We study the influence of its microstructure of an Al–Cu–Mg alloy sprayed by the electric-arc method in the intact state and with oxide-ceramic coating on its corrosion resistance in synthetic weakly acid media. It is shown that the increase in the temperature of annealing the electric-arc coating results in the growth of Al2Cu intermetallic inclusions acting as cathodic inclusions and increasing the sizes of pores in the oxide-ceramic coatings. These changes promote a significant increase in corrosion currents both in the electric-arc coating and in the oxide-ceramic layer on it. It is shown that the corrosion currents formed in the electric-arc coatings without oxide-ceramic layer are stronger by an order of magnitude.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The procedure of electric-arc spraying of the coatings is one of the most efficient and inexpensive methods for the surface protection of products, including the products made of light alloys. However, these coatings not always guarantee the reliable protection of the surfaces of metal products against wear and, due to their high porosity, against the influence of corrosion media. Hence, the problem of finding the possibilities of increasing the wear and corrosion resistances of the coatings of this kind is an urgent engineering task. However, it should be emphasized that the quality of aluminum coatings strongly depends on the method of their creation and the chemical composition. Only after the heat treatment of the products, the protective action of sprayed coatings significantly increases due to the changes in their structures. However, the relationship between the changes in the structure of coatings made of aluminum alloys and the parameters of corrosion is insufficiently well studied and poorly discussed in the literature.
The works aimed at the improvement of the corrosion and corrosion-erosion resistances of structures by the electric-arc deposition of the layers sprayed from aluminum wires with various compositions with the subsequent plasma-electrolytic oxidation (PEO) are now extensively carried on at the Karpenko Physicomechanical Institute of the Ukrainian National Academy of Sciences.
The multifunctionality of the PEO coatings promotes their applications in various branches of industry. However, the currently existing understanding of the possibilities of this method is far from being complete. The structure and composition of oxide layers are determined not only by the nature of the treated metal (and some other less important internal factors) but also by the external conditions of their formation; first of all, by the composition of electrolytes whose components may appear in the structure of the coating and by the parameters of the mode of treatment affecting the thermal, time, and other characteristics of microdischarges. Therefore, the aim of the present work is to study the influence of the mode of heat treatment (annealing) of Al–Cu–Mg alloys sprayed by the electric-arc method in the intact state and with oxide-ceramic coatings on the microstructure and corrosion resistance of these coatings in the environment of weakly acid rain.
Materials and Methods of investigations
We studied electric-arc coatings (EAC) sprayed from continuous electrode wires of the Al–Cu–Mg grade (3.8–4.5% Cu; 1.2–1.8% Mg; balance Al) with subsequent synthesis of oxide-ceramic layers on its surface in an electrolytic plasma.
The coatings were applied to a pipe 40 mm in diameter made of 20 steel. For the high-quality application of the metal layer of aluminum alloy, the pipe surface was preliminarily activated by its cleaning by the sandblasting method. As a result, the surface roughness was equal to 32 ± 3 μm.
The microstructure of electric-arc coatings (EACs) was changed as a result of annealing at temperatures 200–600°C. The oxide-ceramic coatings were synthesized in an IMPELOM installation in an electrolytic plasma with the following composition: 3 g/liter KOH + 2 g/liter nNa2O·mSiO2 (the balance is distilled water) by the method described in [1]. The current density was 20 A/dm2, the ratio Іc /Ia = 1, and duration of synthesis was 50 min. Prior to the application of the coatings, the specimens were ground up to the appearance of metallic luster. Then they were washed in distilled water and degreased with ethyl alcohol. After the synthesis of oxide-ceramic coatings, the specimens were washed in distilled water and dried.
The characteristics of corrosion processes were studied in the potentiodynamic mode with the help of an SVA-1B-M voltammetric system. The role of reference electrode was played by a silver/silver-chloride electrode of the ÉVL-1M1 type. The rate of changes in the potential was 2 mV/sec. The polarization curves were used to find the corrosion rate and obtain information about the parameters of corrosion processes. The corrosion rate was found by the extrapolation of linear sections of the polarization curves to the corrosion potential. In the electrochemical studies, the working part of the specimen surface was split into cylindrical cells 2 cm2 in area.
The role of corrosive environment was played by a weakly acid rain with the following composition:
In this solution, the specimens were held for 40 days. The ambient temperature was 18–25°C.
Results and Discussion
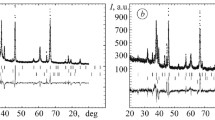
In the course of spraying, the alloying components are completely or partially dissolved in aluminum without separation of the intermetallic phases (in particular, iron-containing), which are always present in the Al–Cu–Mg monolithic alloy. The microstructure of the coating made of Al–Cu–Mg alloy after heat treatment is formed by a solid solution of copper in aluminum and secondary inclusions of various intermetallic compounds, such as CuAl2, Al2CuMg, Mg2Si, (Mn, Fe)Al6, and AlSiMnFe, which was confirmed by the data of X-ray spectral microanalysis (see Table 1 and Fig. 1).
After the formation of primary crystals of the aluminum solid solution, the Al2Cu phase is separated. The concentrations of iron and silicon admixtures are on a quite low level. Therefore, they have practically no effect on the microstructure.
Note that, as the temperature of annealing of the coating increases within the range 200–600°C, the amount of the Al2Cu phase increases and its morphology changes. On the basis of the results of metallographic investigations, we plotted the dependence of the size of intermetallic inclusions on the annealing temperature (Fig. 2).
The open porosity of EAC is a significant factor affecting the corrosion behavior of both EAC and the substrate material. Thus, the corrosive medium penetrates through pores to the substrate and creates favorable conditions for corrosion under the film. The corrosion products are accumulated on the EAC–substrate interface and lead to the exfoliation of the coating.
The increase in the annealing temperature (200–600°C) results in the heterogeneity of the structure due to the increase in the sizes of intermetallic inclusions in the alloy (Figs. 1 and 2). In the presence of electrolytes, numerous microgalvanic couples start to operate on the surface, which causes the corrosion and fracture of the anodic phases.
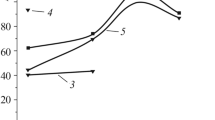
At the onset of interaction of the annealed EAC with a corrosive medium (3 h), the corrosion resistance is lower than after the long-term holding in the medium (Figs. 3 and 4). However, the character of corrosion processes does not change.
It was discovered that, as the duration of holding of the EACs annealed at different temperatures (Fig. 3) increases, the corrosion currents become twice weaker and the maximum decrease was observed for the coating annealed at 600°C.
In the course of long-term investigations (Fig. 4), we established that, for 40 days, the stationary potential of the coatings annealed at different temperatures does not attain the potential of 20 steel equal to –540 mV in a weakly acid rain. In other words, the sprayed coating shifts the potential to the negative side and, hence, creates the cathodic protection of the substrate.
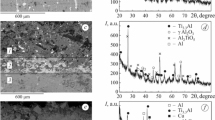
The oxide-ceramic layers formed on thick (400–500 μm) Al–Cu–Mg EACs formed on the steel substrate have a typical structure depicted in Fig. 5.
It is clear that, in the course of PEO, the electrolyte penetrates into the coating and is accumulated in its pores. Hence, the formation of the oxide-ceramic layer runs as if from the inside [2].
By analyzing the results of potentiodynamic investigations of the PEO coatings without and with annealing at 200°C (Fig. 6, curves 1 and 2), it was established that the electrochemical corrosion runs with an insignificant predominance of the anodic control (ba ≥ bc). Then, after annealing at 400 and 500°C (Fig. 6, curves 3 and 4), the corrosion process runs under the mixed control (bc ≈ ba) and, finally, after annealing at 600°C (Fig. 6, curve 5), the corrosion process occurs with the cathodic control (bc > ba).
Moreover, a gradual shift of the potential toward the anodic region is accompanied by a quite slow increase in the rate of dissolution without clearly manifested domain of passivation.
In Fig. 7 in the form of a histogram, we present the results of corrosion-electrochemical studies of the PEO coatings on Al–Cu–Mg EAC for different temperatures of annealing.
It was established that the corrosion currents for specimens with EAC and PEO layers are weaker than for the intact alloy by an order of magnitude. The oxide-ceramic layers are electrochemically inert [3,4,5]. In this case, the metallic substrate, i.e., 20 steel, suffers corrosion fracture due to the penetration of aggressive media through the pores of the oxide-ceramic layer and EAC. The decrease in the corrosion rate in these systems is explained by the fact that the total porosity of oxide-ceramic layers formed in the plasma-electrolytic mode does not exceed 5% and the diameters of pores are small [5,6,7,8]. Thus, the pores become closed with corrosion products, which decreases the access of aggressive media to the substrate.
Conclusions
We study the influence of the temperature of annealing on the corrosion behavior of electric-arc Al–Cu–Mg coatings both in the intact state and with PEO coatings. We establish the relationships between the changes in the sizes of structural components of the Al–Cu–Mg coating and the corrosion resistance of PEO layers in the environment of synthetic weakly acid rain (pH 4.5). It is shown that the increase in the temperature of annealing of EAC causes an increase in the size of Al2Cu intermetallic inclusions playing the role of cathodic inclusions and leading to an increase in the sizes of pores in the oxide-ceramic coating. As a result of these changes, we observe a significant increase in corrosion currents both in the EAC and in the oxide-ceramic layer covering the EAC, although the EAC without oxide-ceramic layers is characterized by corrosion currents higher by an order of magnitude.
References
V. M. Posuvailo, M. D. Klapkiv, M. M. Student, H. V. Pokhmurska, and Y. Y. Sirak, “Gibbs energy calculation of electrolytic plasma channel with inclusions of copper and copper oxide with Al-base,” Mater. Sci. Eng., 181, 157–168 (2017).
M. D. Klapkiv, H. M. Nykyforchyn, and V. M. Posuvailo, “Properties of synthesized oxide-ceramic coatings in electrolyte plasma on aluminium alloys,” Surf. Coat. Techn., 100–101, 219–225 (1998).
I. L. Knunyants (editor), Chemical Encyclopedia [in Russian], Sovetskaya Éntsiklopediya, Moscow (1988), Vol. 1.
W. Dietzel, M. Klapkiv, H. Nykyforchyn, V. Posuvailo, and C. Blawert, “Porosity and corrosion properties of electrolyte plasma coatings on magnesium alloys,” Fiz.-Khim. Mekh. Mater., 40, No. 5, 13–17 (2004); English translation : Mater. Sci., 40, No. 5, 585–590 (2004).
J. A. Curran and T. W. Clyne, “Thermo-physical properties of plasma electrolytic oxide coatings on aluminium,” Surf. Coat. Techn., 199, 168–176 (2005).
I. P. Shatskyi, L. Ya. Ropyak, and M. V. Makoviichuka, “Strength optimization of two-layer coating for the particular local loading condition,” Strength Mater., 48, No. 5, 726–730 (2016).
I. B. Ivasenko, V. M. Posuvailo, M. D. Klapkiv, V. A. Vynar, and S. I. Ostap’yuk, “Express method for determining the presence of defects of the surface of oxide-ceramic coatings,” Fiz.-Khim. Mekh. Mater., 45, No. 3, 123–127 (2009); English translation : Mater. Sci., 45, No. 3, 460–464 (2009).
M. M. Student, V. V. Shmyrko, M. D. Klapkiv, I. M. Lyasota, and L. N. Dobrovol’ska, “Evaluation of the mechanical properties of combined metal-oxide-ceramic layers on aluminum alloys,” Fiz.-Khim. Mekh. Mater., 50, No. 2, 116–121 (2014); English translation : Mater. Sci., 50, No. 2, 290–295 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 53, No. 6, pp. 42–47, November–December, 2017.
Rights and permissions
About this article
Cite this article
Student, M.M., Posuvailo, V.M., Veselivs’ka, H.H. et al. Corrosion Resistance of Plasma-Electrolytic Layers on Alloys and Coatings of the Al–Cu–Mg System for Various Modes of Heat Treatment. Mater Sci 53, 789–795 (2018). https://doi.org/10.1007/s11003-018-0137-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-018-0137-8