Abstract
Antimicrobial peptides (AMPs) are promising candidates for the development of new drugs. However, thorough studies on the toxicity of these molecules are scarce, which is a gap, as host toxicity is one of the main reasons for nonapproval of the drug by regulatory agencies. This work aimed to evaluate the toxicity of three AMPs isolated from Capsicum annuum leaves, named CaCPin-II, CaCDef-like and CaCLTP2. The AMP toxicological profile was evaluated by in vitro cytotoxicity against mammalian cells and systemic in vivo toxicity using Galleria mellonella larvae as study model. AMP cytotoxicity was evaluated in a broad panel of human cell lines, namely, vascular endothelium, cervical adenocarcinoma, prostatic epithelium, mammary epithelium and fibroblasts, and in murine macrophages. Cell viability was evaluated through metabolic activity, a gold standard method for assessing viability due to the speed, robustness and reliability of the results. To elucidate the toxicity mechanism of the peptides, their ability to bind to the cell surface and to permeabilize membranes was evaluated by measuring the zeta potential and the absorption of the SYTOX® Green fluorescent probe, respectively. The AMPs did not decrease cell viability or permeabilize the membranes of the cell lines at the tested concentrations. Only CaCLTP2 had the ability to interact with the cell surface, but it was not able to permeabilize them. The in vivo systemic toxicity was evaluated by the survival rate of the G. mellonella larvae inoculated with peptides. CaCPin-II showed in vivo toxicity, as the larval survival rate after the test was 60% lower than that of the controls. The results suggest that these peptides have potential as antimicrobial agents because they have low or no toxicity to mammalian cells and can serve as a framework for drug development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial peptides (AMPs) are part of innate defense or produced as a microorganism competition strategy to limit the growth of others (Moretta et al. 2021) and are ubiquitous in nature, existing not only in microorganisms, but also in animals and plants (Seyfi et al. 2020). AMPs are expressed by specific genes constitutively or inducible by specific external factors (Lei et al. 2019), forming short ribosomally synthesized peptides of L-amino acids (Carvalho and O, Gomes 2012). Most of them are small cationic peptides that have low molecular masses, commonly in the range of 6 to 100 amino acids, amphipathic design and are collected according to their sequence homology, functional similarities and three-dimensional structure (Seyfi et al. 2020; Huan et al. 2020; Mookherjee et al. 2020). Structural classes of plant AMPs are mainly represented by the groups of defensins, thionins, lipid transfer proteins (LTPs) and cyclotides that have their N- and C-termini bound and, therefore, are cyclic peptides (Ojeda et al. 2019; Zasloff 2019). As part of the first line of host defense against pathogen attack, AMPs have biocidal activities against bacteria, fungi, viruses, parasites and insects but also have other biological activities, such as antitumor and modulators of the immune system, representing one of the oldest innate defense components in evolutionary history (Wei and Zhang 2022). Therefore, these peptides with spread-spectrum activities emerge as good candidates for drug development (Koo and Seo 2019; Lewies et al. 2019).
The interest in AMP research is due to their multiple mechanisms of action and multiple targets, which include direct action in membranes but also action in intracellular targets, in addition to being able to act in synergy with conventional drugs (Lewies et al. 2019). Therefore, these molecules can destroy pathogens by membrane permeation, but they differ on the intracellular target. Furthermore, these molecules do not induce the emergence of drug-resistant pathogens, as resistance involves a drastic change in the phospholipid composition of the membrane, affecting many systems in turn (Zhang et al. 2021). These peptides have selective toxicity and a low rate of microbial resistance induction and do not trigger stress response and mutagenesis pathways in bacteria (Lewies et al. 2019; Rodríguez-Rojas et al. 2014). Although naturally occurring and synthetic AMPs have shown promising results for new drugs, some disadvantages have been pinpointed for AMP-based therapies, including chemical and physical instability, low pharmacokinetic characteristics, short half-life in vivo, proteolytic degradation and toxicity (Cardoso et al. 2020; Chen and Lu 2020). The toxicity of AMPs to mammalian cells is still the major obstacle in their development and clinical applications (Khabbaz et al. 2021). To overcome these shortcomings, it is essential to further explore the mechanisms of action of AMPs to understand their activity and biotoxicity (Wei and Zhang 2022).
Drug development requires several steps with complex and important tests that guarantee the selection and approval of only effective and nontoxic molecules (Robles-Loaiza et al. 2022; Pognan et al. 2023). Selectivity is one of the main requirements for their progression into the clinic. Although many molecules are constantly discovered, only a tiny fraction is converted into secure and effective therapeutic molecules (Mohs and Greig 2017). The pharmacologic and biochemical characteristics of the drug candidate are established using an extensive range of in vitro and in vivo test procedures. It is also a regulatory requirement that the drug is administered to animals to assess its safety (Tamimi and Ellis 2009). Drug toxicity remains a latent problem, and peptides are no exception to this rule (Khan et al. 2018). Several bioactive peptides have shown toxicity, especially hemotoxicity (Ruiz et al. 2014; Greco et al. 2020). Therefore, toxicity to healthy eukaryotic cells remains a major bottleneck in the approval rate of new pharmaceutical peptides (Gupta et al. 2015). In vitro, in vivo and ex vivo toxicity evaluation is an essential step in the development of potential new drugs, including the half-lethal dose (LD50) and half‐hemolytic activity (HC50) (Wei and Zhang 2022; Cunha et al. 2017). More than 3000 AMPs have been discovered, but only some nonribosomally synthesized peptides have been approved by the U.S. Food and Drug Administration (FDA), for example, gramicidin, daptomycin, vancomycin, oritavancin, dalbavancin, telavancin, telaprevir and colistin (also known as polymyxin E), all of which are mostly used for topical medications. Most of these peptides are usually very limited in their usefulness for clinical applications. In fact, many AMPs failed prior to or during clinical trials (Lei et al. 2019; Chen and Lu 2020). At the same time, dozens of AMPs are in clinical development around the world, mainly focusing on treatments for skin infections caused by bacterial and fungal pathogens, and in some clinical trials, AMPs are also being used as treatments for cancer and systemic infections (Koo and Seo 2019). Currently, a defensin derivative is the only example of a plant AMP that is in preclinical trials (Hein et al. 2022).
CaCPin-II, CaCDef-like and CaCLTP2 are AMPs from Capsicum annuum cv. Carioquinha leaves that have been isolated and characterized as a protease inhibitor, a defensin-like and a LTP (Lipid Transfer Protein), respectively. These AMPs displayed molecular masses between 3.5 and 6.5 kDa and caused morphological and physiological alterations in four opportunistic species of the genus Candida, such as growth inhibition, reduced cell viability, pseudohyphal formation, agglutination, oxidative stress and cell membrane permeabilization. CaCDef-like and CaCLTP2 have low or no hemolytic activity at concentrations that cause antifungal effects (Cherene et al. 2023a, b). CaCPin-II has α-amylase and protease inhibitory activity (Cherene et al. 2023a, b) and insecticidal activity against Callosobruchus maculatus larvae (Cherene et al. 2023a, b). In this work, we screened the in vitro cytotoxicity of these AMPs against a panel of mammalian cell lines to complement the initial results of peptide hemotoxicity and to better characterize their cytotoxicity. Furthermore, we performed initial systemic in vivo toxicity studies using Galleria mellonella larvae as a model.
Materials and methods
Cell Culture
Immortalized cell lines from human breast cancer MDA-MB-231 (ATCC® HTB-26™), SKBR3 (ATCC® HTB-30™) and MCF7 (ATCC® HTB-22™); human fibroblasts Hs68 (ATCC® CRL-1635™); human epithelial cervical cancer HeLa (ATCC® CCL-2™); human prostatic cell lines RWPE-1 (ATCC® CRL-11609D™) and PC-3 (ATCC® CRL-1435™); human cerebral microvascular endothelial cell HBEC-5i (ATCC® CRL-3245™) and murine macrophage RAW 264.7 (ATCC® TIB-61™) were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). MDA-MB-231, Hs68, HeLa and RAW 264.7 cells were cultured as a monolayer in Dulbecco’s modified Eagle’s medium (DMEM). SKBR3 and MCF7 cells were cultured as a monolayer in McCoy′s 5 A and Eagle’s minimum essential medium (EMEM) media, respectively. RWPE-1 and PC-3 cells were cultured as a monolayer in defined keratinocyte SFM and Ham’s F-12 K (Kaighn’s) media, respectively. All the abovementioned media were supplemented with 10% fetal bovine serum (FBS) (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (Gibco/Thermo Fisher Scientific, Waltham, MA, USA). HBEC-5i were cultured in T-flasks precoated with attachment factor protein (Gibco/Thermo Fisher Scientific, Waltham, MA, USA) using DMEM: F12 medium with 10% FBS, 1% penicillin–streptomycin and 40.0 μg/mL endothelial cell growth supplement (Sigma‒Aldrich, Spain). All cells were grown in a humidified atmosphere of 5% CO2 at 37 °C (MCO-18AIC (UV), Sanyo, Japan), and the medium was changed every other day.
Obtaining CaCPin-II, CaCDef-like and CaCLTP2 Peptides
CaCPin-II, CaCDef-like and CaCLTP2 peptides were purified from C. annuum cv. Carioquinha leaves as described by Cherene et al. (2023a, b).
In Vitro Cytotoxicity Assay
Peptides in vitro cytotoxicity against mammalian cell lines were evaluated by measuring the reducing activity in cells using the CellTiter-Blue® cell viability assay (Promega, Madrid, Spain) according to the manufacturer’s instructions. Briefly, cells were allowed to grow until ~80% confluence in a 75-T flask under standard conditions as mentioned above. Cells were then carefully detached and seeded at 1.0 × 105 cells.mL− 1 in 96-well flat-bottomed plates (Corning, USA) of 100 μL per well volume. After 24 h, the medium was removed, and adhered cells were incubated with 100 μL of CaCPin-II, CaCDef-like and CaCLTP2 dissolved in complete serum-free medium for culturing of the cell line at concentrations in the range of 1.56–200.0 μg.mL− 1 for an additional 24 h in a humidified atmosphere of 5% CO2 at 37 °C. Then, 20 μL of CellTiter-Blue® Reagent was added to each well and incubated for 3 h under the same conditions. Fluorescence intensity was measured with λexc = 560 nm and λem = 590 nm in a Varioskan Lux microplate reader (Thermo Fisher, Spain). Medium without peptides and 1% Triton X-100-containing medium were used as positive controls (100%, normal reducing activity) and negative controls (0%, nonviable cells), respectively. Reducing activity (%) was determined by the following expression:
Experiments were performed in three independent biological replicates performed on separate passages of cells and on separate days, and the mean is presented with standard deviation in the graphs.
Zeta Potential Measurements
Zeta potential measurements were performed as described previously (Oliveira et al. 2022). Briefly, cells were harvested from confluent cell cultures by trypsinization, washed, resuspended in 1x PBS buffer, and diluted to a final concentration of 1.0 × 105 cell·mL− 1 (in 1x PBS). Cell suspensions with peptides at concentrations in the range of 25.0–200.0 μg.mL− 1 were prepared. Samples with and without peptides were loaded into disposable zeta cells with gold electrodes and allowed to equilibrate for 30 min at 37 °C. Each experiment consisted of a set of 15 measurements with 40 subruns performed on a Malvern Zetasizer Nano ZS (Malvern, UK) at a constant voltage of 40 V, with a 90 s pause between measurements. The complete experiment was carried out at least two times using independent cellular suspensions, a control (untreated cells) was performed each day, and the mean is presented with standard deviation in the graphs. Statistical analysis were carried out with GraphPad Prism software (version 8.0 for Windows) and one-way analysis of variance (ANOVA); p < 0.05 was considered statistically significant.
Plasma Membrane Permeabilization
Plasma membrane permeabilization was investigated by SYTOX® Green Nucleic Acid Stain uptake according to the methodology described previously (Almeida et al. 2021), with modifications. Briefly, cells were allowed to grow until ~80% confluence in a 75-T flask under standard conditions. Cells were then carefully detached and seeded at 1.0 × 105 cells.mL− 1 in 96-well flat-bottomed plates, with 100 μL of peptides at concentrations in the range of 6.25–200.0 μg.mL− 1 dissolved in serum-free DMEM without phenol red for 24 h in a humidified atmosphere of 5% CO2 at 37 °C. Then, 1 μM Sytox green was added to each well and incubated for 10 min under the same conditions. Fluorescence intensity was measured with λexc = 485 nm and λem = 520 nm in a Varioskan Lux microplate reader. Medium without peptides and 0.1% Triton X-100-containing medium were used as negative controls (untreated cells) and positive controls, respectively. Membrane permeabilization (%) was determined by the expression:
Experiments were performed on different days using independent cell cultures, and the mean is presented with standard deviation in the graphs. Statistical analysis were carried out with GraphPad Prism software (version 8.0 for Windows) and one-way analysis of variance (ANOVA); p < 0.05 was considered statistically significant.
ROS Induction Detection Assay
The ability of the peptides to induce intracellular ROS formation was assessed using a DCFDA/H2DCFDA-Cellular ROS Assay Kit (ab113851, Abcam, USA) and performed according to the manufacturer’s instructions with minor changes. Briefly, cells were allowed to grow until ~80% confluence in a 75-T flask under standard conditions, as mentioned above. Cells were then carefully detached and seeded at 1.0 × 105 cells.mL− 1 in 96-well flat-bottomed plates, with 100 μL of peptides at concentrations in the range of 6.25–200.0 μg.mL− 1 dissolved in serum-free DMEM without phenol red for 24 h in a humidified atmosphere of 5% CO2 at 37 °C. One hour before the end of the assay, 2x concentrated DCFDA solution (20 μM) was prepared, and 100 μL was added to each well and incubated for 45 min under the same conditions. Fluorescence intensity was measured with λexc = 485 nm and λem = 535 nm in a Varioskan Lux microplate reader. Medium without peptides and 50 μM tert-butyl hydrogen peroxide (tbHP)-containing medium were used as negative controls (untreated cells) and positive controls, respectively. ROS increase (%) was determined by the expression:
Experiments were performed on different days using independent cell cultures, and the mean is presented with standard deviation in the graphs. Statistical analysis were carried out with GraphPad Prism software (version 8.0 for Windows) and one-way analysis of variance (ANOVA); p < 0.05 was considered statistically significant.
In Vivo Toxicity Study in Galleria mellonella
To evaluate the systemic in vivo toxicity of the peptides, we used G. mellonella larvae as a study model, as described by Mylonakis et al. (Mylonakis et al. 2005), with modifications. Thirty last-instar G. mellonella larvae of similar weight and size (250 and 350 mg) were used in each of the treatment and control groups. Ten randomly chosen larvae of the needed weight were used per group, and assays were performed in duplicate (n = 20). Peptide solutions (400 μg. mL− 1) using PBS as the vehicle were prepared, and 10 μL was injected with a Hamilton syringe into the hemocoel of each larva through the last left proleg (dose of ~13 μg/g larva). Larvae that received the injection of 10 μL of vehicle (PBS) and larvae that only received the injection needle injury were used as general viability controls. Then, larvae were incubated in Petri dishes at 37 °C, and the number of dead larvae was counted every 24 h for a period of 7 days. Larvae were considered dead when they showed no movement in response to touch. Percent survival curves were plotted, and estimates of differences in survival (log rank Mantel-Cox and Breslow-Wilcoxon tests) were analyzed by the Kaplan-Meier method using GraphPad software (version 8.0 for Windows).
Results
In Vitro Cytotoxicity Assay
The in vitro cytotoxicity of CaCPin-II, CaCDef-like and CaCLTP2 was initially evaluated by measuring the metabolic activity of a panel of nine cell lines after incubation with the three peptides. For this purpose, the metabolic activity of nine different mammalian cell lines (MDA-MB-231, MCF7, SKBR3, HBEC-5i, HeLa, Hs68, RWPE-1, PC-3 and RAW 264.7) was assessed using the CellTiter-Blue® assay. The results indicate that the three peptides exhibited low toxicity toward the tested cell lines at concentrations up to 200 μg.mL− 1, as shown in Fig. 1 for the human cell lines MDA-MB-231, MCF7, SKBR3, HBEC-5i, HeLa and Hs68, as well as for the RWPE-1, PC-3 and RAW 264.7 cell lines (data not shown).
Effect of CaCPin-II, CaCDef-like and CaCLTP2 peptides on the metabolic activity of human cells. Cells from human breast cancer MDA-MB-231 (A), MCF7 (B), SKBR3 (C); human cerebral microvascular endothelial cell HBEC-5i (D); human epithelial cervical cancer HeLa (E) and human fibroblasts Hs68 (F) were seeded in 96-well plates containing medium (1.0 × 105 cells.mL− 1) and grown for 24 h at 37 °C. After this period, the cells were incubated with the peptides at concentrations ranging from 200.0 to 1.56 μg.mL− 1. Metabolic activity was measured with the addition of CellTiter-Blue®, and fluorescence was measured at 590 nm
Zeta Potential
To elucidate CaCPin-II, CaCDef-like and CaCLTP2 peptide interactions with cell plasma membranes, the zeta potential of the cells was determined by electrophoretic light scattering using SKBR3, HeLa and RAW 264.7 cell lines as the study model. No significant increases were detected in zeta potential values for SKBR3, HeLa and RAW 264.7 cells after 30 min incubation with CaCPin-II (Fig. 2A) and CaCDef-like (Fig. 2B), in contrast with CaCLTP2 (Fig. 2C). The zeta potential of SKBR3 and HeLa cells incubated with 100 μg.mL− 1CaCLTP2 was increased by approximately 40% compared to that of untreated cells. There was no significant difference in the zeta potential of these cells incubated with 100 and 200 μg.mL− 1CaCLTP2. The cell line with which CaCLTP2 interacted with the membrane most efficiently was RAW 264.7 cells. The zeta potential of RAW 264.7 cells incubated with 25 μg.mL− 1CaCLTP2 was increased by 44% compared to untreated cells, and there was no significant difference in zeta potential measures of cells incubated with concentrations of up to 200 μg.mL− 1CaCLTP2. The data show that CaCLTP2 can interact with the cell surface of these cell lines.
Peptide interactions with cell surfaces. Zeta potential measurements of the human breast cancer cell line SKBR3, human epithelial cervical cancer HeLa and murine macrophage RAW 264.7 cells were performed in the absence and presence of CaCPin-II (A), CaCDef-like (B) and CaCLTP2 (C) at increasing peptide concentrations (25 to 200 μg.mL− 1). Dose response graphs were constructed, and * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 compared to the negative control (0 μg.mL− 1) determined by Tukey test
Plasma Membrane Permeabilization
After zeta potential results, together with the fact that CaCPin-II and CaCLTP2 peptides have hemolytic activity on sheep erythrocytes (Cherene et al. 2023a, b), we decided to evaluate the membrane integrity of SKBR3, HeLa and RAW 264.7 cells after incubation with CaCPin-II and CaCLTP2. To investigate whether these peptides can damage membranes, cells were incubated with peptides. Following a period of 24 h, the permeabilization of the plasma membrane was investigated using Sytox Green. Figure 3 shows that both untreated and peptide-treated cells did not present Sytox fluorescence, demonstrating that CaCPin-II and CaCLTP2 did not damage membranes.
CaCPin-II and CaCLTP2 membrane permeabilization assay. Membrane permeabilization (%) of human breast cancer cell line SKBR3 (A), human epithelial cervical cancer HeLa (B) and murine macrophage RAW 264.7 (C). Cells were incubated with peptides at increasing concentrations for 24 h (6.25 to 200 μg.mL− 1), and Sytox Green probe was added after this period. Negative control: cells not treated. Positive control (C+): 0.1% Triton. The fluorescence intensity was measured at 520 nm. The results presented are mean values obtained over three experiments, each performed in triplicate. Dose response graphs were constructed, and **** p < 0.0001 compared to the negative control (0 μg.mL− 1) determined by Tukey test
ROS Induction
To confirm that CaCPin-II, CaCDef-like and CaCLTP2 peptides do not induce toxicity to cells, ROS-inducing activity was investigated in vitro using three human cell lines, MDA-MB-231, HeLa, and PC-3, as a study model. Cells were incubated with increasing concentrations of peptides for 24 h, and the data showed that peptides at concentrations up to 200 μg.mL− 1 did not generate oxidative stress in these cell lines, as shown in Fig. 4.
ROS induction detection assay cells treated with CaCPin-II, CaCDef-like and CaCLTP2. Human breast cancer MDA-MB-231 (A), human epithelial cervical cancer HeLa (B) and human prostatic PC-3 (C) cells were incubated with peptides for 24 h (1.0 × 105 cells.mL− 1), and the DCFDA probe was added after this period. Negative control: cells not treated. Positive control: tbHP. The fluorescence intensity was measured at 535 nm. Numerical values on the x-axis represent the concentration of peptides (μg.mL− 1). The results presented are mean values obtained over three experiments, each performed in triplicate. Dose response graphs were constructed, and **** p < 0.0001 compared to the positive control (0 μg.mL− 1) determined by Tukey test
In Vivo Toxicity
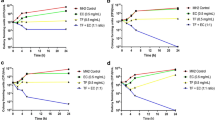
To assess the correlation of in vitro observations with an in vivo situation, CaCPin-II, CaCDef-like and CaCLTP2 peptides were used for systemic toxicity studies in animals, with G. mellonella larvae as the study model. The peptide concentration used in the in vivo test was twice as high as the maximum concentration used in the in vitro cytotoxicity tests. After injecting the peptides into the larval hemocoel, we observed that CaCDef-like did not cause the death of any larvae over the 7 days of monitoring (Fig. 5). However, CaCPin-II was the peptide that showed the greatest in vivo toxicity for G. mellonella larvae, causing a significant (P = 0,0016) and progressive reduction in the survival rate, with 3% death on day 3 to 40% death on day 6, and after that day, there were no more deaths until the end of the monitoring of the larvae. CaCLTP2 caused the death of 8% of the larvae on the second day after the injection, and after that period, the survival rate of the larvae remained the same until the last day of monitoring. There was no death of larvae that received PBS injection during the 7-day monitoring, and 7% of the larvae that received only needle injury (mock inoculation) died on day 6 of monitoring, but this reduction in survival rate was not significant (P = 0,2484).
Peptides in vivo systemic toxicity to G. mellonella. Kaplan-Meier plots of G. mellonella larvae survival after injection of CaCPin-II, CaCDef-like and CaCLTP2 (400 μg.mL− 1 or ~13 μg peptide/g larvae) using PBS as vehicle. There was no killing of larvae that received PBS. The assays were performed in duplicate (n = 20)
Discussion
Despite the fact that AMPs are often described as promising candidates for the development of new therapies against drug-resistant bacterial and fungal infections, some pharmacological characteristics hinder clinical development (Lei et al. 2019). In particular, toxicity has been poorly addressed although AMP-based drug candidates need to show low or no toxicity to mammalian cells to advance in clinical tests (Mohs and Greig 2017; Greco et al. 2020). Hemolysis is a versatile tool for rapid initial toxicity assessment due to its ease associated with isolating erythrocytes (Farag and Alagawany 2018), and it is not uncommon for erythrocytes from different species of mammals to be used in the initial screening phase (Greco et al. 2020). CaCPin-II, CaCDef-like and CaCLTP2 hemolytic activity on sheep erythrocytes has already been described. CaCPin-II has an HC50 of 270 μg mL− 1, CaCDef-like has no hemolytic activity at concentrations up to 400 μg mL− 1, and CaCLTP2 showed a weak hemolytic effect at a concentration of 200 μg mL− 1, causing 1.7% hemolysis in tests using sheep erythrocytes (Cherene et al. 2023a, b). However, isolated and washed erythrocytes are more vulnerable cells than adhered cells in in vitro culture, and different methods of testing hemolytic activity can generate significant variations in results for the same compound (Greco et al. 2020; Helmerhorst et al. 1999). Therefore, it is important to study the in vitro cytotoxic activity of a compound using other cell lines and standardized methods to obtain more accurate results.
We evaluated cell viability by quantifying the metabolic conversion of resazurin to resorufin. Virtually all toxicological and pharmacological studies include at some point the assessment of cell viability and/or metabolic activity, and resazurin reduction is probably the most widely used method to assess the metabolic activity of cells (Vieira-da-Silva and Castanho 2023). We observed an increase in metabolic reduction of resazurin, suggesting that peptides may be causing reducing stress in these cells lines, as demonstrated in another study that evaluates the cytotoxicity of synthetic AMPs using the ability of treated cells to reduce resazurin (Greco et al. 2020). The results of the cytotoxic activity of the peptides showed that the cells tested did not have their metabolic activity reduced, even in the presence of CaCPin-II, which has weak hemolytic activity on sheep erythrocytes already described. Our results align with those of a study comparing the hemotoxicity of some synthetic AMPs using erythrocytes from different mammalian species and the cytotoxicity with immortalized human keratinocytes (HaCaT), human liver cancer cells (HepG2) and human epithelial cervical cancer cells (HeLa). This study showed that all these cell lines have more tolerance for the tested peptides in comparison to human erythrocytes, and there was no direct relationship between hemolytic activity and cytotoxicity (Greco et al. 2020).
Membrane permeabilization and increased ROS production are among the most common modes of action of many AMPs, which might lead to programmed cell death (PCD) pathway activation (Aerts et al. 2007; Kulkarni et al. 2009). ROS generally play an important role in cellular signaling and are produced in cells by means of normal physiological processes or by enzymatic and nonenzymatic mechanisms associated with pathological processes (Camini et al. 2017). Increased oxidative stress appears in the early stages of the apoptotic process (Kowaltowski et al. 2009) and has already been described as a mechanism of action employed by plant defensins and several other AMPs (Mello et al. 2011; Soares et al. 2017), as with Candida tropicalis yeasts treated with CaCPin-II and CaCDef-like (Cherene et al. 2023a, b). Our data suggest that CaCPin-II, CaCDef-like and CaCLTP2 peptides do not induce a pathological response in human cells since they do not lead to an increase in ROS in MDA-MB-231, HeLa and PC-3 cell lines.
The amphipathic and cationic character of AMPs and their secondary structure are the main factors that determine the interactions between AMPs and their targets (Buck et al. 2019; Choi et al. 2016). The toxic effects of AMPs usually involve electrostatic interactions with the cell surface. Most AMPs have positive net charges at neutral pH, and their cellular selectivity is associated with their high affinity for the anionic lipid components of the membranes of microorganisms (Huang 2006) and tumor cells (Harris et al. 2011; Schweizer 2009). The outer layer of the cell membrane of mammalian cells, on the other hand, is closer to neutral and consists mainly of zwitterionic phospholipids and cholesterol; the latter further stabilizes the membranes to the action of AMPs (Huang 2006; Sok et al. 1999). Furthermore, intracellular targets for AMPs have also been described, adding even more complexity to the mechanisms of action of these peptides (Wei and Zhang 2022). Therefore, to assess whether CaCPin-II, CaCDef-like and CaCLTP2 peptides can interact with the outer surface of mammalian cell membranes, the zeta potential of SKBR3, HeLa and RAW 264.7 cell lines was determined. Our data show that the peptides, except CaCLTP2, had no electrostatic interaction with the surface of the membranes of these mammalian cells. Nonspecific LTPs (nsLTPs) are a class of AMPs found in all land plants. LTPs have a tunnel-like hydrophobic cavity that enables them to bind and transport various types of lipids (Edqvist et al. 2018; Melnikova et al. 2023). Thus, the cell membrane is considered a potential target for LTP antimicrobial action via hydrophobic and electrostatic interactions (Finkina et al. 2016).
No single general mechanism can be applied to explain the membrane effects of all cationic AMPs. Membrane permeabilization caused by cationic AMPs can occur by direct interaction with the membrane, as shown by four main models established to describe membrane-pore formation, i.e., barrel-stave, toroidal-pore, carpet and aggregate models or by indirect intracellular action mode for acting in the regulation of important enzymes (Wei and Zhang 2022). Plant defensins (Mello et al. 2011; Soares et al. 2017; Gebara et al. 2020) and lipid transfer proteins (LTPs) (Finkina et al. 2016; Salminen et al. 2016) can permeabilize the membranes of microorganisms. Plant protease inhibitors can also affect cell membranes since they inhibit enzymes involved in important cellular events (Rudzińska et al. 2021). As many plant AMPs can permeabilize microorganism cell membranes and CaCPin-II, CaCDef-like and CaCLTP2 are able to permeabilize the cell membrane of some Candida species (Cherene et al. 2023a, b), we evaluated the ability of these peptides to permeabilize mammalian cell membranes with an unwanted toxic effect. Furthermore, CaCPin-II and CaCLTP2 also have weak hemolytic activity (Cherene et al. 2023a, b). Our data suggest that peptides do not permeabilize mammalian cell membranes, while in the membrane permeabilization assay with the SYTOX Green probe, there was no increase in fluorescence in SKBR3, HeLa or RAW 264.7 cells lines incubated with these peptides. Although CaCLTP2 interacted with the surface of these cell lines according to the zeta potential assay data, this peptide did not cause membrane permeabilization. In this way, we suggest that the hemolytic effect already described for CaCPin-II and CaCLTP2 may have occurred because erythrocytes are more vulnerable than other mammalian cell lines grown in vitro. Together, these data suggest that the peptides have some selective toxicity.
To obtain a more accurate toxicological analysis, it is important to correlate the in vitro toxicity results with an in vivo situation. In vivo tests allow the assessment of systemic toxicity, which is more complex and not achievable through cellular assays. Factors associated with the metabolism of compounds, such as absorption, biotransformation, distribution and excretion, may not be simulated in cell culture tests (Vliet 2011; Allegra et al. 2018). For natural and synthetic membrane-active AMPs, hemolytic and cytotoxic experiments are often insufficiently backed up to provide an accurate prediction of an in vivo situation (Greco et al. 2020). Cationic and amphiphilic compounds, such as AMPs, are prone to rapidly associate with both major exogenous transport plasma proteins serum albumin and α-1 glycoprotein. Recent studies show the various forms of binding of cationic AMPs with these carriers, which reduces the bioavailability of AMP so that it exerts its in vivo bioactivity, and this is a parameter not accounted for in cytotoxic experiments (Svenson et al. 2007; Sivertsen et al. 2014). Thus, some AMPs that showed cytotoxic activity may not have any pronounced toxic effects in vivo (Greco et al. 2020) while having physiological toxicity not revealed by cellular assays. We performed in vivo systemic toxicity tests using G. mellonella larvae as a study model. G. mellonella (greater wax moth) larvae are an established and widely used model for drug discovery, in vivo toxicity tests and host‒pathogen interaction studies as an intermediate invertebrate model between in vitro and mammalian in vivo trials (Serrano et al. 2023). They reflect aspects of the complexity present in mammals, such as an innate immune system that is structurally and functionally similar to that of mammals, and are accepted as an ethical alternative for research (Allegra et al. 2018; Piatek et al. 2021; Cutuli et al. 2019). The data obtained regarding the in vivo toxicity of the peptides are in agreement with the data already described for each of them. The protease inhibitor CaCPin-II, despite not showing in vitro toxicity for the mammalian cells tested, showed toxicity in the in vivo test. The CaCPin-II toxicity for G. mellonella larvae may be related to at least one of its properties already described in the literature: its ability to inhibit important enzymes and its insecticidal activity (Cherene et al. 2023a, b). Several Pin-II protease inhibitors have already been isolated and have shown insecticidal activity (Mishra et al. 2010; Yadav et al. 2021). Although some plant defensins (Kovaleva et al. 2020) and LTPs (Maximiano and Franco 2021) have already been described with insecticidal activity, the defensin-like CaCDef-like and the nsLTP CaCLTP2 did not show in vivo toxicity to G. mellonella larvae. The CaCDef-like peptide has no cytotoxicity for any of the mammalian cell lines tested and is not hemolytic (Cherene et al. 2023a, b). In fact, some of the few AMPs that are currently in clinical testing are defensins (Hein et al. 2022). PvD1 is a plant defensin that shows antifungal activity against four different Candida species and does not show in vivo toxicity to G. mellonella larvae (Skalska et al. 2020). Pezadeftide, a derivative of a plant defensin, is in phase IIa clinical trials for the treatment of fungal nail disease (Hein et al. 2022). Plant LTPs present a broad range of versatile bioactivities, and most studies have shown low or no toxicity to mammalian cells, but the biggest challenges pointed out for the use of LTPs in human health are the difference between the results found in in vitro assays and in preclinical or clinical tests, since these molecules can trigger side effects, such as strong allergic reactions (Edqvist et al. 2018; Maximiano and Franco 2021).
Our data suggest that CaCPin-II, CaCDef-like and CaCLTP2 may have biotechnological applications for the development of new drugs and therapies. Due to its in vivo toxicity and hemolytic activity, a CaCPin-II protease inhibitor can be used as a framework for the development of bioinspired AMPs with higher selective toxicity and for the development of antimicrobials and insecticides that do not involve direct application in mammals. CaCDef-like and CaCLTP2 should thus be safe for animal use, as they showed little or no toxicity. Our data point to the CaCDef-like peptide as the safest antifungal candidate, as it had good results in all tests. However, for this purpose, other toxicological tests using mammals as a study model are necessary. As CaCLTP2 was able to bind to the cell surface without causing damage, we suggest that this peptide can still be used for the development of drug systems.
Data Availability
No datasets were generated or analysed during the current study.
References
Aerts AM, François IEJA, Meert EMK et al (2007) The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J Mol Microbiol Biotechnol 13:243–247. https://doi.org/10.1159/000104753
Allegra E, Titball RW, Carter J, Champion OL (2018) Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 198:469–472. https://doi.org/10.1016/j.chemosphere.2018.01.175
Almeida CV, de Oliveira CFR, dos Santos EL et al (2021) Differential interactions of the antimicrobial peptide, RQ18, with phospholipids and cholesterol modulate its selectivity for microorganism membranes. Biochim Biophys Acta - Gen Subj 1865https://doi.org/10.1016/j.bbagen.2021.129937.
Buck AK, Elmore DE, Darling LEO (2019) Using fluorescence microscopy to shed light on the mechanisms of antimicrobial peptides. Future Med Chem 11:2445–2458. https://doi.org/10.4155/fmc-2019-0095
Camini FC, da Silva Caetano CC, Almeida LT, de Brito Magalhães CL (2017) Implications of oxidative stress on viral pathogenesis. Arch Virol 162:907–917. https://doi.org/10.1007/s00705-016-3187-y
Cardoso MH, Orozco RQ, Rezende SB et al (2020) Computer-aided design of antimicrobial peptides: are we Generating Effective Drug candidates? Front Microbiol 10:1–15. https://doi.org/10.3389/fmicb.2019.03097
Carvalho A, de O, Gomes VM (2012) Plant defensins and defensin-like peptides - Biological activities and Biotechnological Applications. Curr Pharm Des 17:4270–4293. https://doi.org/10.2174/138161211798999447
Chen CH, Lu TK (2020) Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9. https://doi.org/10.3390/antibiotics9010024
Cherene MB, Taveira GB, Almeida-Silva F et al (2023a) Structural and biochemical characterization of three antimicrobial peptides from Capsicum annuum L. var. annuum leaves for anti – Candida Use. https://doi.org/10.1007/s12602-023-10112-3. Probiotics Antimicrob Proteins
Cherene MB, Ferreira SR, dos Santos L A, et al (2023b) Insecticidal activity of Capsicum annuum L. leaf proteins on cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae) development. J Asia Pac Entomol 26:1–11. https://doi.org/10.1016/j.aspen.2023.102158
Choi H, Rangarajan N, Weisshaar JC (2016) Lights, Camera, Action! Antimicrobial peptide mechanisms imaged in space and time. Trends Microbiol 24:111–122. https://doi.org/10.1016/j.tim.2015.11.004
Cutuli MA, Petronio Petronio G, Vergalito F et al (2019) Galleria mellonella as a consolidated in vivo model hosts: new developments in antibacterial strategies and novel drug testing. Virulence 10:527–541. https://doi.org/10.1080/21505594.2019.1621649
da Cunha NB, Cobacho NB, Viana JFC et al (2017) The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov Today 22:234–248. https://doi.org/10.1016/j.drudis.2016.10.017
Edqvist J, Blomqvist K, Nieuwland J, Salminen TA (2018) Plant lipid transfer proteins: are we finally closing in on the roles of these enigmatic proteins? J Lipid Res 59:1374–1382. https://doi.org/10.1194/jlr.R083139
Farag MR, Alagawany M (2018) Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem Biol Interact 279:78–83. https://doi.org/10.1016/j.cbi.2017.11.007
Finkina EI, Melnikova DN, Bogdanov IV, Ovchinnikova TV (2016) Lipid transfer proteins as components of the plant innate immune system: structure, functions, and applications. Acta Naturae 8:47–61. https://doi.org/10.32607/20758251-2016-8-2-47-61
Freire JM, Gaspar D, Veiga AS, Castanho MARB (2015) Shifting gear in antimicrobial and anticancer peptides biophysical studies: from vesicles to cells. J Pept Sci 21:178–185. https://doi.org/10.1002/psc.2741
Gebara RdaS, Taveira GB, de Azevedo dos Santos L et al (2020) Identification and characterization of two defensins from Capsicum annuum fruits that exhibit antimicrobial activity. Probiotics Antimicrob Proteins 12:1253–1265. https://doi.org/10.1007/s12602-020-09647-6
Greco I, Molchanova N, Holmedal E et al (2020) Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-69995-9
Gupta S, Kapoor P, Chaudhary K et al (2015) Peptide Toxicity Prediction. In: Computational Peptidology. pp 143–57
Harris F, Dennison SR, Singh J, Phoenix DA (2011) On the selectivity and efficacy of defense peptides with respect to Cancer cells. Med Res Rev 33:190–234. https://doi.org/10.1002/med.20252
Hein MJA, Kvansakul M, Lay FT et al (2022) Defensin-lipid interactions in membrane targeting: mechanisms of action and opportunities for the development of antimicrobial and anticancer therapeutics. Biochem Soc Trans 50:423–437. https://doi.org/10.1042/BST20200884
Helmerhorst EJ, Reijnders IM, Van Hof ’T W, et al (1999) A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. FEBS Lett 449:105–110. https://doi.org/10.1016/S0014-5793(99)00411-1
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:1–21. https://doi.org/10.3389/fmicb.2020.582779
Huang HW (2006) Molecular mechanism of antimicrobial peptides: the origin of cooperativity. 1758:1292–1302. https://doi.org/10.1016/j.bbamem.2006.02.001
Khabbaz H, Karimi-Jafari MH, Saboury AA, BabaAli B (2021) Prediction of antimicrobial peptides toxicity based on their physico-chemical properties using machine learning techniques. BMC Bioinformatics 22:1–11. https://doi.org/10.1186/s12859-021-04468-y
Khan F, Niaz K, Abdollahi M (2018) Toxicity of biologically active peptides and future safety aspects: an update. Curr Drug Discov Technol 15:236–242. https://doi.org/10.2174/1570163815666180219112806
Koo HB, Seo J (2019) Antimicrobial peptides under clinical investigation. Pept Sci 111. https://doi.org/10.1002/pep2.24122
Kovaleva V, Bukhteeva I, Kit OY, Nesmelova IV (2020) Plant defensins from a structural perspective. Int J Mol Sci 21:1–23. https://doi.org/10.3390/ijms21155307
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343. https://doi.org/10.1016/j.freeradbiomed.2009.05.004
Kulkarni MM, Mcmaster WR, Kamysz W, Mcgwire BS (2009) Antimicrobial peptide-induced apoptotic death of Leishmania results from Calcium-dependent, caspase-independent mitochondrial toxicity *. J Biol Chem 284:15496–15504. https://doi.org/10.1074/jbc.M809079200
Lei J, Sun LC, Huang S et al (2019) The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11:3919–3931
Lewies A, Du Plessis LH, Wentzel JF (2019) Antimicrobial peptides: the Achilles’ heel of Antibiotic Resistance? Probiotics Antimicrob Proteins 11:370–381. https://doi.org/10.1007/s12602-018-9465-0
Li S, Wang Y, Xue Z et al (2021) The structure-mechanism relationship and mode of actions of antimicrobial peptides: a review. Trends Food Sci Technol 109:103–115. https://doi.org/10.1016/j.tifs.2021.01.005
Maximiano MR, Franco OL (2021) Biotechnological applications of versatile plant lipid transfer proteins (LTPs). https://doi.org/10.1016/j.peptides.2021.170531. Peptides 140:
Mello EO, Ribeiro SFF, Carvalho AO et al (2011) Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr Microbiol 62:1209–1217. https://doi.org/10.1007/s00284-010-9847-3
Melnikova DN, Finkina EI, Bogdanov IV et al (2023) Features and possible applications of plant lipid-binding and transfer proteins. Membr (Basel) 13:1–17
Mishra M, Tamhane VA, Khandelwal N et al (2010) Interaction of recombinant CanPIs with Helicoverpa armigera gut proteases reveals their processing patterns, stability and efficiency. Proteomics 10:2845–2857. https://doi.org/10.1002/pmic.200900853
Mohs RC, Greig NH (2017) Drug discovery and development: role of basic biological research. Alzheimer’s Dement Transl Res Clin Interv 3:651–657. https://doi.org/10.1016/j.trci.2017.10.005
Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ (2020) Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19:311–332. https://doi.org/10.1038/s41573-019-0058-8
Moretta A, Scieuzo C, Petrone AM et al (2021) Antimicrobial peptides: a New Hope in Biomedical and Pharmaceutical Fields. Front Cell Infect Microbiol 11:1–26. https://doi.org/10.3389/fcimb.2021.668632
Mylonakis E, Moreno R, Khoury JB, El et al (2005) Galleria mellonella as a Model System to study Cryptococcus neoformans Pathogenesis. Infect Immun 73:3842–3850. https://doi.org/10.1128/IAI.73.7.3842
Ojeda PG, Cardoso MH, Franco OL (2019) Pharmaceutical applications of cyclotides. Drug Discov Today 24:2152–2161. https://doi.org/10.1016/j.drudis.2019.09.010
Oliveira FD, Cavaco M, Figueira TN et al (2022) The antimetastatic breast cancer activity of the viral protein-derived peptide vCPP2319 as revealed by cellular biomechanics. FEBS J 289:1603–1624. https://doi.org/10.1111/febs.16247
Piatek M, Sheehan G, Kavanagh K (2021) Galleria mellonella: the versatile host for drug discovery, in vivo toxicity testing and characterising host-pathogen interactions. Antibiotics 10. https://doi.org/10.3390/antibiotics10121545
Pognan F, Beilmann M, Boonen HCM et al (2023) The evolving role of investigative toxicology in the pharmaceutical industry. Nat Rev Drug Discov 22:317–335. https://doi.org/10.1038/s41573-022-00633-x
Robles-Loaiza AA, Pinos-Tamayo EA, Mendes B et al (2022) Traditional and computational screening of non-toxic peptides and approaches to improving selectivity. Pharmaceuticals 15. https://doi.org/10.3390/ph15030323
Rodríguez-Rojas A, Makarova O, Rolff J (2014) Antimicrobials, stress and Mutagenesis. PLoS Pathog 10. https://doi.org/10.1371/journal.ppat.1004445
Rudzińska M, Daglioglu C, Savvateeva LV et al (2021) Current status and perspectives of protease inhibitors and their combination with nanosized drug delivery systems for targeted cancer therapy. Drug Des Devel Ther 15:9–20. https://doi.org/10.2147/DDDT.S285852
Ruiz J, Calderon J, Rondón-Villarreal P, Torres R (2014) Analysis of structure and hemolytic activity relationships of antimicrobial peptides (AMPs). Adv Intell Syst Comput 232:253–258. https://doi.org/10.1007/978-3-319-01568-2_36
Salminen TA, Blomqvist K, Edqvist J (2016) Lipid transfer proteins: classification, nomenclature, structure, and function. Planta 244:971–997. https://doi.org/10.1007/s00425-016-2585-4
Schweizer F (2009) Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol 625:190–194. https://doi.org/10.1016/j.ejphar.2009.08.043
Serrano I, Verdial C, Tavares L, Oliveira M (2023) The virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 12:505. https://doi.org/10.3390/antibiotics12030505
Seyfi R, Kahaki FA, Ebrahimi T et al (2020) Antimicrobial peptides (AMPs): roles, functions and mechanism of action. Int J Pept Res Ther 26:1451–1463. https://doi.org/10.1007/s10989-019-09946-9
Sivertsen A, Isaksson J, Leiros HKS et al (2014) Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct Biol 14. https://doi.org/10.1186/1472-6807-14-4
Skalska J, Andrade VM, Cena GL et al (2020) Synthesis, structure, and activity of the Antifungal Plant Defensin PvD1. J Med Chem 63:9391–9402. https://doi.org/10.1021/acs.jmedchem.0c00543
Soares JR, José Tenório de Melo E, da Cunha M et al (2017) Interaction between the plant ApDef1 defensin and Saccharomyces cerevisiae results in yeast death through a cell cycle- and caspase-dependent process occurring via uncontrolled oxidative stress. Biochim Biophys Acta - Gen Subj 1861:3429–3443. https://doi.org/10.1016/j.bbagen.2016.09.005
Sok M, Šentjurc M, Schara M (1999) Membrane fluidity characteristics of human lung cancer. Cancer Lett 139:215–220. https://doi.org/10.1016/S0304-3835(99)00044-0
Svenson J, Brandsdal BO, Stensen W, Svendsen JS (2007) Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J Med Chem 50:3334–3339. https://doi.org/10.1021/jm0703542
Tamimi NAM, Ellis P (2009) Drug development: from concept to marketing! Nephron - Clin Pract 113:125–131. https://doi.org/10.1159/000232592
Van Vliet E (2011) Current standing and future prospects for the technologies proposed to transform toxicity testing in the 21st century. Altex 28:17–44. https://doi.org/10.14573/altex.2011.1.017
Vieira-da-Silva B, Castanho MARB (2023) Resazurin reduction-based assays revisited: guidelines for Accurate Reporting of relative differences on metabolic status. Molecules 28. https://doi.org/10.3390/molecules28052283
Wei D, Zhang X (2022) Biosynthesis, bioactivity, biotoxicity and applications of antimicrobial peptides for human health. Biosaf Heal 4:118–134. https://doi.org/10.1016/j.bsheal.2022.02.003
Yadav NK, Saikhedkar NS, Giri AP (2021) PINIR: a comprehensive information resource for Pin-II type protease inhibitors. BMC Plant Biol 21
Yeaman MR, Büttner S, Thevissen K (2018) Regulated cell death as a therapeutic target for novel antifungal peptides and biologics. Oxid Med Cell Longev 2018.https://doi.org/10.1155/2018/5473817.
Zasloff M (2019) Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol 1117:3–6. https://doi.org/10.1007/978-981-13-3588-4_1
Zhang QY, Yan Z, Bin, Meng YM et al (2021) Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res 8:1–25. https://doi.org/10.1186/s40779-021-00343-2
Acknowledgements
This work was performed at the Universidade de Lisboa and Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF).
Funding
We acknowledge the financial support of the Brazilian agencies CNPq (307590/2021-6), FAPERJ (E-26/200567/2023; E-26/210353/2022; E-26/200.127/2023) and European Union H2020-01 (828774). This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES), finance code 001.
Author information
Authors and Affiliations
Contributions
The study was conceived by MBC, MARBC and VMG. Experimental procedures were carried out by MBC, GBT, MCC, TZAG, LAS, EOM. Data analyses were performed by MBC, GBT, MCC, VLSN, AOC, MARBC. The paper was written by MBC, MCC, GBT, VMG, MARBC. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cherene, M.B., Cavaco, M.C., Neves, V.L.S. et al. Non-toxicity of Plant Candicidal Peptides for Mammalian Cell Lines and Galleria mellonella Model to Improving Selectivity for Clinical Use. Int J Pept Res Ther 30, 28 (2024). https://doi.org/10.1007/s10989-024-10607-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-024-10607-9