Abstract
We aimed to explore the effects of myeloid-derived growth factor (Mydgf) on the regulation of hypoxia/reoxygenation (HR)–induced apoptosis of cardiac microvascular endothelial cells (CMECs). CMECs were exposed to hypoxia for 24 h and reoxygenation for 6 h to establish an HR cell model. Subsequently, an adenovirus was used to overexpress Mydgf in CMECs. Flow cytometry and TUNEL staining were used to detect the extent of apoptosis, whereas qPCR was used to detect the relative expression of Mydgf mRNA. Western blotting was also performed to detect the expression of apoptosis-related proteins and endoplasmic reticulum stress (ERS)–related proteins, including C/EBP Homologous Protein (CHOP), glucose-regulated protein 78 (GRP 78), and cleaved Caspase-12. The endoplasmic reticulum stress agonist tunicamycin (TM) was used to stimulate CMECs for 24 h as a rescue experiment for Mydgf. Flow cytometry revealed that the HR model effectively induced endothelial cell apoptosis, whereas qPCR and western blotting showed that Mydgf mRNA and protein levels decreased significantly after HR treatment (P < 0.05). Overexpression of Mydgf in cells effectively reduced apoptosis after HR. Furthermore, western blotting showed that HR induced a significant upregulation of CHOP, GRP78, and cleaved-Caspase-12 expression in CMECs, whereas HR-treated cells downregulated the expression of CHOP, GRP78, and cleaved-Caspase-12 after Mydgf overexpression. Under HR conditions, TM significantly reversed the protective effect of Mydgf on CMECs. Mydgf may reduce CMEC apoptosis induced by HR by regulating oxidative stress in ERS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reperfusion therapy is the primary treatment method for acute myocardial infarction; however, this process often causes severe myocardial ischemia–reperfusion injury (MIRI) (Simon et al. 2021). Excessive accumulation of reactive oxygen species (ROS) and calcium overload can cause secondary damage to the vascular and myocardial tissues (Deng et al. 2019), where damage to cardiac microvascular endothelial cells (CMECs) is most prominent. Among the various biochemical mechanisms and signaling pathways that may be involved in MIRI, endoplasmic reticulum stress (ERS) is associated with reperfusion-mediated oxidative stress and CMEC apoptosis and necrosis (Tan et al. 2020). Therefore, preventing and reducing the ERS induced by ischemia–reperfusion is an important therapeutic target that may reduce CMEC apoptosis and improve myocardial infarction.
Mydgf is a vascular endothelial cell growth factor secreted by bone marrow mononuclear macrophages, also known as C190rfl0. Human and mouse Mydgf proteins share 97% homology. Korf-Klingebiel et al. (2015) found that Mydgf can reduce cardiomyocyte apoptosis during myocardial ischemia by regulating the P13K/Akt signaling pathway. Additionally, recent studies have found that Mydgf is chiefly located in the endoplasmic reticulum effector protein (Garcia et al. 2016). However, the potential mechanisms of Mydgf involvement in the regulation of ischemia–reperfusion injury in cardiac endothelial cells have not been reported. Therefore, this study further explores the idea that Mydgf regulates CMEC apoptosis, induced by hypoxia and reoxygenation (HR), through mediating ERS.
Experimental methods
Experimental animals and related reagents
C57BL/6 J mice were purchased from Zunyi Medical University. Animal care, surgery, and handling procedures were approved by the Institutional Animal Care and Use Committee of the University of Zunyi’s Medical University.
DMEM, 0.25% trypsin, and type II collagenase were purchased from Gibco (Grand Island, NY). Fetal bovine serum (FBS) was purchased from BI (Cromwell, CT). Penicillin–streptomycin solution was purchased from Soleibao Technology Company (Beijing, China), and Mydgf mRNA primers were purchased from Shanghai Sheng Gong Biological Engineering (Shanghai, China Co., Ltd). The RNA extraction kit was purchased from Takara Biotechnology Co., Ltd. (Dalian, China). The real-time, fluorescence-based quantitative reverse transcription polymerase chain reaction (RT-qPCR) kit was purchased from Life Technologies (Carlsbad, CA). The adenovirus overexpressing the Mydgf gene and the empty vector were purchased from Nanjing KGI Biotech Co., Ltd. (Nanjing, China). The relevant primary antibodies for western blot detection were purchased from Abcam Co., Ltd (Cambridge, UK) and Proteintech Co., Ltd (Wuhan, China). Horseradish peroxidase–labeled secondary antibodies were purchased from Shanghai Biyuntian Biotechnology Co., Ltd. (Shanghai, China). CMECs were tested and authenticated in September 2021, and all experiments were performed using mycoplasma-free cells.
Culture of CMECs and establishment of HR model
The enzyme digestion method was combined with the differential adhesion method to isolate and culture mouse CMECs according to previous research reports (Garcia et al. 2016). Briefly, CMECs were isolated from postnatal C57BL/6 J mice according to a previously published protocol (Wang et al. 2019). Briefly, the mice were euthanized and immersed in 75% alcohol. The ventricular tissue was cut into small pieces and digested with 0.08% trypsin and 0.1% collagenase II at 37 °C under stirring for 40 min. The supernatant was transferred to a 50 mL centrifuge tube containing 10% FBS DMEM to terminate the detachment. The digested solution was filtered through a 200-μm sieve and centrifuged at 1200 rpm for 5 min at room temperature. The supernatant was discarded, and the cell pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and placed in a 5% carbon dioxide incubator at 37 °C for 1 h to separate cardiac fibroblasts. The non-adherent cell suspension was centrifuged at 1200 rpm for 5 min, the supernatant was discarded, and endothelial cell culture medium (ECM, Procell, Wuhan, China) was added to resuspend the cell pellet, and placed in a poly lysine pre-coated culture flask to continue culturing. After 48 h, the cell culture medium was changed, and cell growth was observed under a microscope.
In previous studies, we have performed immunofluorescence and flow cytometry identification of cultured CMECs (Wang et al. 2019). In this study, we also used immunofluorescence for the identification of surface markers of CMECs. Briefly, cultured cells were fixed in 4% paraformaldehyde for 30 min, then permeabilized with 1% Triton X-100 in PBS for 10 min, and incubated with goat serum at room temperature for 1 h. Afterwards, the samples were incubated overnight at 4 °C with the following primary antibodies diluted with goat serum: anti-CD31 (ab222783; Abcam) and anti-vWF (27,186–1-AP, Proteintech). The next day, the samples were stained with fluorescent secondary antibodies (Alexa Fluor 488 and/or 594; Proteintech) at room temperature for 1 h. The nuclei were stained with 4,6-diamino-2-phenylindole (DAPI, Sigma-Aldrich) for 10 min. Images were collected with a fluorescence microscope (Olympus, Tokyo, Japan). Then, P3-P6 cells were cultured without serum for 12 h for cell synchronization and then placed in a 95% N2 and 5% CO2 incubator at 37 °C for 24 h. The cells were incubated at 37 °C in a 5% CO2 incubator for 6 h to establish a hypoxia and reoxygenation model.
Overexpression of Mydgf in CMECs and treatment of CMECs by tunicamycin (TM)
An adenovirus overexpressing Mydgf infected CMECs for 48 h at an optimal multiplicity of infection of 30. The concentration and duration of action of tunicamycin (TM) were based on previous studies with slight modifications (Haas et al. 2022). Briefly, CMECs were placed on a well to obtain appropriate confluence (60–70%). The cells after growth were incubated in a fresh medium for 24 h. Then, cells were rinsed twice with phosphate-buffered saline (PBS, pH 7.4, Gibco Life Technologies). The cells were then exposed to 0.5 μg/mL of TM solution (Sigma Aldrich, St. Louis, MO) in DMSO (diluted in the culture medium to obtain final concentrations) for 24 h. Subsequently, the cells were washed twice with PBS and collected for detection of apoptosis and ERS-related proteins by western blot.
Real-time, fluorescence-based RT-qPCR
Total RNA from CMECs was extracted using TRIzol, and a UV spectrophotometer was used to detect the purity and concentration of RNA. The Takara RT-qPCR kit was used to reverse transcribe RNA into antisense DNA (cDNA), while qPCR analysis was performed according to the instructions of the SYBR Premix Ex Taq II kit. The reaction conditions were as follows: pre-denaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing at 64 °C for 8 s, and extension at 72 °C for 10 s for a total of 40 cycles. Relative gene expression was normalized to β-actin using the standard 2−△△Ct quantification method. The primer sequences are listed in Table 1.
TUNEL assay
CMECs (1 × 105) were inoculated into Petri dishes and rinsed twice with pre-cooled PBS, after which 500 μL of 4% paraformaldehyde was added to fix the cells at room temperature for 1 h. After rinsing with PBS, the cells were ruptured by 500 μL of 0.1% Triton X-100 for 2 min and stained with TUNEL reaction mixture in a 5% CO2 incubator at 37 °C for 1 h. Nuclei were stained with DAPI for 5 min, and fluorescence microscopy (Olympus, Tokyo, Japan) was used to randomly collect five visual fields for ImageJ statistical analysis.
Flow cytometry
Apoptosis was detected according to the kit’s detection instructions, and after mixing the reagents and cells thoroughly, they were incubated at room temperature in the dark for 15 min and analyzed by flow cytometry (Millipore Guava, Burlington, MA).
ROS was detected in the cells according to each grouping condition based on the operation steps of 2,7-dichlorofluorescein diacetate (DCFH-DA) staining (Sigma). Briefly, cells were incubated with a DCFH-DA probe for 30 min at 37 °C, washed three times with PBS, trypsinized, and centrifuged. Fluorescence intensity was analyzed by flow cytometry.
Western blot
The protein was extracted and quantitated, and the same amount of protein was separated by 12% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Burlington, MA). The membrane was sealed with 5% skim milk and incubated with a primary antibody against the target protein. PVDF membranes were then incubated with HRP-conjugated secondary antibody at room temperature for 1 h. Protein signals were assessed by enhanced chemiluminescence (ECL) reagent on a ChemiDoc MP system, and the grayscale value of the target band was analyzed using the ImageJ software. Antibody information is listed in Table 2.
Statistical analysis
Statistical analyses were conducted using the SPSS 21.0 statistical software package (IBM, Armonk, NY) and GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA). The data were normally distributed and expressed as means ± SD. One-way analysis of variance (ANOVA) was used to compare multiple groups. In addition, P-values less than 0.05 were considered statistically significant.
Results
Effect of hypoxia/reoxygenation-induced CMEC apoptosis on the expression of Mydgf
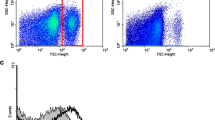
Mydgf can reduce myocardial cell apoptosis and improve heart function after myocardial infarction (Korf-Klingebiel et al. 2015). In addition, recent studies have found that Mydgf is involved in cardiomyocyte proliferation and mediates regeneration and repair after heart injury (Wang et al. 2020). However, it is unclear whether Mydgf is involved in regulating the HR-induced apoptosis of CMECs. Cells were observed crawling out of the adherent tissue under an inverted microscope, and the CMECs at day 3 and passage 1 (P1) showed cobblestone-like growth. The immunofluorescence assay revealed that cultured CMECs expressed the endothelial cell markers CD31 and vWF (Fig. 1A–C). In this study, CMECs were exposed to hypoxia for 24 h and reoxygenation for 6 h to simulate in vitro ischemia–reperfusion injury. Flow cytometry revealed a significantly elevated level of CMEC apoptosis in the HR group compared with the normal (Nor) group (P < 0.05; Fig. 2A). Furthermore, RT-qPCR and western blots showed that the expression levels of Mydgf mRNA and protein in the HR group were significantly less than those of the Nor group (P < 0.05; Fig. 2B and C).
Expression of Mydgf after HR induced CMEC apoptosis. (A) Apoptosis was detected by flow cytometry. Left: representative dot plots of cell apoptosis after Annexin V/PI dual staining. The left upper quadrant (% gated) shows necrotic cells (Annexin V−/PI+); the upper right quadrant (% gated) shows late apoptotic cells (Annexin V+/PI+); the lower left quadrant (% gated) shows normal live cells (AnnexinV−/PI−); and the lower right quadrant (% gated) shows early apoptotic cells (Annexin V+/PI−). Right: Histogram of the percentage of normal, apoptotic, and necrotic cells. Apoptotic cells represent total apoptotic cells, including both early and late apoptotic cells; n = 3. (B) RT-qPCR detected the relative expression of Mydgf mRNA. (C) The protein expression of Mydgf was detected by western blots. n = 3; ∗P < 0.05 compared with the Nor group.
Mydgf overexpression improves HR-induced CMEC apoptosis
To verify the potential anti-apoptotic effects of Mydgf in CMECs, we used an adenovirus to induce Mydgf overexpression. RT-qPCR results showed that compared with the Nor and HR groups, Mydgf mRNA levels were significantly increased after transfection with the adenovirus carrying the Mydgf gene (P < 0.05; Fig. 3A). Flow cytometry and western blotting were used to detect ROS levels and apoptosis-related protein expression in the cells. The results showed that compared with the Nor group, the expression of ROS in HR cells was increased (P < 0.05; Fig. 3B and C). Compared with the HR group, ROS levels in the Mydgf overexpression group were significantly decreased (P < 0.05). Western blotting showed that HR can decrease Mydgf expression in CMECs, while Mydgf overexpression can increase the protein level of Mydgf in cells under HR conditions. At the same time, we also detected the expression of apoptosis-related proteins and found that Mygdf overexpression could reduce the expression of pro-apoptotic proteins, Bax, and cleaved Caspase-3, induced by HR, as well as promote the expression of the anti-apoptotic protein Bcl-2 (P < 0.05; Fig. 3D–H). The TUNEL assay was used to further detect the extent of apoptosis in CMECs. The results showed that, compared with the Nor group, apoptosis in the HR group significantly increased, while apoptosis in the Mydgf overexpression group significantly decreased (Fig. 4A and B). These results indicate that Mydgf could improve HR-induced apoptosis and oxidative stress in CMECs.
Mydgf reduces HR-induced ROS and improves cell apoptosis. (A) RT-qPCR to detect the relative expression of Mydgf mRNA. (B–C) Intracellular ROS was detected by flow cytometry. P2 represents the percentage of fluorescence intensity in different conditions. (D) Representative western blot images of Mydgf, Bcl-2, Bax, cleaved Caspase-3, and Caspase-3. (E) Histogram of relative expression of mydgf protein. F Histogram of relative expression of Bcl2 protein. (G) Histogram of relative expression of Bax protein. (H) Histogram of relative expression of cleaved Caspase-3 and Caspase-3 protein. n = 3; ∗P < 0.05 compared with the Nor group. #P < 0.05 compared with the HR group.
Mydgf improves endoplasmic reticulum stress (ERS) induced by HR
Recent studies have found that Mydgf is mainly located in the endoplasmic reticulum and that ERS is involved in regulating cell apoptosis. To clarify whether Mydgf regulates apoptosis by mediating ERS, we further examined the expression of ERS-related proteins.
Western blot results (Fig. 5A–D) showed that compared with the Nor group, the expression levels of the ERS-related proteins CHOP, cleaved Caspase 12, and GRP78 in HR cells were significantly increased (P < 0.05). Additionally, compared with the HR group, the expression levels of these proteins in the Mydgf overexpression group decreased significantly (P < 0.05). The expression level of total Caspase-12 in the cells did not differ among the groups (P > 0.05). These results suggest that Mydgf may alleviate HR-induced ERS.
Mydgf inhibits HR-induced apoptosis by improving endoplasmic reticulum stress
To verify that Mydgf can improve HR-induced apoptosis by resisting ERS, CMECs overexpressing Mydgf were treated with ERS agonist TM under HR conditions. The results of the western blot analysis showed that, under HR conditions, Mydgf overexpression could reduce the expressions of apoptosis-related proteins, and ERS-related proteins cleaved caspase 3 and GRP78, while TM treatment significantly increased the expressions of cleaved caspase 3 and GRP78. Importantly, in Mydgf-overexpressing cells, concomitant TM treatment reversed the anti-ERS and apoptosis effects of Mydgf (P < 0.05; Fig. 6A–C). This indicates that the ability of Mydgf to improve HR-induced apoptosis and ERS can be reversed with TM treatment, further suggesting that Mydgf could improve HR-induced CMEC apoptosis by regulating ERS.
Discussion
In this study, we aimed to explore the potential roles of Mydgf in CMEC apoptosis after ischemia–reperfusion injury in vitro. Mydgf was found to reduce the HR-induced apoptosis of CMECs by regulating the oxidative stress pathway of the endoplasmic reticulum.
Acute myocardial infarction (AMI) is a life-threatening, critical illness. At present, the most effective treatment for AMI is timely reperfusion therapy; however, blood flow reperfusion is often accompanied by severe oxidative stress, calcium disturbances, and inflammatory reactions or, collectively, ischemia–reperfusion injury (Gandhi et al. 2022). In particular, this type of injury exacerbates damage to cardiac microcirculation, resulting in the lack of effective blood perfusion after reperfusion therapy (Berndt et al. 2021). Improving cardiac microcirculation after ischemia–reperfusion therapy is a potentially life-saving strategy, although it remains to be effectively implemented. It is well known that CMECs are structurally closely connected with cardiomyocytes, which mediate the exchange of substances and energy between microcirculation and cardiac tissue, and play a decisive role in maintaining the blood perfusion of the heart and the normal metabolism of myocardial tissue (Hsieh et al. 2006; Bulluck et al. 2016). Metabolism disorder of CMECs results in cardiac dysfunction (Wang et al. 2018a, b). Therefore, it may be of significance to explore the mechanism of protecting CMECs under HR in the search for a treatment of ischemic heart injury.
Previous studies on bone marrow transplantation and recombinant protein treatment have shown that Mydgf can significantly reduce the area of myocardial infarction and improve cardiac contractility in mice (Korf-Klingebiel et al. 2015). In addition, Mydgf not only reduces ischemia–reperfusion injury in vivo, but also reduces the death of myocardial cells from serum starvation, promotes the proliferation of human coronary artery endothelial cells (HCAECs), and improves their ability to form tubes in vitro (Zhao et al. 2020). Mydgf can also mediate neutrophil interstitial movement and inflammation by regulating the hypoxia-inducible factor 1-alpha (HIF-1α) pathway to improve tissue damage (Houseright et al. 2021). Moreover, Mydgf can activate the Cyclin D1 and STAT3 signaling pathways to promote the proliferation of human umbilical vein endothelial cells and reduce hydrogen peroxide-induced apoptosis (Berndt et al. 2021). This suggests that Mydgf is involved in cytoprotection and can promote cardiomyocyte proliferation to improve cardiac function after myocardial infarction. However, whether Mydgf is involved in regulating HR-induced apoptosis in CMECs and the related mechanisms remain widely unknown. In this study, it was found that, after HR, the apoptosis rate of CMECs cultured in vitro increased significantly, while the expression of Mydgf, according to mRNA and protein levels, in the cells decreased significantly. By overexpressing Mydgf in the cells, HR-induced apoptosis was significantly improved; however, the mechanism of this improvement needs to be elucidated by future studies.
The endoplasmic reticulum (ER) is responsible for the folding, synthesis, modification, and quality control of various proteins in cells (Wang et al. 2018a, b). Pathological stimuli such as inflammation, oxidative stress, and ischemia–reperfusion, can activate the ER oxidative stress signaling pathway, mediating cell apoptosis and necrosis (Tang et al. 2019). ERS also plays an important role in MIRI. Studies have shown that ischemia–reperfusion injury can activate ERS-related effector protein expression, such as GRP78 and CHOP, and lead to irreversible myocardial tissue injury (Deng et al. 2019). Yet, drug intervention or gene regulation to reduce ERS could improve the cardiac damage caused by ischemia–reperfusion (Chen et al. 2021). Recent studies have shown that Mydgf is a permanent protein in the ER (Garcia et al. 2016), which prompted us to question whether Mydgf can reduce HR-induced apoptosis by regulating ERS. Our study revealed that the expression of ERS-related proteins in CMECs, including CHOP, GRP78, and cleaved Caspase-12, was significantly increased after HR. However, the overexpression of Mydgf in CMECs significantly inhibited the expression of these ERS-related proteins. The ERS activator TM was used to treat the cells in a rescue experiment, where TM could effectively inhibit Mydgf and cause an increased expression of ERS-related proteins. Based on the results of the rescue experiment, Mydgf may improve HR-induced apoptosis by regulating ERS.
In summary, our study highlights that Mydgf overexpression in CMECs may improve HR-induced apoptosis and that this effect could be through the inhibition of oxidative stress in the endoplasmic reticulum.
Data availability
The data used to support the findings of this study are included within the article.
References
Berndt R, Albrecht M, Rusch R (2021) Strategies to overcome the barrier of ischemic microenvironment in cell therapy of cardiovascular disease. Int J Mol Sci 22(5):2312
Bulluck H, Yellon DM, Hausenloy DJ (2016) Reducing myocardial infarct size: challenges and future opportunities. Heart 102(5):341–348
Chen Q, Thompson J, Hu Y, Lesnefsky EJ (2021) Chronic metformin treatment decreases cardiac injury during ischemia-reperfusion by attenuating endoplasmic reticulum stress with improved mitochondrial function. Aging 13(6):7828–7845
Deng T, Wang Y, Wang C, Yan H (2019) FABP4 silencing ameliorates hypoxia reoxygenation injury through the attenuation of endoplasmic reticulum stress-mediated apoptosis by activating PI3K/Akt pathway. Life Sci 224:149–156
Gandhi S, Garratt KN, Li S, Wang TY, Bhatt DL, Davis LL, Zeitouni M, Kontos MC (2022) Ten-year trends in patient characteristics, treatments, and outcomes in myocardial infarction from National Cardiovascular Data Registry Chest Pain-MI Registry. Circ Cardiovasc Qual Outcomes 15(1):e008112
Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A (2016) Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res 109(3):397–408
Houseright RA, Miskolci V, Mulvaney O, Bortnov V, Mosher DF, Rindy J, Bennin DA, Huttenlocher A (2021) Myeloid-derived growth factor regulates neutrophil motility in interstitial tissue damage. J Cell Biol 220(8):e202103054
Hsieh PC, Davis ME, Lisowski LK, Lee RT (2006) Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol 68:51–66
Haas MJ, Warda F, Bikkina P, Landicho MA, Kapadia P, Parekh S, Mooradian AD (2022) Differential effects of cyclooxygenase-2 (COX-2) inhibitors on endoplasmic reticulum (ER) stress in human coronary artery endothelial cells. Vascul Pharmacol 02(142):e106948
Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, Napp LC, Bauersachs J, Ganser A, Brinkmann E, Reimann I, Kempf T, Niessen HW, Mizrahi J, Schönfeld HJ, Iglesias A, Bobadilla M, Wang Y, Wollert KC (2015) Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med 21(2):140–149
Simon JN, Vrellaku B, Monterisi S, Chu SM, Rawlings N, Lomas O, Marchal GA, Waithe D, Syeda F, Gajendragadkar PR, Jayaram R, Sayeed R, Channon KM, Fabritz L, Swietach P, Zaccolo M, Eaton P, Casadei B (2021) Oxidation of protein kinase A regulatory subunit PKARIα protects against myocardial ischemia-reperfusion injury by inhibiting lysosomal-triggered calcium release. Circulation 143(5):449–465
Tan Y, Mui D, Toan S, Zhu P, Li R, Zhou H (2020) SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol Ther Nucleic Acids 22:696–707
Tang V, Fu S, Rayner BS, Hawkins CL (2019) 8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response. Redox Biol 26:101274
Wang S, Binder P, Fang Q, Wang Z, Xiao W, Liu W, Wang X (2018a) Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol 175(8):1293–1304
Wang Y, Li Y, Feng J, Liu W, Li Y, Liu J, Yin Q, Lian H, Liu L, Nie Y (2020) Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration. Theranostics 10(20):9100–9112
Wang Y, Zhao RZ, Liu WW, Wang ZL, Rong JD, Long XP, Liu ZJ,Ge JB, Shi B (2019) Exosomal circHIPK3 released from hypoxia-pretreated cardiomyocytes regulates oxidative damage in cardiac microvascular endothelial cells via the miR-29a/IGF-1 Pathway. Oxidative Med Cellu Longev 7954657
Wang YY, Han XT, Fu MQ, Wang JF, Song Y, Liu Y, Zhang JJ, Zhou JM, Ge JB (2018b) Qiliqiangxin attenuates hypoxia-induced injury in primary rat cardiac microvascular endothelial cells via promoting HIF-1 alpha-dependent glycolysis. J Cell Mol Med 22(5):2791–2803
Zhao L, Feng S, Wang S, Fan M, Jin W, Li X, Wang C, Yang Y (2020) Production of bioactive recombinant human myeloid-derived growth factor in Escherichia coli and its mechanism on vascular endothelial cell proliferation. J Cell Mol Med 24(2):1189–1199
Funding
This study was supported by a scientific research project from the National Natural Science Foundation of China (Grant Nos. 81860061 and 82160057). This work was also supported by the Guizhou Science and Technology Foundation (ZK[2021]353).
Author information
Authors and Affiliations
Contributions
Yan Wang and Bei Shi conceived and designed the experiments;
Yu Zhang and Jiao Li performed the experiments;
Ranzhun Zhao and Xianping Long analyzed the data;
Chaofu Li, Weiwei Liu, and Wenming Chen prepared figures;
Yan Wang drafted the paper;
Bei Shi revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zhang, Y., Li, J. et al. Role of Mydgf in the regulation of hypoxia/reoxygenation-induced apoptosis in cardiac microvascular endothelial cells. In Vitro Cell.Dev.Biol.-Animal 58, 669–678 (2022). https://doi.org/10.1007/s11626-022-00709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-022-00709-3