Abstract
Studies have demonstrated that the high-mobility group 1B protein (HMGB1) could regulate endothelial progenitor cell (EPC) homing, but the effect of HMGB1 on EPC apoptosis and associated mechanisms are still unclear. The aim of this study was to investigate the effects of HMGB1 on EPC apoptosis and the possible involvement of the endoplasmic reticulum (ER) stress pathway. EPC apoptosis was determined by flow cytometry. The expressions of PERK, eIF2α, and CHOP were detected by western blotting. Additionally, the effects of PERK shRNA on the biological behaviors of EPCs were assessed. Our results showed that incubation of EPCs with HMGB1 (0.1–1 μg/ml) for 12–48 h induced apoptosis as well as activated ER stress transducers, as assessed by up-regulating PERK protein expression and eIF2α phosphorylation in a dose or time-dependent manner. Moreover, HMGB1-mediated EPC apoptosis and CHOP expression were dramatically suppressed by PERK shRNA or a specific eIF2α inhibitor (salubrinal). Importantly, a blocking antibody specifically targeted against RAGE (anti-RAGE antibody) markedly inhibited HMGB1-induced EPC apoptosis and ER stress marker protein (PERK, eIF2α, and CHOP) expression levels. Our novel findings suggest that HMGB1 triggered EPC apoptosis in a manner of RAGE-mediated activation of the PERK/eIF2α pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelial progenitor cells (EPCs) play critical roles in various human diseases such as atherosclerosis and vascular repair at sites of ischaemia (myocardial infarction, peripheral artery disease, and stroke). EPC dysfunction is proved to be associated with thrombotic complication, atherosclerosis, and other cardiovascular diseases [1–3]. A reduced number of EPC is an independent predictor of morbidity and mortality of cardiovascular diseases [4]. Risk factors of atherosclerosis, such as diabetes, aging, hypercholesterolemia, hypertension and smoking, could impair the function of EPCs partly via promoting EPC apoptosis [5–7]. However, the underlying mechanism of EPC apoptosis remains unclear.
High-mobility group 1B protein (HMGB1), a 30 kDa nuclear protein, can be released by inflammatory cells or necrotic cells to trigger inflammation [8], which plays an important role in regulating development of atherosclerosis. Recent studies have indicated that HMGB1 can alter EPC functions and survival. Such as, HMGB1 attracts endothelial progenitor cells and hematopoietic stem cells to the sites of tissue injury and tumors in order to promote neovascularization [9]. More recently, Hayakawa et al. reported that astrocytic HMGB1 promoted endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery [10]. Besides, HMGB1 also inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway [11]. However, the effect of HMGB1 on EPC apoptosis and associated mechanisms are still unclear.
The endoplasmic reticulum (ER) is an organelle that has an essential role in multiple cellular processes. A variety of insults can interfere with ER function, leading to the accumulation of unfolded and misfolded proteins in the ER. The ER stress will be activated as long as the ER transmembrane sensors detect the accumulation of unfolded proteins. If ER stress is prolonged or overwhelming, however, it can induce cell apoptosis or death. Recent studies have suggested that ER stress-initiated apoptosis are implicated in the pathophysiology of various human diseases, including atherosclerosis and diabetes mellitus [12, 13]. Therefore, ER stress response pathway has been considered to be a critical system that determines whether cells survive or die [14]. The ER stress is triggered by three upstream proteins: inositol-requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA-like endoplasmic reticulum kinase (PERK, also known as EIF2AK3) [15]. Among these signal transducers, PERK acts as a pivotal mediator of cell survival in response to ER stress through phosphorylating eukaryotic translation initiation factor 2 alpha (eIF2α) to regulate C/EBP homologous protein (CHOP) transcription, which is one of the most thoroughly investigated molecules among those involved in ER-initiated apoptotic signaling [16]. Deletion of the CHOP gene protects cells against death induced by pharmacological ER stressors and accumulation of defectively folded proteins and ischemia [17].
Therefore, the aim of this study was to investigate whether HMGB1 can induce EPC apoptosis and the relationship with PERK/ eIF2α pathway.
Methods
Isolation, cultivation, and characterization of EPCs
EPCs were cultured as described previously [18, 19]. Briefly, 40 ml of peripheral blood samples anticoagulated with heparin (25 U/ml) of healthy volunteers was collected in sterile blood packs. Peripheral blood mononuclear cells (PBMNCs) were isolated using density gradient centrifugation with Histopaque 1077 (Sigma-Aldrich, St. Louis, MO, USA). After washing with phosphate-buffered saline (PBS) for three times, 1 × 107 PBMCs were plated on fibronectin-coated 6-well plate. The cells were cultured in endothelial basal medium-2 (EBM-2) (Lonza, Basel, Switzerland) supplemented with EGMTM-2-MV SingleQuots™ containing 5% fetal bovine serum, vascular endothelial growth factor (VEGF), fibroblast growth factor-2, epidermal growth factor, insulin-like growth factor, and ascorbic acid. After 4 days of culture, the non-adherent cells were removed by washing with PBS and fresh medium was applied and culture was maintained through 7 days. To confirm whether the adherent cells were EPCs, the expressions of endothelial marker proteins were measured by flow cytometry. The cells were detached with 1 mM EDTA in PBS and incubated for 15 min with fluorescein isothiocyanate (FITC)-conjugated anti-kinase insert domain-containing receptor (KDR) (Sigma-Aldrich, St. Louis, MO, USA), phycoerythrin (PE)-conjugated anti-CD31, and FITC-conjugated anti-CD34 (BD Pharmingen, San Diego, CA, USA). Isotype-matched antibodies served as controls. After incubation, cells were fixed with 1% paraformaldehyde, and quantitative analysis was performed measuring 20, 000 cells per sample. In addition, to detect the uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein (ac-LDL) (Molecular Probes), cells were incubated with ac-LDL (2.4 μg/ml) at 37 °C for 1 h. Then, the cells were fixed with 2% paraformaldehyde for 10 min and incubated with FITC-labeled Ulex europaeus agglutinin 1 (UEA-1) (10 μg/ml) (Sigma-Aldrich, St. Louis, MO, USA) for 1 h. Dual-staining cells positive for both UEA-1 and ac-LDL were identified as EPCs and counted per well. Two or three independent investigators blinded to the treatment assignment evaluated the number of EPCs per well by counting randomly selected high-power fields. The study protocol was approved by the local Ethics Committee of Xiangya Hospital, Central South University, Changsha, Hunan, China and the written informed consent was obtained from all the subjects.
Experimental protocol
Firstly, to investigate the effect of HMGB1 on ER stress and apoptosis in EPCs, the dose and time experiments were performed. The apoptosis rate of EPCs, PERK, and eIF2α protein expression were subsequently measured. Secondly, in the following parallel studies, to further determine the critical effect of the PERK/ eIF2α pathway in HMGB1-induced EPC apoptosis, PERK shRNA or 100 μM salubrinal (eIF2α-specific inhibitor, Santa Cruz Biotechnology, Dallas, Texas, USA) was used prior to exposure to HMGB1 (R&D Systems Inc, Minneapolis, MN, USA). The apoptosis rate of EPCs and CHOP protein expression were subsequently determined. Furthermore, to examine the role of RAGE in HMGB1-induced apoptosis, EPCs were pretreated for 30 min with an anti-RAGE Ab (20 μg/ml, Biovision, Milpitas, CA, USA) or IgG Ab (60 μg/ml, Biovision, Milpitas, CA, USA) before exposing to HMGB1. The apoptosis rate of EPCs, PERK, eIF2α, and CHOP protein expression were subsequently determined.
PERK shRNA transfection in EPCs
To inhibit the expression of PERK, we designed a short hairpin RNA (shRNA) targeting the PERK transcript. The synthesized oligonucleotides which contain specific target sequence, a loop, the reverse complement of the target sequence, a stop codon for U6 promoter, and two sticky ends were cloned into pGCSIL-GFP lentivirus vector according to the manufacturer’s instructions (Santa Cruz Biotech, Dallas, Texas, USA). The target sequence in the oligonucleotide for suppressing PERK was 5′-AGCGCGGCAGGTCATTAGTAA-3′, which was generated as described previously [20]; the negative control siRNA sequence was 5′-TTCTCCGAACGTGTCACGT-3′. The resulting plasmid was named as pGCSIL-GFP-shPERK (PERK shRNA) and pGCSIL-GFP-shNC (Non-target shRNA). The resulting constructs allow for the transient and stable expression of the siRNA. Retroviruses carrying the PERK siRNA were generated by co-transfection of recombinant plasmids PERK shRNA and non-target shRNA, respectively, with the lentiviral helper plasmids (pHelper 1.0 and pHelper 2.0) into 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 48 h of incubation, the culture medium containing recombinant virus was harvested and purified by a 0.45-µm filter. Target EPCs were infected with recombinant virus supplemented with 20 µg/ml polybrene for a spin infection procedure (Clontech, Mountain View, CA, USA). Cells were collected for detecting the inhibitory effects after 2–4 days of infection. The infection efficiency was evaluated by PERK expression using western blotting analysis.
Apoptosis determination with Annexin V-propidium iodide (PI) double staining assay
Cells were collected and washed with PBS twice and were resuspended in 250 μl binding buffer. Next, 5 μl FITC-Annexin V (Millipore, Bedford, Massachusetts, USA) and 10 μl PI (20 μg/ml) (Millipore, Bedford, Massachusetts, USA) were diluted per 100 μl cell suspension. The cells were incubated at room temperature for 15 min. After 400 μl PBS was added to the mixed solution, the samples were analyzed using flow cytometry (BD, Franklin Lakes, New Jersey, USA). The percent of apoptosis was expressed as a ratio of apoptotic cells to total cells.
Western blotting analysis
Cells were lysed for 30 min at 4°C in lysis buffer. The total cell protein concentration was determined using a bicinchoninic acid method. Total protein (50 to 100 μg) was resolved using SDS–polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane, and subjected to immunoblotting analysis. Primary antibodies for PERK (1:400, Abgent), eIF2α (1:1000, Cell Signaling Technology), p-eIF2α (1:1000, Cell Signaling Technology), CHOP (1:1000, Cell Signaling Technology), and β-actin (1:5000, Cell Signaling Technology), and horseradish peroxidase-conjugated secondary antibodies were used (1:5000, Santa Cruz Biotech, Dallas, Texas, USA). The bands were visualized using enhanced chemiluminescence reagents and analyzed with a gel documentation system (Bio-Rad Gel Doc1000 with Multi-Analyst version 1.1). All of the results were representative of at least three independent experiments.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). Statistical analysis was performed by ANOVA followed by the Student–Newman–Keuls test for multiple comparisons. All statistical analyses were performed using the SPSS 13.0 (SPSS Inc., Chicago, IL, USA) software package. A two-tailed P value < 0.05 was considered to be statistically significant.
Results
Characterization of EPC
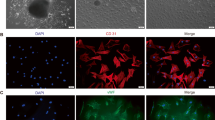
Flow cytometry was applied to appraise EPC after 7 days of culture. Among the attached cells, the expression rates of CD31, CD34, and KDR were 90.5, 96.0, and 73.1%, respectively (Fig. 1 a-c). Over 90% of adherent cells took up Dil-ac-LDL and bound FITC-UEA-1 (Fig. 1 d-f), indicating that they expressed scavenger receptor for ac-LDL and ligand for UEA-1.
HMGB1 induces apoptosis and activated PERK/ eIF2α pathway in EPCs
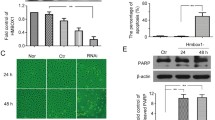
In order to determine whether HMGB1 can induce EPC apoptosis, EPCs were treated with 0, 0.01, 0.1, and 1 μg/ml recombinant HMGB1 for 24 h or treated with 1 μg/ml HMGB1 for 12, 24, and 48 h. As a result, HMGB1 could induce EPC apoptosis, increase protein expression of ER stress sensors PERK, and triggered phosphorylation of eIF2α in a dose- and time-dependent manner (Fig. 2). These results suggested that EPC apoptosis and PERK/eIF2α pathway are activated by HMGB1 in a concentration- and time-dependent manner. Based on these findings and those of previous studies, we selected the optimal concentration of HMGB1 (1 μg/ml) for use in subsequent experiments.
HMGB1 triggers ER stress activation and apoptosis in EPCs. EPCs were treated with 0, 0.01, 0.1, and 1 μg/ml HMGB1 for 24 h, and exhibited apoptotic effects in dose-dependent manner (a) as well as PERK/eIF2α pathway activation (b). Meanwhile, when EPCs treated with 1 μg/ml HMGB1 for 0 h, 12 h, 24 h, and 48 h, EPCs exhibited apoptotic effects in time-dependent manner (c) and PERK/eIF2α pathway activation (d). The data were expressed as the means ± SEM, n = 6 each. * P < 0.05, ** P < 0.01, compared with the control group
PERK/ eIF2α pathway is involved in HMGB1-induced apoptosis in EPCs
As PERK/eIF2α pathway is activated by HMGB1 in EPCs, so we next conducted experiments using knockdown methodology to confirm the critical role of PERK/ eIF2α pathway in HMGB1-induced EPC apoptosis. The results indicated that PERK gene silencing by PERK shRNA markedly inhibited HMGB1-induced apoptosis of EPCs and attenuated the elevation of CHOP expression induced by HMGB1, while the non-target shRNA had no effects (Fig. 3a, b). Besides, in order to confirm whether HMGB1 induced EPC apoptosis via eIF2α phosphorylation, the specific eIF2α inhibitor salubrinal was used. Treatment of the cells with salubrinal (100 μM) significantly abrogated the HMGB1-induced apoptosis and CHOP protein expression, while salubrinal (100 μM) alone had no effect on above change (Fig. 3c, d).
The role of PERK/eIF2α pathway in HMGB1-induced EPC apoptosis. PERK shRNA markedly suppressed HMGB1-induced EPC apoptosis (a) through its downstream effector CHOP (b). Meanwhile, eIF2α inhibitor (Salubrinal) also markedly suppressed HMGB1-induced EPC apoptosis (c) via its downstream effector CHOP (d). The data were expressed as the means ± SEM, n = 6 each. ** P < 0.01, compared with the control group. ++ P < 0.01, compared with HMGB1 group
The role of RAGE in HMGB1-induced apoptosis of EPCs
To further determine the relationship between RAGE and PERK/ eIF2α pathway in mediating HMGB1-induced apoptosis in EPC, we used a specific blocking antibody targeted against RAGE (anti-RAGE antibody). As shown in Fig. 4, treatment with anti-RAGE antibody (20 μg/ml) markedly suppressed the apoptosis of EPCs induced by HMGB1 as well as the expression of the ER sensors such as PERK, p-eIF2α, and CHOP. However, treatment with a non-specific IgG (60 μg/ml) had no effect on EPC above these. These results suggested that upon binding to RAGE, HMGB1 triggered PERK/ eIF2α pathway to activate CHOP-dependent apoptosis pathways and subsequently caused EPC apoptosis (Fig. 5).
HMGB1-induced ER stress and apoptosis in EPCs is RAGE-dependent. HMGB1-induced EPC apoptosis was abolished by anti-RAGE antibody (a). Moreover, HMGB1-induced ER stress markers expressions (PERK, p-eIF2α, and CHOP) were also suppressed by anti-RAGE antibody (b, c). The data were expressed as the means ± SEM, n = 6 each. ** P < 0.01, compared with the control group. ++ P < 0.01, compared with HMGB1 group
Discussion
In this study, we reported that ER stress is induced by HMGB1 in human EPC and modulate the balance between survival and apoptosis induced by HMGB1. The present study revealed that (1) HMGB1 triggers ER stress and induce EPC apoptosis; (2) HMGB1-induced apoptosis of EPCs is via PERK/eIF2α pathway; (3) RAGE is involved in HMGB1-induced EPC apoptosis, which activate sequential PERK/eIF2α pathway.
Evidences from clinical and experimental studies have shown that EPCs are valuable in the repair of blood vessel injury, in the prevention of the development of ischemia, and in the acceptance of prosthetic grafts [21–23]. The population and function of EPCs are influenced by cigarette smoking, hypertension, diabetes mellitus, dyslipidemia, aging, cardiovascular disease (CVD), and so on [24]. Reduced EPC population and EPC dysfunction are considered to contribute to endothelial dysfunction and associated with risk factors for atherosclerosis and cardiovascular disease progression [1–5]. Therefore, the elucidation of the factors which affect the apoptosis of EPCs will be beneficial to the prevention and the therapy of cardiovascular disease. In the present study, to our knowledge, we are the first to determine that exposing EPCs to HMGB1 results in apoptosis. We found that EPCs treated with 0, 0.01, 0.1, or 1 μg/ml HMGB1 for 24h or treated with 1 μg/ml HMGB1 for 12, 24, and 48 h induced apoptosis in dose- and time-dependent manner. More interestingly, HMGB1 (0, 0.01, 0.1, and 1 μg/ml) for 24h and 1 μg/ml HMGB1 (12, 24, and 48 h) also increased protein expression of ER stress sensors PERK, and triggered phosphorylation of eIF2α in a concentration- and time-dependent manner. These results suggest that EPC apoptosis and PERK/eIF2α pathway are activated by HMGB1 in a concentration- and time-dependent manner. However, the precise molecular mechanism by which HMGB1 induces EPC apoptosis remains unclear.
The endoplasmic reticulum (ER) is a major signal transducing organelle that senses and responds to changes in homeostasis. ER stress is caused by disturbances in the structure and function of the ER, accumulated by misfolded proteins and determine the survival or death of cells [25]. In response to ER stress, the unfolded protein response (UPR) is initiated by the activation of three molecules: PERK, IRE1, and ATF6 [13, 14]. PERK activation leads to phosphorylation of eIF2α, which facilitates the recovery pathways during transient ER stress by mediating translational up-regulation of ATF4 to augment CHOP (also known as GADD153) expression. CHOP mediates the transcriptional repression and activation of proteins involved in the ER-initiated apoptotic pathway in order to repress the expression of anti-apoptotic Bcl-2 [15, 16]. The severity and length of ER stress determine whether cells survive or die. Our results demonstrate that treatment of EPCs with HMGB1 resulted in activation of ER stress, which is characterized by the activation of the ER stress sensors PERK, as well as the subsequent eIF2α phosphorylation, CHOP protein expression, and in turn induce EPC apoptosis. Pharmacological manipulations that reduce the ER stress response such as treatment with salubrinal (eIF2α inhibitor) attenuate the pro-apoptotic effects of HMGB1 and inhibit PERK, CHOP protein, expression and eIF2α phosphorylation. Additionally, PERK gene silencing inhibited the HMGB1-induced activation of PERK, the subsequent phosphorylation of eIF2α, and CHOP expression suggesting that HMGB1 triggers ER stress in EPCs via up-regulation of phosphorylation of eIF2α and CHOP. Finally, PERK gene silencing also inhibited EPC apoptosis induced by HMGB1. Collectively, these findings suggest that the HMGB1 induces endothelial cell apoptosis triggered by PERK/eIF2α pathways.
RAGE is a transmembrane protein that belongs to the immunoglobulin superfamily of cell surface receptors and has been isolated as a receptor for HMGB1. RAGE signaling accounts for both the physiological and pathophysiological consequences of HMGB1/cell surface interactions [26, 27]. Recently, Chavakis et al. found that HMGB1 activates integrin-dependent homing of endothelial progenitor cells via RAGE receptor [28]. In keeping with our hypothesis, extracellular HMGB1 regulates cells through RAGE, which is by far the most widely studied HMGB1 receptor at the cell surface. Our study demonstrated that HMGB1 induced EPC apoptosis in a manner that is mediated by RAGE activation. This conclusion is based on our observation that pretreatment of EPCs with a RAGE blocking anti-RAGE antibody markedly suppressed HMGB1-induced EPC apoptosis and pro-apoptotic protein CHOP expression, while pretreatment with a negative control IgG antibody had no effect on the HMGB1-induced apoptosis. Moreover, we postulated that HMGB1 might trigger ER stress via the RAGE pathway. Indeed, our study provides evidence of this process. In the present study, we demonstrated that the anti-RAGE antibody markedly attenuates HMGB1-induced PERK protein expression and their subsequent signaling through eIF2α.
In conclusion, the present study indicates that HMGB1 triggers apoptosis of EPCs by inducing the expression of CHOP via RAGE-mediated stimulation of PERK/ eIF2α stress pathway. These findings may explain the important mechanism through which HMGB1 causes EPC dysfunction and provide an alternative target for drug development and clinical treatment for vascular diseases.
References
Ruan C, Shen Y, Chen R, Wang Z, Li J, Jiang Y (2013) Endothelial progenitor cells and atherosclerosis. Front Biosci (Landmark 18:1194–1201
Lin CP, Lin FY, Huang PH, Chen YL, Chen WC, Chen HY, Huang YC, Liao WL, Huang HC, Liu PL, Chen YH (2013) Endothelial progenitor cell dysfunction in cardiovascular diseases: role of reactive oxygen species and inflammation. Biomed Res Int 2013:845037
Du F, Zhou J, Gong R, Huang X, Pansuria M, Virtue A, Li X, Wang H, Yang XF (2012) Endothelial progenitor cells in atherosclerosis. Front Biosci (Landmark 17:2327–2349
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353(10):999–1007
Peplow PV (2014) Influence of growth factors and cytokines on angiogenic function of endothelial progenitor cells: a review of in vitro human studies. Growth Factors 32(3–4):83–116
Chiang CH, Huang PH, Chiu CC, Hsu CY, Leu HB, Huang CC, Chen JW, Lin SJ (2014) Reduction of circulating endothelial progenitor cell level is associated with contrast-induced nephropathy in patients undergoing percutaneous coronary and peripheral interventions. PLoS One 9(3):e89942
Du G, Song Y, Zhang T, Ma L, Bian N, Chen X, Feng J, Chang Q, Li Z (2014) Simvastatin attenuates TNF–α–induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med 34(1):177–182
Asavarut P, Zhao H, Gu J, Ma D (2013) The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol Taiwan 51(1):28–33
Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M (2006) Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 176(1):12–15
Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH (2012) Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A 109(19):7505–7510
Meng E, Guo Z, Wang H, Jin J, Wang J, Wang H, Wu C, Wang L (2008) High mobility group box 1 protein inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway. Stem Cells Dev 17(4):805–813
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110(10):1389–1398
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7(12):1013–1030
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14(1):20–28
Minamino T, Kitakaze M (2010) ER stress in cardiovascular disease. J Mol Cell Cardiol 48(6):1105–1110
Iurlaro R, Muñoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283(14):2640–2652
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18(24):3066–3077
Zheng H, Fu G, Dai T, Huang H (2007) Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol 50(3):274–280
Chen J, Song M, Yu S, Gao P, Yu Y, Wang H, Huang L (2010) Advanced glycation endproducts alter functions and promote apoptosis in endothelial progenitor cells through receptor for advanced glycation endproducts mediate overpression of cell oxidant stress. Mol Cell Biochem 335(1–2):137–146
Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 120(1):127–141
Zhang M, Malik AB, Rehman J (2014) Endothelial progenitor cells and vascular repair. Curr Opin Hematol 21(3):224–228
Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG (2005) Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension 46(1):7–18
Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S (2002) Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res 90(10):E89–E93
Aragona CO, Imbalzano E, Mamone F, Cairo V, Lo Gullo A, D’Ascola A, Sardo MA, Scuruchi M, Basile G, Saitta A, Mandraffino G (2016) Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease. Stem Cells Int 2016:8043792
Malhotra JD, Kaufman RJ (2011) ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol 3(9):a004424
Rouhiainen A, Kuja-Panula J, Tumova S, Rauvala H (2013) RAGE-mediated cell signaling. Methods Mol Biol 963:239–263
Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM (2013) Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal 25(11):2185–2197
Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S (2007) High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res 100(2):204–212
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31270992 and No. 81670453) and China Postdoctoral Science Foundation (2015M582350).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Ji-Peng Zhou and Ying Luo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, Q., Yang, Z., Zhou, JP. et al. HMGB1 induces endothelial progenitor cells apoptosis via RAGE-dependent PERK/eIF2α pathway. Mol Cell Biochem 431, 67–74 (2017). https://doi.org/10.1007/s11010-017-2976-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-2976-2