Abstract
The aim of the study was to investigate the possible association of AluI and RsaI polymorphisms of estrogen receptor β (ER-β) gene and 23-bp nucleotide repeat polymorphism of estrogen-related receptor α (ERRα) gene with bone mineral density (BMD) in postmenopausal Egyptian women. Two-hundred postmenopausal osteoporotic women as cases and 180 healthy age-matched postmenopausal women as controls were genotyped by PCR fragment length polymorphism for AluI, allele-specific PCR for RsaI, and by sizing of PCR products on agarose gels for ERRα repeats. sRANKL levels were estimated by ELISA. BMD measurements for spine and femoral neck were performed by dual energy X-ray absorptiometry. A significant difference between women with osteoporosis and controls regarding allele and genotype distributions of AluI G/A (OR 2.37, 95 % CI 1.77–3.18 and p < 0.001 for A allele) and ERRα polymorphisms (for the two repeats allele OR 2.08, 95 % CI 1.09–4.00, and p = 0.02). Osteoporotic women with the AluI AA + GA genotype or with the EERα 2,2 genotype had significantly lower BMD than did women with the other genotypes. Moreover, there was a significant increase of the mean values of sRANKL in carriers of AluI A, RsaI A alleles and in patients having 2,2 genotypes of ERRα (p < 0.001, p < 0.001, p = 0.02, respectively). We demonstrated an association of ER-β AluI G/A and ERRα 23-repeats polymorphisms with BMD in postmenopausal Egyptian women. A possible effect of ER-β and ERRα polymorphisms on the levels of sRANKL was estimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common skeletal disorder characterized by low bone mass, low bone mineral density (BMD), and deteriorated skeletal microarchitecture, with consequent increase in bone fragility and fracture risk [1]. Osteoporosis is a multifactorial disease with a strong genetic component. Although a number of factors including hormonal, environmental and nutritional have been shown to affect BMD; twin and family studies have revealed that at least 40–70 % of the variation of BMD is accounted for by genetic factors [2]. Among many candidate genes, estrogen receptor (ER) genes have been proven to regulate bone mass [3].
The concept that estrogen deficiency is critical to the pathogenesis of osteoporosis was based initially on the fact that postmenopausal women, whose estrogen levels naturally decline, are at the highest risk for developing the disease. However, the heterogeneity of osteoporosis may be due not only to differences in the production of estrogen, but also to changes in receptors, signal transduction mechanisms, nuclear transcription factors, and enzymes that produce or inactivate local regulators [3]. Estrogen acts through binding to ER-α and ER-β; both receptors are highly expressed in bone [4]. The human ER-α and ER-β are the products of separate genes present on distinct chromosomes (locus 6q25.1 and locus14q22-24, respectively). ER-α and ER-β genes comprise eight exons separated by seven intronic regions and spans more than 140 kb and approximately 40 kb, respectively [5].
Genetic screening of ER-α and ER-β genes has revealed the existence of several polymorphic sites. ER-α gene polymorphisms have been extensively studied for their associations with bone mass and osteoporosis risks in several populations [6–9]. A number of studies have found an association between a dinucleotide (CA) repeat polymorphism in intron 5 of ER-β gene and BMD phenotypes [10–12]. However, there are few studies about the association of the common polymorphisms, AluI (1730 G/A, rs4986938) located at exon 8 or RsaI (1082 G/A, rs1256049) located at exon 5 of ER-β gene and BMD [13, 14].
An orphan nuclear receptor, estrogen-related receptor α (ERRα), with sequence homology to ER-α and ER-β, was shown to be highly expressed throughout the osteoblast developmental sequence. Despite its inability to bind estrogens, this receptor may interact with ER-α and ER-β, or act directly to alter bone cell function. ERRα gene is located on chromosome 11q12 and comprises seven exons separated by six introns, which span about 20 kb [15]. Laganière et al. [16] reported a 23-base pair (bp) sequence referred to as ERRα 23 (rs3217060), located at position −682 in the promoter region of the ERRα gene. ERRα 23 is a 23-bp nucleotide repeat found in one to four copies in human chromosomes. It was mainly found in two copies of a 23-bp element; however, about 12 % of the population had three or four copies. Two studies found this variant to be associated with BMD in premenopausal women [17, 18]; however up to date, no reports concerning the role of this polymorphism in postmenopausal osteoporotic women have been reported as yet.
One of potential sites of action of estrogen/ER pathway includes effects on osteoblastic cells to alter their production of receptor activator of nuclear factor-kappa-B ligand (RANKL) [3]. It was also shown that RANKL levels were increased on the surface of bone marrow cells from early postmenopausal women who are estrogen deficient [19]. It is now clear that RANKL is the key final effector osteoclastogenic cytokine. Bone cells appear to express the membrane-bound form of RANKL, and thus, osteoblasts must physically interact with osteoclasts precursors in order to activate RANK [20]. Soluble RANKL (sRANKL) can be produced by activated T lymphocytes and is as active as membrane-bound RANKL in binding to RANK [21].
The aim of this study was to evaluate the association of AluI G/A and RsaI G/A polymorphisms of ER-β gene and 23-bp nucleotide repeat polymorphism of ERRα gene with BMD in Egyptian postmenopausal women, and to determine the effect of these polymorphisms on serum levels of sRANKL.
Subjects and methods
This case–control study included two-hundred postmenopausal osteoporotic women evaluated in the Internal Medicine and Radiology Departments of Zagazig University Hospitals. One-hundred and eighty age-matched healthy postmenopausal women with normal BMD were recruited from the same demographic area and taken as controls. Women were considered postmenopausal if they had no menstruation for at least 12 months. All the subjects underwent BMD measurements by dual energy X-ray absorptiometry at lumbar spine (vertebrae L1-L4) and at the femoral neck. On the basis of T score of dual energy X-ray absorptiometry, they were classified as osteoporotic or normal BMD according to the World Health Organization definition and diagnosis of osteoporosis [22]. T score of both lumbar spines and femoral neck was taken to differentiate between women with normal BMD (T score ≥ −1 at both sites) and osteoporotic women (T score ≤ −2.5 at both sites).
Women with endocrinological disorders (such as hypo- or hyperparathyroidism, hyperthyroidism, Cushing’s syndrome, and diabetes mellitus) or other diseases affecting BMD such as chronic disorders of liver and kidney, rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, and other skeletal diseases (e.g., Paget’s disease, osteogenesis imperfecta) were excluded from the study. Women who were on medications known to affect bone density and metabolism e.g., corticosteroids, anticonvulsants, bisphosphonates, or calcitonin; and women with hormone replacement therapy, premature menopause <40 years, or underwent bilateral oophorectomy were also excluded. None of the women was a smoker and their daily work comprises usual physical activities. The study was approved by the ethics committee of Zagazig University and informed consent was obtained from all the subjects included in the study. All subjects were interviewed for their personal and medical history, including age, menarche and menopause and submitted to thorough clinical examination including height and weight measurements and assessment of body mass index (BMI) [weight in Kg/surface area in m2]. Baseline characteristics of the studied groups are shown in Table 1.
DNA isolation and determination of ER-β and ERRα genotypes
Genomic DNA was extracted from EDTA whole blood using a spin column method according to the manufacturer recommendations (QIAamp Blood Kit; Qiagen GmbH, Hilden, Germany). The AluI ER-β gene polymorphism was detected by PCR restriction fragment length polymorphism analysis according to Meng et al. [23]. PCRs were performed in a 25 µl volume containing 100 ng of DNA template, 12.5 µl one step PCR mixture containing 1 unit Taq polymerase, 10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris-HCl (pH 8.75), 0.1 % Triton X-100, 0.1 mg/ml BSA and 200 µM dTNPs (Bio-Basic Inc., Ontario, Canada), and 0.2 µM of each primer (Biosynthesis Inc., Lewisville, Tx, USA). The PCR product was digested with restriction endonuclease AluI (New England Biolabs, Ipswich, MA, USA).
In all subjects, allele-specific PCR was performed to detect the RsaI variants of ER-β according to Silva et al. [24]. Two reactions per subject were run, using a specific primer for either the polymorphic A variant or for the wild-type G variant, together with an upstream and a downstream primers. PCR conditions were established to generate both a control fragment and a shorter, allele-specific band in the presence of the variant and only the control fragment in its absence. Allele-specific PCR of the RsaI polymorphism was performed in a total volume of 25 µl containing 200 ng DNA, 12.5 µl one step PCR mixture (Bio-Basic Inc., Ontario, Canada), and 0.2 µM of each of the primers RsaI forward (Fw), RsaI reverse (Rev), and either RsaI Rev A or RsaI Rev G.
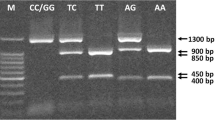
The ERRα repeats polymorphism was detected by sizing of PCR products on agarose gel according to Laflamme et al. [17] with some modifications. 25 µl reaction mixtures were containing 200 ng genomic DNA, 12.5 µl one step PCR mixture, and 0.2 µM of each primer. The ER-β genotype was identified by electrophoresis of PCR products in 2 % agarose gel and ERRα genotype in 3 % agarose gel. The gel was visualized under UV trans-illuminator with a 100-bp ladder (SiZer™-100 DNA Marker, iNtRON Biotechnology, Seongnam, Korea) for both ER-β genotype and a 20-bp ladder (SiZerTM-20 DNA Marker, iNtRON Biotechnology, Seongnam, Korea) for ERRα genotype. The primers, PCR conditions, and DNA fragment sizes are listed in Table 2.
Measurement of serum sRANKL
Centrifuged serum was frozen and stored at −80 °C until assay. Determinations of sRANKL levels were performed by ELISA (Immundiagnostik, Bensheim, Germany). The sensitivity, intra-assay and inter-assay coefficient of variation were 0.04 pmol/l, 5, and 7 %, respectively.
Statistical analysis
Continuous variables were expressed as means ± SD. The means of the two groups were compared by student t test. The statistical significances of differences in frequencies of variants between the groups were tested using the Chi square test. In addition, odds ratios (ORs) and 95 % confidence intervals (CIs) were calculated as a measure of the association of the ER-β AluI, RsaI, and ERRα 23 repeats alleles with osteoporotic women. Interaction between ER-β and ERRα genes were determined based on Bayesian algorithm using the Phase program. A difference was considered significant if p was <0.05. Each of the association studies had 80% power to detect a gene conferring a genotype relative risk of 1.5 at the 5 % significance level.
Results
Baseline characteristics of the studied groups
There were insignificant difference as regard age (p = 0.34) and menopause (p = 0.34) between osteoporotic women and controls. Osteoporotic women had statistically significant lower mean values of T score and BMD of both lumbar spine and femoral neck (p < 0.001) as compared to control women, but there was no statistical significance between both groups as regard BMI, age at first menarche, number of children, and serum calcium levels (Table 1).
Genotype and allele distributions of ER-β and ERRα gene polymorphisms
The genotype and allele frequencies of the ER-β and ERRα gene polymorphisms studied women are shown in Table 3. Our results showed significant differences between osteoporotic women and controls regarding the genotype and allele distributions of the ER-β AluI 1730 G/A polymorphism. The AA genotype for the AluI G/A polymorphism was significantly more frequent in osteoporotic women than in controls (37.5 vs. 16.7 %, p < 0.001), and there was a statistically significant increase in the A allele in the osteoporotic women as compared to controls (p < 0.001, OR 2.37 and 95 % CI 1.77–3.18). The frequency of AluI GG genotype was significantly higher in controls than in osteoporotic women (47.2 vs. 26 %, respectively, p < 0.001).
Concerning ER-β RsaI 1082 G/A polymorphism, there were no significant differences of genotype or allele frequencies between patients and controls (p = 0.24, OR 1.3 and 95 % CI 0.83–2.04 for the A allele).
The ERRα genotype frequencies in this sample were as follows: 1, 1 repeats (0 %), 1, 2 (0 %), 1, 3 (0 %), 1, 4 (0 %), 2, 2 (85.5 %), 2, 3 (12.8 %), 2, 4 (1.1 %), 3,3 (0.6 %), 3, 4 (0 %), and 4, 4 (0 %) for controls and 1, 1 (0 %), 1, 2 (0 %), 1, 3 (0 %), 1, 4 (0 %), 2, 2 (93 %), 2,3 (6 %), 2, 4 (0.5 %), 3, 3 (0.5 %), 3, 4 (0 %), and 4, 4 (0 %) for osteoporotic women. The 2, 2 genotype was significantly more frequent in osteoporosis patients than in controls (93 vs. 85.5 %, p = 0.02) and OR, and 95 % CI for the 2 repeats allele of ERRα = 2.08 (1.09–4.00), p = 0.02. On the other hand, the 2, 3 genotype frequency was significantly higher in the control group than in osteoporosis one (12.8 vs. 6 %, p = 0.02).
Interaction between ER-β and ERRα genes
We studied the osteoporosis susceptibility in relation to various combinations of genotypes. We found that patients who were carriers of haplotype (AluI AA, RsaI AA and ERRα 2,3 repeats 2,2 genotypes) had an increased risk of osteoporosis (OR and 95 % CI 3.3 (1.9–5.6), p < 0.001).
The association of ER-β and ERRα gene polymorphisms with characteristics of osteoporotic women
Further, we analyzed the relation between ER-β and ERRα genotypes with the characteristics and the BMD measures in osteoporotic women. There was no significant difference in age, BMI, age at menarche, age at menopause, and number of children between the different genotypes of ER-β polymorphisms. The mean values of lumbar spine BMD were significantly lower in carriers of AluI A mutated alleles in comparison with GG genotype patients (p = 0.01). Also the average femoral neck BMD of the patients with mutated alleles AluI A was significantly lower than those with GG genotypes (p = 0.03). For ER-β RsaI polymorphism, no effect of different RsaI genotypes on both lumbar spine and femoral neck BMD was found (p = 0.06 and 0.11, respectively) (Table 4).
For ERRα polymorphism, because of some genotypes were rare or absent and for association analyses, ERRα genotypes were regrouped into those having 2,2 genotype for contrast with those having 2,3; 2,4 and 3,3 genotypes, as in the previous report of Laflamme et al. [17]. We found that the long repeats (2,3 + 2,4 + 3,3) genotypes of ERRα 23 were associated with a higher BMI than the short repeats 2,2 genotype (p = 0.006). Moreover, the values of both lumber and femoral neck BMD were significantly lower in the short repeat 2,2 genotype than in the long repeats genotype patients (p < 0.001 for both) (Table 4).
Serum sRANKL in all studied groups
Mean values of serum sRANKL levels were significantly higher in osteoporotic women than in controls (p < 0.001) (Table 1). When we studied the relation between ERRα and ER-β genotypes and sRANKL levels, we found a significant increase of the mean values of sRANKL in carriers of AluI A, RsaI A alleles and in women having 2,2 genotype of ERRα (p < 0.001, p < 0.001, p = 0.02 respectively) (Table 4).
Discussion
Since estrogens have important effects on bone mass and bone remodeling, ER genes have been tested for association with osteoporosis and fracture risk. Most of the studies that investigated the relationships between BMD and ER genes have been focused on polymorphisms in ER-α gene that is robustly expressed in reproductive tissues. The results obtained, even if compelling, for ER-α gene involvement in osteoporosis or fracture were not conclusive; likely because of confounding factors, i.e., the ethnic- and gender-distribution of ER-α gene polymorphisms [7–9]. After the identification of ER-β gene and its gene product ER-β, which is more abundant than the ER-α one in trabecular bone, several studies have also evaluated polymorphisms of ER-β gene as risk markers for osteoporosis [10–12]. The rational in studying the ER-β gene as a candidate gene for osteoporosis lays on the fact that ER-β is robustly expressed by developing human bone, especially the cancellous bone compartment [4]. As well as studies on cell cultures revealed that there is a greater than nine fold increase in ER-β expression in cultured human osteoblasts during bone mineralization, whereas ER-α levels remain unchanged during this process [25]. Furthermore, experimental studies utilizing ER-β gene knockout mouse models remarked the important involvement of ER-β in bone formation [26]. In addition, ER-β was shown to be more potent than ER-α in mediating estrogen-induced repression of tumor necrosis factor α expression, which is associated with the pathogenesis of osteoporosis [27].
In this study, we have evaluated the association of AluI 1730 G/A and RsaI 1082 G/A polymorphisms of ER-β gene with BMD in postmenopausal women. Our results showed significant differences between osteoporotic women and control women regarding the genotype and allele distributions of the ER-β AluI 1730 G/A polymorphism. The homozygous AA genotype and the A allele for the AluI G/A polymorphism were significantly more frequent in osteoporotic patients than in controls. Interestingly, the frequency of homozygous wild AluI GG genotype was significantly higher in controls than in osteoporotic women. Moreover, mean values of lumbar spine BMD and femoral neck BMD were significantly lower in carriers of AluI A mutated alleles in comparison with GG genotypes among osteoporotic patients.
Concerning ER-β RsaI 1082 G/A polymorphism, there were no significant differences of genotype or allele frequencies between osteoporotic women and controls and also there was no effect of different RsaI genotypes on both lumbar spine and femoral neck BMD. Our results are in consistence with Curro et al. [13] who found that ER-β AluI polymorphism was associated with BMD in a cohort of Caucasian osteopenic and postmenopausal women living in Sicily (Italy). In particular, they observed a significant, negative correlation between the mutated homozygous genotype AA1730 and lumbar spine BMD values in comparison to subject with GA or GG genotypes. Interestingly, 85 % of patients with homozygous genotype AA1730 in their study presented the smallest lumbar spine BMD values, thus suggesting that this genetic variant may likely contribute to bone loss worsening in postmenopausal women. Moreover, they demonstrated that the negative effect of AA1730 genotype on BMD has been reinforced by longer times since the onset of menopausal status, accordingly with a progressive reduction in estrogen synthesis. However, several previous investigations did not find any significant association of ER-β AluI 1730 G/A [28–30] and RsaI 1082G/A polymorphisms with either spine or hip BMD [14, 28].
In our study, the recruited participants were a homogenous population regarding age, race, ethnic origin, and menopausal status. The human ER-β synthesis shows variability in a gender, age, and cell-type dependent manner. Specifically, it has been suggested that the worsening effects of ER-β polymorphisms on female bone mass become more evident at lower estrogen concentrations, as occurring in postmenopausal women, than at higher estrogen concentrations, as seen in premenopausal women [13, 29]. To date, several ER-β gene polymorphisms have been tested for their association with BMD and changes in bone turnover markers. However, only few studies reported a positive association between ER-β gene variants and BMD or fracture risk [10–12], while other results were inconclusive [28, 30] or showed a dissimilar effect at different ER-β gene loci [29]. The contradictory results conferred by different studies on a specific candidate gene could be attributed to different sample size, cross-sectional study approaches as well as differences in race/ethnicity, age, and gender in the populations studied; and also, the heterogeneity of selected populations encompassing healthy individuals with significantly different ages and hormonal milieu, ranging from fertile to pre or peri-menopausal status [28, 29]. The crucial role of hormonal status is also supported by the findings on the lack of association of ER-β AluI polymorphism with BMD in a population of Caucasian men, underlining the gender-related differences effects of estrogens [30]. Thus, different genetic backgrounds and environmental effects and/or their interaction could explain the diversity in the results reported by different study groups. Although the exact mechanism behind the observed associations with BMD is still not fully understood, it has been hypothesized that the ER-β AluI polymorphism, consisting in a G/A1730 transition in the 3′-Untraslated region, could alter mRNA stability and protein levels [31], leading, in turn, to a reduced synthesis of ER-β. This would indicate that this AluI ER-β gene variant is likely implicated in the distribution and density of ER-β in woman body organs, primarily affecting the interaction of estrogen to ERs on trabecular bone. In this context, the use of natural molecules, i.e., soy isoflavone derivatives, mainly targeting ER-β receptor has already been demonstrated successfully in improving both BMD and biochemical bone markers in osteopenic postmenopausal women [32, 33].
In this study, the association of 23-bp nucleotide repeat polymorphism in ERRα gene promoter with BMD was evaluated as well. We found that the 2,2 genotype was significantly more frequent in osteoporotic women than in controls. On the other hand, the 2,3 genotype frequency was significantly higher in the control group than in osteoporosis one. Also, we found that the values of both lumber and femoral neck BMD were significantly lower in the short repeat 2,2 genotype than in the long repeats (2,3 + 2,4 + 3,3) genotypes among osteoporotic women. Our results are in agreement with Laflamme et al. [17] who reported that this polymorphism was associated with a significant difference in lumbar spine BMD in a sample of premenopausal women. They also found that women with long variants (three repeats) of the ERRα promoter polymorphism showed a higher lumbar spine BMD, whereas the short variant genotype (two repeats) which is more common and present in about 89 % of the population is associated with lower BMD and likely increased risk of bone fracture. However, contradictory to our results, Giroux et al. [18] found no association of 23-bp nucleotide repeat polymorphism in ERRα gene promoter and BMD. A functional study done by Laganière et al. [16] implicated that the 23-nucleotide-long sequence includes a functional ERR-response element. Indeed, they showed that an interdependent ERRα/PGC-1α (peroxisome proliferator activated receptor γ coactivator-1α)-based transcriptional pathway targets this ERRα polymorphic element to dictate the level of ERRα expression. Also, they had shown that a higher number of repeats lead to a higher expression of ERRα. Interestingly, ERRα is highly expressed in bone at all developmental stages, from the earliest precursors to the most mature osteoblasts in mineralized nodules. Furthermore, in vitro studies using rat calvaria cells indicated that inhibition of ERRα expression through antisense oligonucleotide treatment results in a significant decrease in mineralized bone nodule number. In the same manner, ERRα overexpression by transient transfection with CMV- ERRα construct resulted in a significant increase in the number of mineralized bone nodules formed [15]. Despite significant amino acid homology in the ligand binding domain with the ERs, ERRα does not bind estradiol or other natural ligands and is therefore still considered as an orphan receptor. However, the synthetic ER agonist diethylstilbestrol was identified as an ERRα antagonist [34] and the structural basis for the antagonistic action of this synthetic compound has recently been solved showing that ERRα can be a target for therapeutic intervention [35].
The evidence is now extremely strong supporting a final effector role of the RANK/RANKL/OPG system in osteoclast formation and the regulation of bone resorption. The binding of RANKL to its receptor (RANK) induces differentiation, activation, and the prevention of osteoclast apoptosis, leading to enhanced bone resorption and bone loss [36]. In the present study, serum sRANKL was significantly higher in osteoporotic women than controls. Oelzner et al. [37] demonstrated that high-serum levels of RANKL were associated with osteoporosis in patients with rheumatoid arthritis. Gurban et al. [38] showed that levels of sRANKL and RANKLOBL in primary cultures of osteoblasts are significantly higher in postmenopausal osteoporotic women demonstrating osteoclast activation. A monoclonal antibody against RANKL was shown to produce prolonged inhibition of bone resorption in postmenopausal women [39].
We also studied the effect of ER-β and ERRα polymorphisms on the serum levels of sRANKL (as one of effectors molecules of the estrogen/ER pathway). To the best of our knowledge, our study is the first to demonstrate the influence of the ER polymorphisms on serum RANKL concentrations. We found a significant increase of sRANKL levels in carriers of AluI A, RsaI A alleles and in women having 2,2 genotype of ERRα.
There are a few limitations of our study. Identifying significant associations of genetic variants with a complex qualitative trait, such as osteoporosis, may need a larger sample size. In the current study, a replication sample is lacking to further confirm the association between ER-β and ERRα polymorphisms and osteoporosis.
In conclusion, we demonstrated an association of ER-β AluI G/A and ERRα 23-repeats polymorphisms with BMD in postmenopausal Egyptian women. These polymorphisms can be considered one of the genetic markers for predicting osteoporosis and can be used to make possible early therapeutic interventions in women at high risk for osteoporosis. Also, we suggested the possible relation of ER-β and ERRα polymorphisms with the levels of sRANKL; the final effect or osteoclastogenic molecule.
References
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007) Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177:575–580
You L, Chen L, Pan L, Chen JY (2013) New insights into the gene function of osteoporosis. Front Biosci 1:1088–1097 (Landmark Ed)
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325
Bord S, Horner A, Beavan S, Compston J (2001) Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab 86:2309–2314
Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA (1997) Human estrogen receptorbeta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 82:4258–4265
Gennari L, Merlotti D, De Paola V, Calabrò A, Becherini L, Martini G, Nuti R (2005) Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGe review. Am J Epidemiol 161:307–320
Jeedigunta Y, Reddy PR, Kolla VK, Munshi A, Ananthapur V, Narasimulu G, Akka J (2010) Association of estrogen receptor α gene polymorphisms with BMD and their effect on estradiol levels in pre- and postmenopausal women in south Indian population from Andhra Pradesh. Clin Chim Acta 411:597–600
Tanriover MD, Tatar GB, Uluturk TD, Erden DD, Tanriover A, Kilicarslan A, Oz SG, Yurter HE, Sozen T, Guvenm GS (2010) Evaluation of the effects of vitamin D receptor and estrogen receptor 1 gene polymorphisms on bone mineral density in postmenopausal women. Clin Rheumatol 29:1285–1293
Wang KJ, Shi DQ, Sun LS, Jiang X, Lü YY, Dai J, Chen DY, Xu ZH, Jiang Q (2012) Association of estrogen receptor alpha gene polymorphisms with bone mineral density: a meta-analysis. Chin Med J 125:2589–2597 (Engl)
Honma N, Mori S, Zhou H, Ikeda S, Mieno MN, Tanaka N, Takubo K, Arai T, Sawabe M, Muramatsu M, Ito H (2013) Association between estrogen receptor-β dinucleotide repeat polymorphism and incidence of femoral fracture. J Bone Miner Metab 31:96–101
Geng L, Yao Z, Yang H, Luo J, Han L, Lu Q (2007) Association of CA repeat polymorphism in estrogen receptor beta gene with postmenopausal osteoporosis in Chinese. J Genet Genomics 34:868–876
Scariano JK, Simplicio SG, Montoya GD, Garry PJ, Baumgartner RN (2004) Estrogen receptor beta dinucleotide (CA) repeat polymorphism is significantly associated with bone mineral density in postmenopausal women. Calcif Tissue Int 74:501–508
Curro M, Marini H, Alibrandi A, Ferlazzo N, Condello S, Polito F, Adamo EB, Atteritano M, Anna RD, Altavilla D, Bitto A, Squadrito F, Ientile R, Caccamo D (2011) The ESR2 AluI gene polymorphism is associated with bone mineral density in postmenopausal women. J Steroid Biochem Mol Biol 127:413–417
Arko B, Prezelj J, Komel R, Kocijancic A, Marc J (2002) No major effect of estrogen receptor beta gene RsaI polymorphism on bone mineral density and response to alendronate therapy in postmenopausal osteoporosis. J Steroid Biochem Mol Biol 81:147–152
Bonnelye E, Aubin JE (2005) Estrogen receptor-related receptor alpha: a mediator of estrogen response in bone. J Clin Endocrinol Metab 90:3115–3121
Laganière J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguère V (2004) Polymorphic autoregulatory hormoneresponse element in the human estrogen related receptor α (ERRα) promoter dictates PGC-1α control of ERRα expression. J Biol Chem 279:18504–18510
Laflamme N, Giroux S, Loredo-Osti JC, Elfassihi L, Dodin S, Blanchet C, Morgan K, Giguère V, Rousseau F (2005) A frequent regulatory variant of the estrogen-related receptor alpha gene associated with BMD in French-Canadian premenopausal women. J Bone Miner Res 20:938–944
Giroux S, Elfassihi L, Cole DE, Rousseau F (2008) Replication of associations between LRP5 and ESRRA variants and bone density in premenopausal women. Osteoporos Int 19:1769–1775
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL (2003) Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111:1221–1230
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146
Kanamaru F, Iwai H, Ikeda T, Nakajima A, Ishikawa I, Azuma M (2004) Expression of membrane-bound and soluble receptor activator of NF-kappaB ligand (RANKL) in human T cells. Immunol Lett 94:239–246
World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level: summary meeting report. Available at:http://www.who.int/chp/topics/Osteoporosis.pdf. Accessed 6 Feb 2012
Meng J, Mu X, Wang YM (2013) Influence of the XbaI polymorphism in the estrogen receptor-α gene on human spermatogenic defects. Genet Mol Res 12:1808–1815
Silva RC, Costa IR, Bordin BM, Silva CT, Souza SR, Júnior CL, Frare AB, Moura KK (2011) RsaI polymorphism of the ERβ gene in women with endometriosis. Genet Mol Res 10:465–470
Arts J, Kuiper GG, Janssen JM, Gustafsson JA, Löwik CW, Pols HA, van Leeuwen JP (1997) Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SVHF cells. Endocrinology 138:5067–5070
Vico L, Vanacker JM (2010) Sex hormones and their receptors in bone homeostasis :insights from genetically modified mouse models. Osteoporos Int 21:365–372
An J, Ribeiro RCJ, Webb P, Gustafsson JA, Kushner PJ, Baxter JD, Leitman DC (1999) Estradiol repression of tumour necrosisfactor-α transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc Natl Acad Sci USA 96:15161–15166
Efstathiadou Z, Koukoulis G, Stakias N, Challa A, Zintzaras E, Tsatsoulis A (2006) Correlation of estrogen receptor beta gene polymorphisms with spinal bonemineral density in peri- and post-menopausal Greek women. Maturitas 53:380–385
Ichikawa S, Koller DL, Peacock M, Johnson ML, Lai D, Hui SL, Johnston CC, Foroud TM, Econs MJ (2005) Polymorphisms in the estrogen receptor beta (ESR2) gene are associated with bone mineral density in Caucasian men and women. J Clin Endocrinol Metab 90:5921–5927
Khosla S, Riggs BL, Atkinson EJ, Oberg AL, Mavilia C, Del Monte F, Melton LJ, Brandi ML (2004) Relationship of estrogen receptor genotypes to bone mineral density and to rates of bone loss in men. J Clin Endocrinol Metab 89:1808–1816
Putnik M, Zhao C, Gustafsson JA, Dahlman-Wright K (2009) Effects of two common polymorphisms in the 3′untranslated regions of estrogen receptor β on mRNA stability and translatability. BMC Genet 10:55. doi:10.1186/1471-2156-10-55
Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D’Anna R, Cannata ML, Corrado F, Cancellieri F, Faraci M, Marini R, Adamo EB, Wilson S, Squadrito F (2008) OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res 23:715–720
Bitto A, Polito F, Squadrito F, Marini H, D’Anna R, Irrera N, Minutoli L, Granese R, Altavilla D (2010) Genistein aglycone: a dual mode of actionanti-osteoporotic soy isoflavone rebalancing bone turnover towards bone formation. Curr Med Chem 17:3007–3018
Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguère V (2001) Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev 15:833–838
Greschik H, Flaig R, Renaud JP, Moras D (2004) Structural basis for the deactivation of the estrogen-related receptor gamma by diethylstilbestrol or 4-hydroxytamoxifen and determinants of selectivity. J Biol Chem 279:33639–33646
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055
Oelzner P, Franke S, Lehmann G, Eidner T, Müller A, Wolf G, Hein G (2007) Soluble receptor activator of NFkappa B-ligand and osteoprotegerin in rheumatoid arthritis-relationship with bone mineral density, disease activity and bone turnover. Clin Rheumatol 26:2127–2135
Gurban CV, Mederle O (2011) The OPG/RANKL system and zinc ions are promoters of bone remodeling by osteoblast proliferation in postmenopausal osteoporosis. Rom J Morphol Embryol 52:1113–1119
Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R (2012) Bench to bedside:elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov 11:401–419
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoukry, A., Shalaby, S.M., Etewa, R.L. et al. Association of estrogen receptor β and estrogen-related receptor α gene polymorphisms with bone mineral density in postmenopausal women. Mol Cell Biochem 405, 23–31 (2015). https://doi.org/10.1007/s11010-015-2391-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2391-5