Cell 18(3):263–272
Edgcomb SP, Carmel AB, Naji S, Ambrus-Aikelin G, Reyes JR, Saphire AC, Gerace L, Williamson JR (2012) DDX1 is an RNA-dependent ATPase involved in HIV-1 Rev function and virus replication. J Mol Biol 415(1):61–74
Han C, Liu Y, Wan G, Choi HJ, Zhao L, Ivan C, He X, Sood AK, Zhang X, Lu X (2014 Sep) The RNA-binding protein DDX1 promotes primary microRNA maturation and inhibits ovarian tumor progression. Cell Rep 8(5):1447–1460
Alves-Cruzeiro JM, Mendonça L, Pereira de Almeida L, Nóbrega C (2016) Motor dysfunctions and neuropathology in mouse models of spinocerebellar ataxia type 2: a comprehensive review. Front Neurosci 10:572
Bardoni B, Castets M, Huot ME, Schenck A, Adinolfi S, Corbin F, Pastore A, Khandjian EW, Mandel JL (2003) 82-FIP, a novel FMRP (fragile X mental retardation protein) interacting protein, shows a cell cycle-dependent intracellular localization. Hum Mol Genet 12(14):1689–1698
Popow J, Englert M, Weitzer S, Schleiffer A, Mierzwa B, Mechtler K, Trowitzsch S, Will CL, Lührmann R, Söll D, Martinez J (2011) HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science 331(6018):760–764
Tanaka N, Meineke B, Shuman S (2011) RtcB, a novel RNA ligase, can catalyze tRNA splicing and HAC1 mRNA splicing in vivo. J Biol Chem 286(35):30253–30257
Popow J, Jurkin J, Schleiffer A, Martinez J (2014) Analysis of orthologous groups reveals archease and DDX1 as tRNA splicing factors. Nature 511(7507):104–107
Jurkin J, Henkel T, Nielsen AF, Minnich M, Popow J, Kaufmann T, Heindl K, Hoffmann T, Busslinger M, Martinez J (2014) The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J 33(24):2922–2936
Kosmaczewski SG, Edwards TJ, Han SM, Eckwahl MJ, Meyer BI, Peach S, Hesselberth JR, Wolin SL, Hammarlund M (2014) The RtcB RNA ligase is an essential component of the metazoan unfolded protein response. EMBO Rep 15(12):1278–1285
Lu Y, Liang FX, Wang X (2014) A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol Cell 55(5):758–770
Ray A, Zhang S, Rentas C, Caldwell KA, Caldwell GA (2014) RTCB-1 mediates neuroprotection via XBP-1 mRNA splicing in the unfolded protein response pathway. J Neurosci 34(48):16076–16085
Acknowledgements
This research was funded by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (25650063 to TS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ozeki, K., Sugiyama, M., Akter, K.A. et al. FAM98A is localized to stress granules and associates with multiple stress granule-localized proteins. Mol Cell Biochem 451, 107–115 (2019). https://doi.org/10.1007/s11010-018-3397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3397-6