Abstract
Mitogen-activated protein kinase (MAPK) pathway-dependent linker phosphorylation of Smad2/3 and subsequent formation of Smad2/3/4 complex and its nuclear translocation are crucial for dysregulated transforming growth factor beta (TGF)-β/Smad signaling in liver fibrosis. Abrogation of this critical step of TGF-β/Smad signaling leading to liver fibrosis could provide new insights for future therapy, but the mechanisms remain incompletely understood. In pursuit, we investigated the subcellular expression and nuclear trafficking of the rate limiting Smad2/3/4 complex in exogenous TGF-β1-stimulated myofibroblasts (MFBs) using three MAPK-specific inhibitors. Our results showed that exogenous TGF-β1 stimulation of MFBs produced both increased protein expression and nuclear translocation of phosphorylated (p)-Smad2C/L, oncogenic pSmad3L, Smad4, importin7/8 (Imp7/8), and plasminogen activator inhibitor (PAI)-1 (Protein and mRNA), while decreased Smad7 protein expression. However, the MAPK-specific inhibitors differentially reversed these observations; for instance, ERK-specific inhibitor blocked the expression and nuclear translocation of pSmad2C/L, while both JNK and p38-specific inhibitors blocked the expression and nuclear translocation of pSmad2C/L and oncogenic pSmad3L. The MAPK-specific inhibitors had no significant effect on the total protein expression of Smad4, but rather significantly blocked its nuclear translocation. All the MAPK-specific inhibitors restored Smad7 expression and also decreased Imp7/8 and PAI-1 (Protein and mRNA) expression. Evidently, the MAPK-specific inhibitors blocked Smad2/3/4 complex formation via restoration of inhibitory Smad7 expression and blockade of Smad3L phosphorylation, while they blocked nuclear translocation of Smad2/3/4 complex through inhibition of Imp7/8 leading to decreased PAI-1 (Protein and mRNA) expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver fibrosis, also commonly known as hepatic fibrosis is a chronic inflammatory disease of the liver characterized by trans-differentiation of quiescent hepatic stellate cells (HSCs) into myofibroblasts (MFBs) leading to overproduction of extracellular matrix (ECM) in liver sinusoidal space [1]. As a result, there is complete imbalance between ECM biosynthesis and degradation setting the stage for various liver-related clinical conditions [2]. Liver fibrosis has therefore been acknowledged to be the underlying factor in almost all known liver diseases of multiple etiologies including viral hepatitis, alcoholic liver diseases, non-alcoholic steatohepatitis, cholestatic liver disease, and an essential stage of liver cirrhosis as well as a precursor of hepatocellular carcinoma (HCC) [3–7]. Liver fibrosis leading to other liver diseases has poor prognosis, mainly due to its multiple etiologies coupled with complicity of many signaling pathways such as MAPK-regulated TGF-β/Smad pathway [8, 9].
In consequence, there is a dire need for scientific investigations that aim at not only increasing our knowledge about liver fibrosis but also will explore targets that can be used for future therapies against liver fibrosis and its attendant complications (Cirrhosis leading to HCC). Pursuant to this, we have studied the modulation of the TGF-β/Smad pathway by three MAPK-specific inhibitors [10]. The MAPK-specific inhibitors represent a class of anti-cancer drugs, which are at various clinical stages of investigations, and they have multi-target inhibitory effects on the MAPK pathway as well as the TGF-β/Smad pathway.
In the present study, our focus is on how the MAPK-specific inhibitors disrupt Smad2/3/4 complex formation and its nuclear translocation, giving that Smad2/3/4 complex is a crucial rate limiting factor in the overall signaling of TGF-β. In an earlier study, we have reported the involvement of TGF-β/Smad signaling pathway in liver fibrosis [11], keloid fibroblast cells [12], HepG2 cells [13], DEN-induced HCC in rats [14] and quite recently, we showed that the MAPK-specific inhibitors ameliorate HCC phenotypic hallmarks (Cell proliferation and invasion) in HepG2 cells by down-regulating transcriptional output of PAI-1 mRNA [10]. On the basis of these previous results coupled with the increasing need for new therapeutic targets, we investigated the effects of the MAPK-specific inhibitors [PD98059 (ERK-specific inhibitor), SP600125 (JNK-specific inhibitor), and SB203580 (p38-specific inhibitor)] on the subcellular expression patterns of Smad2, Smad3, Smad4, and nuclear translocation of rate limiting Smad2/3/4 complex in MFBs stimulated by exogenous TGF-β1.
Materials and methods
Cell origin, culture, and MAPK inhibitors’ treatment
Hepatic stellate cells (HSCs) were isolated from the liver of adult male Sprague–Dawley (SD) rats (450–500 g) obtained from the Experimental Animal Center of Anhui Medical University, by liver perfusion in situ using collagenase IV and separation from liver tissue using pronase-E digestion as previously described [9, 15]. The operation on animals was approved by the Association of Laboratory Animal Sciences and the Center for Laboratory Animal Sciences at Anhui Medical University. The purity of HSCs was greater than 90 % as assessed by cell typical morphological features including the presence of vitamin A droplets. HSCs were cultured on a plastic dish and activated into MFBs as previously described [8]. MFBs were grown as sub-confluent cultures in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA.) supplemented with 10 % fetal bovine serum (FBS, Sijiqing, Zhejiang Tianhang Biological Technology Co., LTD; Zhejiang, China), and maintained in a humidified 5 % CO2 incubator at 37 °C. The experiments were performed at the log phase of growth after the cells had been plated for 24 h. The MFBs were starved overnight in serum-free medium, in the absence or presence of 10 µmol/L of each of the three MAPK inhibitors (ERK-specific inhibitor [PD98059], JNK-specific inhibitor [SP600125], and p38-specific inhibitor [SB203580]; obtained from Calbiochem company) for 5 h, and subsequently treated with TGF-β1 (9 pmol/L) (R&D Systems, Inc., Minneapolis, USA) for 1 h. The cells of the control group were treated with an equal volume of serum-free medium.
Immunofluorescence analysis
To detect the effects of the MAPK inhibitors on intracellular localization of Smad2/3, the MFBs were seeded at a density of 5 × 106/L on slides in a 24-well plate and then treated under the indicated conditions. The cells were fixed with 4 % paraformaldehyde, permeabilized with 0.1 % saponin, and blocked with 0.5 % bovine serum albumin; then incubated with each primary antibody overnight at 4 °C. The cells were incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody for 2 h at room temperature, washed 3 times with phosphate buffer saline (PBS) for 5 min each time, incubated with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) for 10 min at room temperature for nuclear staining. Finally, slides were mounted with 80 % phosphoglycerol, viewed and photographed using a fluorescence microscope (Olympus, Tokyo, Japan). Primary antibodies used in the present study included Smad2/3 phosphorylated at the C-terminal region (rabbit anti-pSmad2C antibody) (Cell Signaling Technology, Beverly, MA, USA), Smad2/3 phosphorylated at the link region (rabbit anti-pSmad2L and pSmad3L antibodies) (gifts from Dr K. Matsuzaki, Kansai Medical University, Japan). At least 100 stained cells were analyzed per sample in each experiment.
Western blot analysis
To detect the effects of MAPK inhibitors on the expression of Smad4, Smad7, PAI-1, Imp7, and Imp8, the MFBs were seeded at a density of 1 × 106 cells/25-cm2 culture flasks, and then treated under the indicated conditions. Total protein from MFBs was extracted by Western blot and IP cell lysis liquid (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Cytoplasmic and nuclear proteins in MFBs were separately extracted using a special kit named nucleoprotein and cytoplasm protein extraction kit (KeyGEN BioTECH, Nanjing, China), and the protein level of Smad4 in the cytoplasm and the nucleus was determined by Western blot as previously described [16]. Primary antibodies used included plasminogen activator inhibitor 1 (PAI-1, rabbit anti-PAI-1 antibody) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); Smad4 and Smad7 (mouse monoclonal anti-Smad4 antibody, goat polyclonal anti-Smad7 antibody) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); Imp7/8 (rabbit anti-Importin7 and rabbit anti-Importin8 Abs) (Abcam, Cambridge, UK); mouse monoclonal glyceraldehyde phosphate dehydrogenase (GAPDH) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibodies used in the study included peroxidase-conjugated rabbit anti-goat lgG, goat anti-mouse lgG, and goat anti-rabbit lgG (Zhongshan Jinqiao Company, Beijing, China). Densitometric analysis was carried out using Quantity One software (Bio-Rad, California, USA). The experiments were repeated at least three times.
Real-time reverse transcriptase-polymerase chain reaction
To detect the effects of MAPK inhibitors on transcription level of PAI-1 gene in TGF-β1-activated MFBs, MFBs were seeded at a density of 1 × 106 cells/10-cm culture dish, sequentially cultured in DMEM supplemented with 10 % FBS, then starved for 24 h in serum-free medium in the absence or presence of each of the three MAPK inhibitors at concentrations (1, 3, 10 µmol/L) for 5 h, and subsequently treated with TGF-β1 (9 pmol/L) for 1 h.
Total RNA of cells was extracted using Trizol® reagent (Invitrogen, Carlsbad, California, USA). The concentration of RNA was determined by measuring the absorbance at A260 nm and the purity of RNA was assessed by measuring the absorbance A260–A280 nm ratio. Reverse transcription of RNA was performed using PrimeScript® RT reagent Kit (TaKaRa Code: DRR037A; Takara Biotechnology Co., Ltd, Dalian, China) according to the manufacturer’s instruction, and real-time PCR reaction was carried out on ABI Prism7500 Sequence Detection System Platform (Applied Biosystems, USA) using a special kit named SYBR® Premix Ex TaqTM (Takara Biotechnology Co., Ltd, Takara Code: DRR420A; Dalian, China). The primers for MFBs PAI-1 and β-actin used as the internal control were designed and synthesized by TaKaRa Biotechnology. The primers sequences are as follows:
-
β-actin: Forward-5′GGAGATTACTGCCCTGGCTCCTA-3′,
-
Reverse-5′GACTCATCGTACTCCTGCTTGCTG-3′;
-
PAI-1: Forward-5′GGGCTGTGTGACCTAACAGGAC-3′,
-
Reverse-5′CAGCCGGAAATGACACATTGA-3′.
Real-time RT-PCR analysis was executed in an iCycler iQ Multicolor Detection System (Bio-Rad) by following the manufacturer’s specifications. Threshold cycle (Ct) at which emission rises above baseline was automatically calculated by the real-time RT-PCR system. Each Ct value was normalized to β-actin Ct value and a control sample. Relative quantization was expressed as fold-induction compared with the control conditions. Each experiment was repeated at least three times.
Statistical analyses
Data are presented as the mean ± SD. Statistical analyses were performed by SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA) for windows. Experimental and control groups were compared by one-way ANOVA. P < 0.05 was deemed to be statistically significant.
Results
Effects of three MAPK inhibitors on TGF-β1-induced phosphorylated Smad2/3 protein expression and nuclear translocation in MFBs
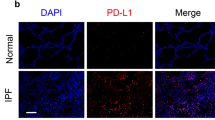
Figure 1 shows representative stained cells by immunofluorescence, highlighting intracellular localization of phosphorylated Smad2/3 proteins. The protein expression of phosphorylated Smad2 at the C-terminal (pSmad2C) and the linker region (pSmad2L), Smad3 at the linker region (pSmad3L) was weak in MFBs without exogenous TGF-β1 stimulation, while not only remarkably increased but also dramatically transferred into the nucleus under TGF-β1 stimulation. All the three MAPK inhibitors decreased the TGF-β1-induced elevation of pSmad2C, pSmad2L, and pSmad3L protein levels by different degrees; ERK-specific inhibitor could inhibit nuclear accumulation of pSmad2C and pSmad3L, JNK-specific inhibitor blocked nuclear translocation of phosphorylated Smad2 at the C-terminal and the linker region, and p38-specific inhibitor decreased nuclear accumulation of pSmad2C, pSmad2L, and pSmad3L.
Effects of the three MAPK-specific inhibitors on TGF-β1-stimulated protein expression and nuclear translocation of phosphorylated Smad2/3 in MFBs. MFBs were starved overnight in serum-free medium, in the absence or presence of 10 μmol/L of ERK-specific inhibitor (PD98059), 10 μmol/L of JNK-specific inhibitor (SP600125), or 10 μmol/L of p38-specific inhibitor (SB203580) for 5 h, respectively; subsequently they were treated with TGF-β1 (9 μmol/L) for 1 h. After they had been fixed, permeabilized, and blocked, the cells were incubated with anti-pSmad2/3 antibody and then fluorescein isothiocyanate-conjugated (FITC) secondary antibody, and viewed and photographed by a fluorescence microscope. DAPI (DNA staining) and merge are indicated. Magnification, ×200
Effects of three MAPK inhibitors on TGF-β1-induced Smad4 protein expression and nuclear translocation in MFBs
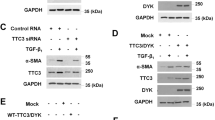
Figure 2 shows the nuclear and cytoplasmic protein levels of Smad4 protein in MFBs after complete subcellular fractionation of MFBs. TGF-β1 treatment markedly increased the nuclear level of Smad4 protein, but was decreased by all the three MAPK-specific inhibitors, especially p38-specific inhibitor, while there was no significant difference between the total protein level of Smad4 in TGF-β1-treated MFBs (model), MAPK inhibitors-treated MFBs, and control.
Effects of the three MAPK-specific inhibitors on TGF-β1-stimulated protein expression and nuclear translocation of Smad4 in MFBs. MFBs were starved overnight in serum-free medium, in the absence or presence of 10 μmol/L of ERK-specific inhibitor (PD98059), 10 μmol/L of JNK-specific inhibitor (SP600125), or 10 μmol/L of p38-specific inhibitor (SB203580) for 5 h, respectively; subsequently they were treated with TGF-β1 (9 μmol/L) for 1 h. Cell lysates fractioned into cytoplasmic and nuclear portions were analyzed by Western blot using anti-Smad4 antibody (a). Densitometry was performed, normalizing Smad4 to TBP for nuclear extracts and GAPDH for cytoplasmic extracts, and the ratio of Smad4 in the nucleus and the cytoplasm is shown in (b). Columns represent mean ± standard deviation, n = 3. (## P < 0.01 vs. control, **P < 0.01 vs. TGF-β1 group)
Effects of three MAPK inhibitors on Imp7 and Imp8 protein expression in MFBs stimulated by TGF-β1
Figure 3 shows the protein level of Imp7 and Imp8 in TGF-β1-activated MFBs pretreated by three MAPK-specific inhibitors. TGF-β1 significantly increased Imp7 and Imp8 protein expressions, while the three MAPK inhibitors decreased the TGF-β1-induced elevation of Imp7 and Imp8 expression levels. For instance, p38-specific inhibitor potently blocked Imp7 and Imp8, while ERK-and JNK-specific inhibitors blocked Imp8.
Effects of three MAPK-specific inhibitors on TGF-β1-stimulated protein expression of Imp7 and Imp8 in MFBs. MFBs were starved overnight in serum-free medium, in the absence or presence of 10 μmol/L of ERK-specific inhibitor (PD98059), 10 μmol/L of JNK-specific inhibitor (SP600125), or 10 μmol/L of p38-specific inhibitor (SB203580) for 5 h, respectively; subsequently they were treated with TGF-β1 (9 μmol/L) for 1 h. Total proteins of MFBs were extracted using Western blot and IP cell lysis liquid. Expression of Imp7 and Imp8 were monitored by Western blot using anti-Importin7 antibody and anti-Importin8 antibody respectively. Intensities of Imp7 and Imp8 were normalized to GAPDH of the corresponding treatment groups. The ratio of the Imp7 and Imp8 to GAPDH without exogenous TGF-β1 is assigned a value of 1. Columns represent mean ± standard deviation, n = 3. (## P < 0.01 vs. control, **P < 0.01 vs. TGF-β1 group)
Effects of three MAPK inhibitors on Smad7 and PAI-1 protein expression in MFBs stimulated by TGF-β1
Figure 4 shows the protein level of Smad7 and PAI-1 in TGF-β1-activated MFBs pretreated by the three MAPK-specific inhibitors. The three MAPK-specific inhibitors markedly increased Smad7 protein level while TGF-β1 stimulation had no significant effect on Smad7 protein expression (Fig. 4a). TGF-β1 induced elevated PAI-1 protein expression, but the three MAPK-specific inhibitors remarkably decreased the elevated protein level of PAI-1 (Fig. 4b).
Effects of three MAPK-specific inhibitors on TGF-β1-stimulated protein expression of Smad7 and PAI-1 in MFBs. MFBs were starved overnight in serum-free medium, in the absence or presence of 10 μmol/L of ERK-specific inhibitor (PD98059), 10 μmol/L of JNK-specific inhibitor (SP600125) or 10 μmol/L of p38-specific inhibitor (SB203580) for 5 h, respectively; subsequently they were treated with TGF-β1 (9 μmol/L) for 1 h. Total proteins of MFBs were extracted using Western blot and IP cell lysis liquid. Expression of Smad7 and PAI-1 were monitored by Western blot using anti-Smad7 antibody and anti-PAI-1 antibody, respectively. Intensities of Smad7 and PAI-1 were normalized to GAPDH of the corresponding treatment groups. The ratio of the Smad7 or PAI-1 to GAPDH without exogenous TGF-β1 is assigned a value of 1. Columns represent mean ± standard deviation, n = 3. (## P < 0.01 vs. control, *P < 0.05, **P < 0.01 vs. TGF-β1 group)
Effects of three MAPK inhibitors on PAI-1 transcriptional activity in MFBs stimulated by TGF-β1
Figure 5 shows the PAI-1 mRNA level in TGF-β1-activated MFBs pretreated by ERK-specific inhibitor (Fig. 5a), JNK-specific inhibitor (Fig. 5b) and p38-specific inhibitor (Fig. 5c). TGF-β1 significantly increased PAI-1 transcriptional activity in MFBs, but this was decreased by MAPK-specific inhibitors at two concentrations (3 and 10 µmol/L).
Effects of three MAPK-specific inhibitors on TGF-β1-stimulated PAI-1 transcriptional activity in MFBs. MFBs were starved overnight in serum-free medium, in the absence or presence of three concentrations (1, 3, 10 μmol/L) of ERK-specific inhibitor (PD98059), JNK-specific inhibitor (SP600125), or p38-specific inhibitor (SB203580) for 5 h, respectively; subsequently they were treated with TGF-β1 (9 μmol/L) for 1 h. Cellular total RNA was extracted to assess the levels of PAI-1 mRNA in each group by real-time reverse transcription-polymerase chain reaction. The results are expressed as fold increases of PAI-1 mRNA normalized to β-actin. Columns represent mean ± standard deviation, n = 3. (## P < 0.01 vs. control, *P < 0.05, **P < 0.01 vs. TGF-β1 group)
Discussion
In the present study, MFBs were derived from the activation of HSCs obtained from a normal rat liver to mimic the pathological features of activated HSCs in human liver fibrosis. From the results of this study, stimulation of MFBs by exogenous TGF-β1 led to activation of TGF-β/Smad signaling, which was characterized by both increased protein expression and nuclear translocation of pSmad2C/L, oncogenic pSmad3L, Smad4, aided by Imp7/8 and also a decrease in the protein expression of inhibitory Smad7. Collectively, these effects produced increased PAI-1 (Protein and mRNA) expression, which is a prerequisite for pathological roles of TGF-β1 in fibrogenesis and hepatocarcinogenesis. Importantly, the above observations were attributable to exogenous TGF-β1 since they were totally absent in the control group (MFBs without exogenous TGF-β1 stimulation). However notable, was the fact that the three MAPK inhibitors in a differential manner reversed the observations associated with exogenous TGF-β1-stimulated MFBs, indicating that indeed the MAPK pathway regulates the fibrogenic arm of TGF-β/Smad signaling in MFBs.
Previously, we have shown the ability of the MAPK-specific inhibitors to inhibit TGF-β1-induced activation of ERK, JNK, and p38 in HepG2 cells which in that study led to decrease in global Smad2/3/4 complex formation and PAI-1 transcriptional activity [10]. Presently, we report that the MAPK-specific inhibitors do not only decrease global expression of Smad2/3/4 complex but also they block its nuclear translocation by inhibiting the expression of Imp7/8 and their roles in aiding nuclear trafficking of Smad2/3/4 complex.
Mediation of TGF-β signaling by Smad proteins [Smad2 and Smad3 (Receptor mediated Smads), Smad4 (Common Smad), and Smad7 (Inhibitory Smad)] has been explained elsewhere [17–19]. TGF-β is a ubiquitous cytokine crucial in metazoan biology playing dual roles in a cell type and context-dependent fashion, for example, in normal physiological states it plays crucial roles in regulating cell growth, cell proliferation, and apoptosis to maintain cellular homeostasis, however, in dysregulated states such as in liver fibrosis and cancer [20–23]. TGF-β may promote fibrogenesis and oncogenesis through interaction with many signaling pathways, especially the MAPK pathway [9,23]. TGF-β is therefore commonly described as a two-edged sword or a necessary evil in cell and cancer biology [24]. The varied and context-dependent roles of TGF-β signaling in part reflect its canonical and non-canonical signaling. The former which is tumor suppressive in nature, is mediated through receptor-dependent C-terminal phosphorylation of Smad2 and Smad3 leading to decrease in pro-fibrogenic TGF-β target genes (PAI-1, c-myc) but increase in growth inhibitors such as p21WAFI, while the latter which is both pro-fibrogenic and oncogenic is mainly mediated through MAPK-dependent linker phosphorylation of Smad2/3, particularly MAPK-dependent pSmad3L signaling [25]. It is at this locus that the MAPK pathway modulates TGF-β/Smad signaling to increase the risk of fibrogenesis leading to fibrocarcinogenesis. However, crucial to these two signaling modes of TGF-β is the formation of Smad2/3/4 complex and its nuclear translocation, indeed nuclear translocation of Smad2/3/4 complex has been shown to be indispensable for TGF-β/Smad signaling [26]. Intrinsically, by a negative feedback mechanism, inhibitory Smad7 can regulate TGF-β-receptor-mediated phosphorylation of Smad2 and Smad3 to terminate or control Smad2/3/4 complex formation within limits [27]. But like all other mediators of TGF-β signaling in disease pathologies, Smad7 expression suffers down-regulation in dysregulated TGF-β signaling and this was not different from our results as exogenous TGF-β1 stimulation of MFBs caused significant down-regulation of Smad7 compared to the control. Essentially, restoration of Smad7 expression, inhibition of Smad2/3/4 complex formation, and nuclear translocation by any pharmacotherapeutic agent may represent a significant step in abrogating fibrogenic roles of TGF-β/Smad in liver fibrosis. This may also provide a new target for therapeutic exploration as was demonstrated earlier by using the MAPK-specific inhibitors in HepG2 cells [10].
From this study, we report that the MAPK-specific inhibitors had no significant inhibitory effect on global Smad4 protein expression (Fig. 2), but differentially each MAPK-specific inhibitor blocked domain-specific phosphorylation of Smad2 and Smad3, particularly fibrogenic Smad3L to interfere with the formation of Smad2/3/4 complex formation, notably, p38 MAPK-specific inhibitor (SB203580) (Fig. 1). Also, all the MAPK-specific inhibitors restored inhibitory role of Smad7 by increasing its global protein expression (Fig. 4) in a manner that confirms our earlier report from studies of the MAPK-specific inhibitors in HepG2 cells [10]. Interestingly, decrease in the expression of Smad2/3/4 complex formation and nuclear translocation by the MAPK-specific inhibitors correlated with decrease in Imp7/8, suggesting that perhaps nuclear translocation of Smad2/3/4 complex is probably linked with Imp7/8 expression and activity. This observation probably indicates that the ability of the MAPK-specific inhibitors to block nuclear translocation of Smad2/3/4 complex may be mediated through inhibition of Imp7/8. And this observation is in line with earlier reports that have linked Imp7/8 activity with nuclear translocation of activated pSmad2 and pSmad3 [28–30]. Together, inhibition of Smad2/3/4 complex formation and nuclear translocation and inhibition of Imp7/8 by each of the MAPK-specific inhibitors led to a decrease in PAI-1 (Protein and mRNA) expression (Figs. 4, 5), a key target gene of TGF-β. While this observation complements our previous results [10,16], it also emphasizes the significance of the Smad2/3/4 complex and Imp7/8 as possible new targets that should be investigated further for therapy against liver fibrosis and other liver-related clinical conditions.
In conclusion, we report that all the MAPK-specific inhibitors (PD98059 [ERK-specific inhibitor], SP600125 [JNK-specific inhibitor] and SB203580 [p38-specific inhibitor]) disrupted the formation of Smad2/3/4 complex via restoration of inhibitory Smad7 expression and blockade of linker phosphorylation of Sma3L, while they blocked nuclear translocation of Smad2/3/4 complex through inhibition of Imp7/8 protein expression. Collectively, these effects resulted in decreased expression of PAI-1 (Protein and mRNA). Of all the MAPK-specific inhibitors studied, p38-specific inhibitor was most potent.
References
Friedman SL (2010) Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 7:425–436. doi:10.1038/nrgastro.2010.97
Elpek GO (2014) Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol 20:7260–7276. doi:10.3748/wjg.v20.i23.7260
Sun Y, Lu Y, Xie L, Deng Y, Li S, Qin X (2015) Interferon gamma polymorphisms and hepatitis B virus-related liver cirrhosis risk in a Chinese population. Cancer Cell Int 15:35. doi:10.1186/s12935-015-0184-2
Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J, Okazaki K, Seki T (2007) Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 46:48–57. doi:10.1002/hep.21672
Cojocariu CE, Trifan AV, Gîrleanu I, Stanciu C (2014) Alcoholic liver disease–epidemiology and risk factors. Rev Med Chir Soc Med Nat Iasi 118:910–917
Yoon HJ, Cha BS (2014) Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol 6:800–811. doi:10.4254/wjh.v6.i11.800
Stickel F (2015) Alcoholic cirrhosis and hepatocellular carcinoma. Adv Exp Med Biol 815:113–130. doi:10.1007/978-3-319-09614-8_7
Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K (2003) p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology 38:879–889. doi:10.1053/jhep.2003.50384
Yoshida K, Matsuzaki K, Mori S, Tahashi Y, Yamagata H, Furukawa F, Seki T, Nishizawa M, Fujisawa J, Okazaki K (2005) Transforming growth factor-beta and platelet-derived growth factor signal via c-Jun N-terminal kinase-dependent Smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am J Pathol 166:1029–1039
Boye A, Kan H, Wu C, Jiang Y, Yang X, He S, Yang Y (2015) MAPK inhibitors differently modulate TGF-beta/Smad signaling in HepG2 cells. Tumour Biol. doi:10.1007/s13277-014-3002-x
Yang Y, Yang S, Chen M, Zhang X, Zou Y, Zhang X (2008) Compound Astragalus and Salvia miltiorrhiza Extract exerts anti-fibrosis by mediating TGF-beta/Smad signaling in myofibroblasts. J Ethnopharmacol 118:264–270. doi:10.1016/j.jep.2008.04.012
He S, Yang Y, Liu X, Huang W, Zhang X, Yang S, Zhang X (2012) Compound Astragalus and Salvia miltiorrhiza extract inhibits cell proliferation, invasion and collagen synthesis in keloid fibroblasts by mediating transforming growth factor-β/Smad pathway. Br J Dermatol 166:564–574. doi:10.1111/j.1365-2133.2011.10674.x
Liu X, Yang Y, Zhang X, Xu S, He S, Huang W, Roberts MS (2010) Compound Astragalus and Salvia miltiorrhiza extract inhibits cell invasion by modulating transforming growth factor-beta/Smad in HepG2 cell. J Gastroenterol Hepatol 25:420–426. doi:10.1111/j.1440-1746.2009.05981.x
Hu X, Rui W, Wu C, He S, Jiang J, Zhang X, Yang Y (2014) Compound Astragalus and Salvia miltiorrhiza extracts suppress hepatocarcinogenesis by modulating transforming growth factor-β/Smad signaling. J Gastroenterol Hepatol 29:1284–1291. doi:10.1111/jgh.12490
Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K (2002) Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology 35:49–61. doi:10.1053/jhep.2002.30083
He S, Liu X, Yang Y, Huang W, Xu S, Yang S, Zhang X, Roberts MS (2010) Mechanisms of transforming growth factor beta(1)/Smad signalling mediated by mitogen-activated protein kinase pathways in keloid fibroblasts. Br J Dermatol 162:538–546. doi:10.1111/j.1365-2133.2009.09511.x
Bae E, Kim SJ, Hong S, Liu F, Ooshima A (2012) Smad3 linker phosphorylation attenuates Smad3 transcriptional activity and TGF-β1/Smad3-induced epithelial-mesenchymal transition in renal epithelial cells. Biochem Biophys Res Commun 427:593–599. doi:10.1016/j.bbrc.2012.09.103
Meng XM, Huang XR, Xiao J, Chung AC, Qin W, Chen HY, Lan HY (2012) Disruption of Smad4 impairs TGF-β/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int 81:266–279. doi:10.1038/ki.2011.327
Choi MJ, Song KM, Park JM, Kwon MH, Kwon KD, Park SH, Ryu DS, Ryu JK, Suh JK (2014) Effect of SMAD7 gene overexpression on TGF-β1-induced profibrotic responses in fibroblasts derived from Peyronie’s plaque. Asian J Androl. doi:10.4103/1008-682X.142130
Wells RG, Kruglov E, Dranoff JA (2004) Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett 559:107–110. doi:10.1016/S0014-5793(04)00037-7
Hidaka H, Nakazawa T, Shibuya A, Minamino T, Takada J, Tanaka Y, Okuwaki Y, Watanabe M, Koizumi W (2011) Effects of 1-year administration of olmesartan on portal pressure and TGF-beta1 in selected patients with cirrhosis: a randomized controlled trial. J Gastroenterol 46:1316–1323. doi:10.1007/s00535-011-0449-z
Liu ZM, Tseng HY, Tsai HW, Su FC, Huang HS (2015) Transforming growth factor β-interacting factor-induced malignant progression of hepatocellular carcinoma cells depends on superoxide production from Nox4. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2015.03.028
Zhang H, Ozaki I, Mizuta T, Yoshimura T, Matsuhashi S, Eguchi Y, Yasutake T, Hisatomi A, Sakai T, Yamamoto K (2004) Transforming growth factor-beta 1-induced apoptosis is blocked by beta 1-integrin-mediated mitogen-activated protein kinase activation in human hepatoma cells. Cancer Sci 95:878–886
Matsuzaki K, Seki T, Okazaki K (2014) TGF-β signal shifting between tumor suppression and fibro-carcinogenesis in human chronic liver diseases. J Gastroenterol 49:971–981. doi:10.1007/s00535-013-0910-2
Murata M, Yoshida K, Yamaguchi T, Matsuzaki K (2014) Linker phosphorylation of Smad3 promotes fibro-carcinogenesis in chronic viral hepatitis of hepatocellular carcinoma. World J Gastroenterol 20:15018–15027. doi:10.3748/wjg.v20.i41.15018
Dong XM, Yin RH, Yang Y, Feng ZW, Ning HM, Dong L, Zheng WW, Tang LJ, Wang J, Jia YX, Jiang YN, Liu ED, Chen H, Zhan YQ, Yu M, Ge CH, Li CY, Yang XM (2014) GATA-2 inhibits transforming growth factor-β signaling pathway through interaction with Smad4. Cell Signal 26:1089–1097. doi:10.1016/j.cellsig.2014.01.028
Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM (2003) Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 125:178–191
Yao X, Chen X, Cottonham C, Xu L (2008) Preferential utilization of Imp7/8 in nuclear import of Smads. J Biol Chem 283:22867–22874. doi:10.1074/jbc.M801320200
Chen X, Xu L (2011) Mechanism and regulation of nucleocytoplasmic trafficking of smad. Cell Biosci 1:40. doi:10.1186/2045-3701-1-40
Xu L, Yao X, Chen X, Lu P, Zhang B, Ip YT (2007) Msk is required for nuclear import of TGF-{beta}/BMP-activated Smads. J Cell Biol 178:981–994
Acknowledgments
We thank Prof. K Matsuzaki (Department of Gastroenterology and Hepatology, Kansai Medical University, Osaka, Japan) for providing us with the following Abs: Anti-pSmad2L and Anti-pSmad3L. Also, this work was supported by the National Natural Science Foundation of China (No. 81073098, No. 81374012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yufeng Jiang and Chao Wu have been contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, Y., Wu, C., Boye, A. et al. MAPK inhibitors modulate Smad2/3/4 complex cyto-nuclear translocation in myofibroblasts via Imp7/8 mediation. Mol Cell Biochem 406, 255–262 (2015). https://doi.org/10.1007/s11010-015-2443-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2443-x