Abstract
Whereas the traditional approaches of cancer therapy including radiotherapy, chemotherapy, and immunotherapy have failed to properly treat cancer due to the condition of the space inside the tumor with the hypoxic/necrotic regions, abnormality of blood vessels that prevent delivery of enough oxygen, nutrients, and therapeutic agents, also, and the emergence of resistance, finding a new way has still remained a challenge. Antimicrobial peptides with dual anticancer activity have received more attention as a new approach for cancer treatment. Using bacteria and their products including the bacterial peptides have shown promising results in tumor regression or inhibition. Surprisingly, the small peptides derived from N-terminus of the ribosomal proteinL1 (RpL1) of Helicobacter pylori demonstrate the strong anticancer activity including activation of caspase-3, -8 and -9-dependent pathway, inducing apoptosis, arresting the cell cycle at the G0/G1 and G2/M and inhibiting the cell proliferation. Moreover, in addition to the anticancer effects, these peptides exhibit the antibacterial, antifungal, and anti-parasitic activities through membrane disruption, pore-forming, and enhancing the membrane permeability. The present study is a comprehensive review of the anticancer activity as well as antibacterial, antifungal, and anti-parasitic activities of small peptides derived from N-terminus of the ribosomal proteinL1 (RpL1) of H. pylori as an anticancer therapeutic peptide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite great deal of advances in the cancer treatment, it still is one of the major leading causes of death in the world and was responsible for more than 9.6 million deaths in 2018 (Bray et al. 2018; Cancer 2018). Conventional therapies such as surgery, chemotherapy, radiation and hormonal therapies encountered limitations in the treatment of cancer in recent decades. The main problems of these therapies include the lack of specific toxicity, formation of multi-drug resistance and high cost (Siegel et al. 2014, 2016). Therefore, there was an urgent need for developing the new therapeutic agents to overcome the complications of the available drugs. In 1909, William Coley used bacteria in cancer therapy, for the first time in history. He used heat-inactivated Streptococcus pyogenes combined with the Serattia marcescence, called the Coley’s toxins. More than 1000 patients with malignancy were treated with this bacterial mixture. Results in this regard demonstrate the regression of the tumors in many patients and 30 cases were completely treated (Nauts et al. 1946; Wiemann and Starnes 1994).
Recently, bacteria have been used as an anticancer therapy in different forms including, live, attenuated or genetically modified states as well as their products (including, bacterial peptides, bacteriocins, and toxins). Different studies demonstrate promising results in the field of bacterial therapy (Fujimori 2006; Roberts et al. 2014; Song et al. 2018).
One of the challenges in the field of cancer therapy is the hypoxic condition into the tumors that most medicines are unable to function in, while this hypoxic and necrotic region is a favorable niche for the obligation or facultative anaerobic bacteria. Moreover, different studies demonstrate that therapeutic bacterial agents are able to proliferate the tumor and form the necrotic region after entering into the tumor tissue, which results in killing the tumor cells due to starvation and suffocation (Danino et al. 2013). Through several mechanisms, the therapeutic bacteria can regress the tumor cells by the means of (i) releasing the substances, (ii) biofilm formation, (iii) acting as a target delivery vehicle for anticancer agents, (iv) enhancing the human immunity, and (v) invading and colonizing the solid tumor (Song et al. 2018).
Bacterial peptides have attracted significant attention to the treatment of cancer due to their therapeutic advantages. Peptides have high target specificity and selectivity due to positively charges, which selectively target the cancer cells with negative charges and different membrane structures from the normal cells (Boohaker et al. 2012; Marqus et al. 2017). They can prevent the establishment of multidrug-resistance mechanisms and enhance the anticancer effect of conventional therapeutic agents. Other advantages of bacterial peptides include their small size and easy synthesis, being simply modified, wide range of targets, and low accumulation in tissues (Marqus et al. 2017). Non-ribosomal peptide is secondary bioactive metabolites that synthesis via bacterial enzymes is known as a non-ribosomal peptide synthetase (Agrawal et al. 2017). These peptides with the bacterial origin are characterized via special chemical structures such as N-terminally attached fatty acids, N formulated residues, D-amino acids, heterocyclic elements, N- and C-methylated residues and glycosylated amino acids, and also the phosphorylated residues (Sieber and Marahiel 2003). These peptides have dual antimicrobial-anticancer activity through different mechanism including inducing apoptosis, prolifration inhibition, inhibiting the angiogenesis, and disrupting the signaling pathway (including vascular endothelial growth factor receptor 2 (VEGFR2) tyrosine kinase, focal adhesion kinase (FAK), AKT, basic fibroblast growth factor (bFGF) (FGFR-1), and PI3K) (Yamada et al. 2002; Goto et al. 2003; Lee et al. 2005; Karpiński 2012; Karpiński et al. 2013).
L1 protein is able to directly bind to the 23SrRNA. The stalk of L1 protein is very mobile in the ribosome and is involved in E site tRNA release. L1 protein has a role as a translational repressor protein, and also it controls the translation of the L11 operon by binding to its mRNA (Kang et al. 2005). Evidence suggests that small peptides derived from the N-terminus of the ribosomal proteinL1 (RpL1) of Helicobacter pylori have anticancer potential (Pütsep et al. 1999a, b). In addition to anticancer activity, these peptides have several important functional features including, antibacterial, antifungal, anti-parasitic, being neutrophil chemoattractant and able to activate the phagocyte NADPH oxidase in order to produce reactive oxygen species (Zhao et al. 2013; Mai et al. 2015) (Table1). The present study is a comprehensive review of current knowledge concerning the anticancer, antibacterial, antifungal, and anti-parasitic activities of small peptides derived from N-terminus of the ribosomal proteinL1 (RpL1) of H. pylori as an anticancer therapeutic peptide.
Anticancer Activity of RpL1 Derived Peptides
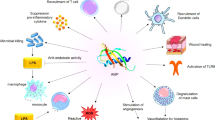
Different studies suggest that the small peptides derived from N-terminus of the ribosomal proteinL1 (RpL1) of H. pylori exert anticancer activity through different mechanisms including (i) activation of caspase-3, -8 and -9-dependent pathway leading to apoptosis, (ii) arresting the cell cycle at the G0/G1 and G2/M, (iii) cell proliferation inhibition, (iv) damaging the mitochondrial function, and (v) increasing the generation of reactive oxygen species (ROS) (Fig. 1). There are several smaller peptides derived from RpL1 with anticancer activity such as HPRP-A1, HPRP-A2, HPA3, HPA3P, and HPA3P2, which will be discussed as follows (Table 2).
HPRP-A1
HPRP-A1 is a 15-mer α-helical cationic peptide derived from N-terminus of ribosomal protein L1 of H. pylori, exhibiting anticancer and antimicrobial activities (Pütsep et al. 1999a, b). These cationic anticancer peptides (ACPs) have different features, which are important for their anticancer activity such as, amphipathicity, secondary structure in their membrane, hydrophobicity, net charge, and oligomerization ability. Some studies suggest that hydrophobicity has an important role in the anticancer activity due to its hydrophobic condition for the cell membrane (Shai 1999; Hoskin and Ramamoorthy 2008; Maher and McClean 2008; Zhao et al. 2013).
Hu et al. used HPRP-A1 co-administration with kla-TAT in a study to enhance the anticancer activity of the combination peptides by the synergistic effect. kla is a pro-apoptotic peptide, which is able to induce apoptosis in the cancer cells through the disruption of mitochondrial membrane after the cell internalization. Moreover, in this study they used cell penetration peptide TAT to increase the cell penetration of kla peptides through the cell membrane. The non-small cell lung cancer (NSCLC) A549 cell line, hepatoma cell line (BEL-7402), breast cancer cell line (MDA-MB-231) and human umbilical vein endothelial cells (HUVECs) were exposed to kla-TAT peptide combined with or without HPRP-A1 peptides (Hu et al. 2018a, b). The result of this study demonstrated that the co-administration significantly increased the anticancer activities of the two peptides, which included disruption of the cell membrane, inducing the caspase-dependent apoptosis, and inducing the cell cycle arrest at the G1. Moreover, the result shows that peptides co-administration, compared to each peptide alone, could induce stronger mitochondrial membrane potential depolarization at 1 and 24 h, respectively, which results in cellular apoptosis (Hu et al. 2018a, b).
In addition, another study in 2019, treated the non-small cell lung cancer (NSCLC) A549 cell line and breast cancer cell line (MCF-7) by kla peptide co-administrated with membrane-active anticancer peptide HPRPA1. kla (KLAKLAK)2, known as an apoptosis-inducing peptide, is able to disrupt mitochondrial membranes and induce cancer cell apoptosis with low cell-penetrating potential for eukaryotic cells (Hao et al. 2019). Another study fused the cell- penetrating peptide TAT to the C-terminus of HPRP-A1. This new hybrid peptide shows a stronger anticancer activity on HeLa cells by inducing caspase activity and increasing apoptosis and also with lower toxicity against human RBC than HPRP-A1. Moreover, in comparison to the HPRP-A1, the construct of HPRP-A1-TAT can quickly cross over the cells and enter the cytoplasm through endocytosis, and then disrupt the cell membrane integrity. Additionally, the result of this study suggests that TAT could protect the HPRP-A1 against degradation due to the high number of positively charged amino acids or the further release of peptides into the cancer cells from the endocytotic vesicles (Hao et al. 2015; Huang 2016).
The effects of co-administration of HPRP-A1 and the tumor homing/penetrating peptide iRGD have been evaluated in another study. iRGD is a peptide known as a cell membrane penetration peptide by targeting the αvβ3 integrins neuropilin-1 (NRP-1) receptors, highly expressed in cancer cells (Hu et al. 2018a, b, c). In this study, the anticancer and penetration activity was examined in vitro and in vivo, which used A549 non-small cell lung cancer cells and A549 xenograft mouse model. These A549 cells were exposed to the concentration of HPRP-A1 with or without of iRGD or RGD for 1 h, 24 h, and 48 h (Hu et al. 2018a, b). In vitro results demonstrate the increase in the cytotoxicity of HPRP-A1 after co-administration with iRGD on both cell line HUVEC and A549 cells. Moreover, co-administration of iRGD and HPRP-A1 results in stronger anticancer activity and tumor specificity against cancer cells like A549 with the over-expression of the NRP-1 receptor compared to the HPRP-A1 alone (Hu et al. 2018a, b).
The other results of these peptides co-administration include: (i) increasing the cellular uptake, (ii) mitochondrial depolarization, (iii) cell membrane destruction, (iv) Reactive oxygen species (ROS) generation, and inducing the apoptosis through a caspase-dependent pathway. Additionally, in-vivo results show that the co-administration of iRGD and HPRP-A1 increased the penetration depth as well as anticancer efficacy in an A549 xenograft mouse model. It also results in tumor growth inhibition in the nude xenograft mice (Hu et al. 2018a, b).
A study used α-helical peptide HPRP-A1 as a combination anticancer therapy with chemical drugs doxorubicin (DOX) and epirubicin (EPI), which were examined at both in-vitro and in-vivo. In this study, the cervical cancer cell line (HeLa) and human liver cancer cell line (HepG2) were exposed to HPRP-A1 alone and DOX/EPI alone or peptide-drug combinations (HPRP-A1/DOX and HPRP-A1/EPI) for 24 h (Zhao et al. 2015a). The in-vitro result shows that the anticancer activity of these drugs is synergistically increased. The HPRP-A1/DOX combination results in the enhancement of apoptosis through activation of caspase-3, -8 and -9-dependent pathway. Moreover, HPRP-A1/DOX combination is able to arrest the cell cycle of HeLa cells at the G0/G1 and G2/M (Zhao et al. 2015a).
The in-vivo studies used HeLa xenograft model in BALB/c nude mice, which received DOX and HPRP-A1 based on the IC50 ratio of HPRP-A1/DOX that was injected directly into the tumors once every two days for 15 days. The in-vivo result demonstrates that the HPRP-A1/DOX combination was more effective than the either drug alone. Even at low concentrations, the growth of the HeLa cell can be significantly inhibited, which is due to the enhancement of cellular apoptosis (Zhao et al. 2015a).
HPRP-A2
Another peptide derived from the N-terminus of ribosomal protein L1 of H. pylori known as an HPRP-A2, which is a synthetic 15-mer cationic peptide with all D-amino acids HPRP-A2, is the enantiomer of HPRP-A1. As well as HPRP-A1, HPRP-A2 also shows a strong anticancer effect on tumor cells with low toxicity against human red blood cells (Schweizer 2009; Zhao et al. 2015a). A study used HPRP-A2 alone and in co-administration with doxorubicin (DOX). In this study, HPRP-A2 “a synthetic 15-mer cationic peptides with all D-amino acids” effectively inhibited the survival of gastric cell lines in a dose-dependent manner. Human gastric cancer cell lines BGC-823 and SGC-7901 went under therapy by HPRP-A2 for 1 h. The result demonstrates that HPRP-A2 can increase the cell membrane permeability as well as inducing the apoptosis in BGC-823 cells (Zhao et al. 2015b). Moreover, HPRP-A2 led to increasing the generation of reactive oxygen species (ROS), activation of caspase-3, -8 and -9 pathway, and arresting the cell cycle at the G1 phase, and a decrease of mitochondrial membrane potential (MMP) caused damages of mitochondrial function. In addition to examining the anticancer effect of HPRP-A2 alone, its effect was examined in combination with DOX. In this regard, the cells were treated with HPRP-A2 and/or Dox. The results demonstrate that in addition to its inherent cytotoxicity, HPRP-A2 is able to be synergized strongly with DOX, resulting in increasing the tumor cell killing in-vitro (Zhao et al. 2015b). In another study, HeLa and HepG2 cell lines were exposed to the HPRP-A2 or DOX/EPI alone, and peptide-drug combination (HPRP-A2/DOX and HPRP-A2/EPI) for 24 h. The results demonstrate that in addition to the anticancer effect, HPRP-A2 alone has a synergistic effect on the cell growth in combination with DOX (Zhao et al. 2015a).
HPA3
HP (2–20) is one of the derived peptides from N-terminus of H. pylori Ribosomal protein L1 (RpL1) with a length of 19 acid amine. HP (2–20), known and identified as a cecropin-like H. pylori peptide is an important factor as a monocyte chemoattractant (Pütsep et al. 1999b; Bylund et al. 2006). Among the different analogues of this peptide HPA3, HPA3P, and HPA3P2 show anticancer as well as antimicrobial activities (Pütsep et al. 1999a; Lee et al. 2002a, b). One of the analogues of HP (2–20) is called HPA3 with the substitution of tryptophan for glutamine and aspartic acid at the positions 17 and 19, respectively. Moreover, in HPA3P, the proline substitution glutamic acid is at position 9, while in HPA3P2, the proline substitutions for glutamic acid and phenylalanine are at positions 9 and 12, respectively (Lee et al. 2013, 2014). As an antimicrobial peptide, HPA3 shows anticancer activity in gastric cancer and leukemia. In a study, it used to target colon cancer cell lines including LoVo, HT-29, SW480, and HCT116 (Cho et al. 2018). All colon cancer cell lines were treated with different concentrations of HPA3, HPA3P, and HPA3P2 for 24 h. Results demonstrate that HPA3P could reduce the cell viability, whereas HPA3 and HPA3P2 were not able to reduce the viability of colon cancer cells. Decreasing the cell viability is due to necrosis, which supported by the release of cellular contents. Furthermore, the results demonstrate that the dropping down of necroptosis proteins such as Receptor Interacting Serine/Threonine Kinase 3 (RIPK3) and Mixed Lineage Kinase Domain Like Pseudokinase (MLKL) attenuated the reductions in the cell viability induced by HPA3P. Additionally, this study suggests that HPA3P can enhance the anticancer activity of chemotherapeutic agents like Fluorouracil (5-FU) (Cho et al. 2018). Furthermore, HPA3P shows anticancer activity in other cancer cell lines, resulting in the reduction of cell viability of other cancer cell lines including ovarian carcinoma (SKOV3), HeLa cell-contaminated amnion (WISH), breast adenocarcinoma (MCF7), breast metastatic carcinoma (MDA-MB-453), gastric adenocarcinoma (AGS), non-small lung carcinoma (H1299), cervical carcinoma (HeLa), and laryngeal squamous cell carcinoma (SNU-1076) cells in human beings (Cho et al. 2018).
Antimicrobial Activity of RpL1 Derived Peptides
Recently, it has been demonstrated that different peptides derived from N-terminus of H. pylori RpL1 have antibacterial, antifungal and anti-parasitic activities, in addition to anticancer activity. The most important antimicrobial mechanisms of RpL1 are discussed in this section.
Antibacterial Activity
Recent studies suggest that HPRP-A1 and HPRP-A2 have a broad spectrum of antibacterial activities against Gram-positive and Gram-negative bacteria. HPRP-A1 and HPRP-A2 are able to disrupt and enhance the permeability of the cell membrane via interaction with the cell membranes, which leads to cell death.
HPRP-A1 is composed of 15 L-amino acid residues that target the bilayer of cell membranes. HPRP-A2 peptide contains all D-amino acids that are more stable than the HPRP-A1 peptide, and it is also resistant to the proteolysis in the plasma and tissue fluid (Matsumoto et al. 2015; Zhao et al. 2015a; Hu et al. 2018c). These peptides are the same as other α-helical The antimicrobial peptides (AMPs) effects on the prokaryotic and eukaryotic cells, which depend on the composition of membrane phospholipids. In eukaryotes membrane containing phospholipids, large amounts of cholesterol and sphingomyelin, it would be able to form the pores in the hydrophobic core of the lipid bilayers and causes eukaryotic cell death. In the prokaryotic cell membrane, which has strong transmembrane potential, these peptides work as a detergent agent to disintegrate cell membranes, as in the “carpet model” (Abraham et al. 2005; Chen et al. 2005b, 2007).
The AMPs role in the destruction of bacterial cell membranes is followed by the mechanism of the “carpet model”. In this regard, peptides interact with the membrane, resulting in the instability of membrane phospholipids due to the peptide location in phospholipids. The peptide concentration is then increased and when it reaches a critical point, causes the disintegration of the membrane due to the lack of energy (Pouny et al. 1992) (Fig. 2). Moreover, HPRP-A1 and HPRP-A2 are able to strongly bind to the LPS, which is the main component of the outer membrane in the Gram-negative bacteria (Chu et al. 2019). Additionally, LPS is responsible for the antimicrobial defense and septic shock by the triggered caspase-11 mediated pyroptosis, when it exposed to the antibiotics or antimicrobial peptides (Rathinam et al. 2019).
The combination therapy of antimicrobial peptides (AMPs) and conventional antibiotics with `synergistic effect have been widely reported. Surprisingly, as the antimicrobial peptides, HPRP-A1 and HPRP-A2 show significant synergistic effects in the co-administration with a disinfectant called chlorhexidine acetate (CHA), resulting in enhancing the antimicrobial activities toward various Gram-negative and Gram-positive bacteria (Zhu et al. 2019). Moreover, as an antimicrobial peptide, HPRP-A2 has been used in combination with silk fibroin (SF) and nanofibrous matrix fabricated by an all-aqueous electrospinning process. The HPRP-A2/SF composite nanofibers show strong antimicrobial activity against both Gram-positive and Gram-negative bacteria. Moreover, this composite nanofibrous matrix has shown great performance in wound healing in animal subjects (Zhang et al. 2019).
Furthermore, several truncated peptides were synthesized to investigate the role or effects of the N- or C-terminal regions of HP (2–20) on antimicrobial activity. Evidence suggests that the full-length of the N-terminal region of HP (2–20) is needed for antimicrobial activity, and also this region facilitates the penetration of peptide into the cell membrane (Park and Hahm 2005). Moreover, circular dichroism (CD) results demonstrate that the α-helical structure of the peptides has a crucial role in the antimicrobial activity (Park et al. 2002, 2007). Additionally, some analogy of HP (2–20) including HPA3, HPA3P, and HPA3NT3 exhibit broad-spectrum antimicrobial activity, while they have less or no hemolytic activity or cytotoxicity on mammalian cell lines. The HPA3 can significantly affect the membrane bilayer structure by the pore-forming activity (Park et al. 2008).
Moreover, the result of an in vivo study demonstrated that HPA3P2 compared to HPA3P has stronger antibacterial activity. In this study, the considered mice were infected with multidrug-resistant Pseudomonas aeruginosa exposed to the one 0.5-mg/kg dose of HPA3P2 or three 0.1-mg/kg doses of HPA3P2, which resulted in 100% survival due to the permeation into the cell membranes and then binding to intracellular RNA and DNA. Also, interaction with Lipopolysaccharides (LPS) led to reducing the production of pro-inflammatory mediators as well as decreasing the damage of lung and liver tissue (Lee et al. 2014).
An investigation used AGS and MKN epithelial cells, to show the regulating role of HP (2–20) in the gastric mucosal healing process through facilitating and stimulating gastric epithelial cell migration, proliferation and up-regulates Vascular endothelial growth factor A (VEGF-A) expression at both transcriptional and translational levels through the interaction with Formyl peptide receptor 2 (FPR2) and FPR3 receptors. The results of this study indicate that HP (2–20) is able to treat the gastric epithelial cells by activating the Extracellular-regulated kinase (ERK), Protein Kinase B (Akt), and Signal transducer and activator of transcription 3 (STAT3) signaling pathways (de Paulis et al. 2009).
Antifungal Activity
There are evidences suggesting that the smaller peptides derived from N-terminus of H. pylori RpL1 have anti-fungal activity. The anti-fungal activity of AMP follows the “barrel-stave model” mechanism (Ehrenstein and Lecar 1977). The monomers of peptides get together on the surface of the outer membrane of the bilayer, which is followed by the interaction of the peptide hydrophilic regions. These regions are vertically inserted into the membrane to interact with the hydrophobic core of the membrane resulting the formation of channels or pores in the fungal membrane. The antifungal activity of HPRP-A1 and HPRP-A2 are depended on the α-helical and amphipathic structure (Ehrenstein and Lecar 1977) (Fig. 2). Moreover, the analogy of HP (2–20), such as HPA3 and HPA3NT3, show antifungal activity against chitin-containing fungi with no hemolytic activity or cytotoxicity on mammalian cell lines (Park and Hahm 2005; Park et al. 2008). Hence, a study used the fungi that do express chitin such as Aspergillus flavus and Aspergillus fumigatus, with the fungi that do not express chitin such as Phytophthora nicotinae and Phytophthora parasitica. The results demonstrate that the analog peptides, HPA3 and HPA3NT3, have highly strong activity against the chitin-harboring fungi, while they have no activity against the strains that do not harbor chitin (Park et al. 2007). Furthermore, several studies suggest that the derived peptides from RpL1 of H.pylori have strong antifungal activity against Candida species, mainly Candida albicans and Candida krusei (Lee et al. 2002a, b; Park and Hahm 2005; Park et al. 2007; Zhu et al. 2019).
Anti-parasitic Activity
HPRP-A1and HPRP-A2 have an anti-parasitic activity against Toxoplasma gondii infection. T. gondii is known as an intracellular parasite, which is the leading cause of severe ocular and neurological diseases in fetuses, newborn infants, and immunosuppressive patients (McLeod et al. 2009; Zare-Bidaki et al. 2016). The critical stage of T. gondii infection is characterized by the generation of tachyzoites, which results in rapid replication in the host cells and destroys them within 24–48 h (El Bissati et al. 2016). There are evidences showing that the viability of tachyzoites was significantly decreased and could not provide adhesion to and invasion of macrophages after the therapy with HPRP-A1/A2. Treatment with these two peptides results in destroying the integrity of tachyzoite membranes, causing membrane disorganization and cytoplasm outflow from tachyzoites (Liu et al. 2019). The results of a study demonstrate that about 90% of tachyzoites were killed after the therapy with 20 μg/ml of HPRP-A1 for 60 min., and 60–70% of tachyzoites were found dead after being exposure to the same concentration of HPRP-A2. Thus the result of this study suggests that HPRP-A1 has more considerable cytotoxic activity against Toxoplasma than HPRP-A2 (Liu et al. 2019).
Several studies suggest that enhancing the host immunity, including responses of Th1 (include TCD4 + producing IFN-γ), responses of Tc1 (include TCD8 + producing IFN-γ), and inducing the pro-inflammatory factors (such as IL-12 and TNF-ɑ), has a crucial role in preventing toxoplasmosis with the long-term effects (Gazzinelli et al. 1994; Cong et al. 2010, 2012; Tan et al. 2010). Surprisingly, the in vivo results show that the intraperitoneal administration of HPRP-A1/A2 led to significant decreasing of the number of tachyzoites and increasing the Th1/Tc1 response, as well as elicited pro-inflammatory cytokines in the mice infected with T. gondii (Liu et al. 2019).
Conclusion
Since the conventional cancer therapy faced limitations such as toxic side effects, and developing resistance to the therapeutic agents, new therapeutics considerations are required in this respect. Using bacteria, especially peptides driven from bacteria, have taken into consideration as a way to treat cancer. In comparison to the conventional therapies, bacterial peptides have advantages as well as disadvantages. From a negative point of view, the most important challenges of bacterial peptides are metabolic instability, weak membrane permeability, rapid clearance (short half-life), poor solubility, and being costly (Chen et al. 2005a; Hu et al. 2011). As a positive point of view, they have selective toxicity for tumor cells with less or without any side effects on normal cells, they are easy to synthesize and modify, and they also have toxicity activity on MDR cancer cells (Johnstone et al. 2000; Chen et al. 2005a).
Recently, some studies suggest that small peptides derived from N-terminus of the RpL1 of H. pylori could be considered as the anticancer therapeutic agent. Different derivatives are known, including HPRP-A1, HPRP-A2, HPA3, HPA3P, HPA3P2, and HPA3NT3, all of which exhibit anticancer activity.
HPRP-A1 possess anticancer activity through activation of caspase-3, -8 and -9-dependent pathway leading to apoptosis, arresting the cell cycle at the G0/G1 and G2/M (Zhao et al. 2015a; Hu et al. 2018a, b). Moreover, it disrupts the cell membranes and significantly inhibits the cell proliferation (Pütsep et al. 1999a). Additionally, membrane-active anticancer peptide HPRP-A1 used in co-administration with tumor homing peptide iRGD, resulting in enhancing the selectivity and anticancer activity, improves the membrane disruption activity and cellular uptake (Hu et al. 2018a, b). Furthermore, HPRP-A1 co-administration with cell penetration peptide TAT leads to improving the anticancer activity (Hu et al. 2018a, b).
HPRP-A2 is also the same as HPRP-A1, being able to activate the caspase-3, -8 and -9 pathway, arrest the cell cycle at the G1 phase, damage of mitochondrial function, and increase the generation of reactive oxygen species (ROS) (Zhao et al. 2015b). The other derivatives including HPA3, HPA3P, and HPA3P2 can also reduce the cell viability by decreasing the necroptosis proteins such as RIPK3 and MLKL (Cho et al. 2018).
It is interesting to know that these H. pylori derived peptides are best known for their antimicrobial characteristics. Their antimicrobial activity is through membrane disruption, strong binding to the LPS of Gram-negative bacteria, pore-forming, and enhancing the membrane permeability, which follows the carpet model mechanism (Chen et al. 2007; Rathinam et al. 2019). In addition to the antibacterial activity, they show strong antifungal activity by the formation of channels or pores in the fungal membrane through the barrel-stave model mechanism (Park et al. 2008).
Furthermore, they have the potential to be used as a preventing or treatment agent against T. gondii infections. Anti T. gondii activity of HPRP-A1/2 is due to the mechanisms (i) through damaging the membrane of the parasite, and (ii) enhancing the host immunity, including the increase in the Th1/Tc1 response and elicited pro-inflammatory cytokines. There are evidences showing that the viability of tachyzoites was significantly decreased and was not able to provide adhesion and invasion of macrophages after the therapy HPRP-A1/A2 (Liu et al. 2019).
Additionally, a study suggests that due to having the potential targeting and disrupting the membrane, HP-A3 can be a virus-cell fusion inhibitory activity. Moreover, in this study, the substitution of Gln and Asp for hydrophobic amino acid, and Trp at position 17 and 19 of HP-A3 caused the increase in the virus-cell fusion inhibitory activity without the hemolytic effect (Woo et al. 2002).
Although the use the bacterial peptide demonstrates a promising result in the field of cancer therapy as similar to RpL1-derived peptides, it is still new and required more intensive future studies to approve them as an anticancer therapeutic agent. Since most of the studies in these peptides have been stopped in the in vitro stage and just a few ones have been transformed from the in vitro to in vivo models, more future studies on in vivo and even clinical trials are needed to investigate the anticancer ability and mechanism of these peptides. Additionally, the anticancer activity of RpL1-derived peptides shows on a few numbers of cancer cell lines. Hence, there is a need to investigate their anticancer activity toward the other cancer cell lines.
References
Abraham T, Lewis RN, Hodges RS, McElhaney RN (2005) Isothermal titration calorimetry studies of the binding of a rationally designed analogue of the antimicrobial peptide gramicidin s to phospholipid bilayer membranes. Biochemistry 44(6):2103–2112
Agrawal S, Acharya D, Adholeya A, Barrow CJ, Deshmukh SK (2017) Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front Pharmacol 8:828
Boohaker JR, Lee MW, Vishnubhotla P, Perez JLM, Khaled AR (2012) The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem 19(22):3794–3804
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 68(6):394–424
Bylund J, Christophe T, Boulay F, Romero A, Hellstrand K, Dahlgren C (2006) A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J Clin Investig 116(5):1457–1457
Cancer, I. A. f. R. o (2018) Latest global cancer data: cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018
Chen J, Xu X-M, Underhill CB, Yang S, Wang L, Chen Y, Hong S, Creswell K, Zhang L (2005a) Tachyplesin activates the classic complement pathway to kill tumor cells. Can Res 65(11):4614–4622
Chen Y, Mant CT, Farmer SW, Hancock RE, Vasil ML, Hodges RS (2005b) Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem 280(13):12316–12329
Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS (2007) Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob Agents Chemother 51(4):1398–1406
Cho E, Lee J-K, Park E, Seo CH, Luchian T, Park Y (2018) Antitumor activity of HPA3P through RIPK3-dependent regulated necrotic cell death in colon cancer. Oncotarget 9(8):7902
Chu M, Qian L, Zhu M, Yao J, Xu D, Chen M (2019) Circumferential strain rate to detect lipopolysaccharide-induced cardiac dysfunction: a speckle tracking echocardiography study. Quant Imaging Med Surg 9(2):151
Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, Maewal A, McLeod R (2010) Human immunome, bioinformatic analyses using HLA supermotifs and the parasite genome, binding assays, studies of human T cell responses, and immunization of HLA-A* 1101 transgenic mice including novel adjuvants provide a foundation for HLA-A03 restricted CD8+ T cell epitope based, adjuvanted vaccine protective against Toxoplasma gondii. Immunome Res 6(1):12
Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, Sette A, Maewal A, El Bissati K, Zhou Y, Suzuki Y (2012) Toxoplasma gondii HLA-B* 0702-restricted GRA720-28 peptide with adjuvants and a universal helper T cell epitope elicits CD8+ T cells producing interferon-γ and reduces parasite burden in HLA-B* 0702 mice. Hum Immunol 73(1):1–10
Danino T, Prindle A, Hasty J, Bhatia S (2013) Measuring growth and gene expression dynamics of tumor-targeted S. typhimurium bacteria. J Vis Exp. 2013:e50540
de Paulis A, Prevete N, Rossi FW, Rivellese F, Salerno F, Delfino G, Liccardo B, Avilla E, Montuori N, Mascolo M (2009) Helicobacter pylori Hp (2–20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J Immunol 183(6):3761–3769
Ehrenstein G, Lecar H (1977) Electrically gated ionic channels in lipid bilayers. Q Rev Biophys 10(1):1–34
El Bissati K, Chentoufi AA, Krishack PA, Zhou Y, Woods S, Dubey JP, Vang L, Lykins J, Broderick KE, Mui E (2016) Adjuvanted multi-epitope vaccines protect HLA-A* 11: 01 transgenic mice against Toxoplasma gondii. JCI Insight 1(15):1–18
Fujimori M (2006) Genetically engineered bifidobacterium as a drug delivery system for systemic therapy of metastatic breast cancer patients. Breast Cancer 13(1):27–31
Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A (1994) Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153(6):2533–2543
Goto M, Yamada T, Kimbara K, Horner J, Newcomb M, Gupta TD, Chakrabarty A (2003) Induction of apoptosis in macrophages by Pseudomonas aeruginosa azurin: tumour-suppressor protein p53 and reactive oxygen species, but not redox activity, as critical elements in cytotoxicity. Mol Microbiol 47(2):549–559
Hao X, Yan Q, Zhao J, Wang W, Huang Y, Chen Y (2015) TAT modification of alpha-helical anticancer peptides to improve specificity and efficacy. PLoS ONE 10(9):e0138911
Hao W, Hu C, Huang Y, Chen Y (2019) Coadministration of kla peptide with HPRP-A1 to enhance anticancer activity. PLoS ONE 14(11):e0223738
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta (BBA) 1778(2):357–375
Hu J, Chen C, Zhang S, Zhao X, Xu H, Zhao X, Lu JR (2011) Designed antimicrobial and antitumor peptides with high selectivity. Biomacromol 12(11):3839–3843
Hu C, Chen X, Huang Y, Chen Y (2018a) Co-administration of iRGD with peptide HPRP-A1 to improve anticancer activity and membrane penetrability. Sci Rep 8(1):2274
Hu C, Chen X, Huang Y, Chen Y (2018b) Synergistic effect of the pro-apoptosis peptide kla-TAT and the cationic anticancer peptide HPRP-A1. Apoptosis 23(2):132–142
Hu C, Huang Y, Chen Y (2018c) Targeted modification of the cationic anticancer peptide HPRP-A1 with iRGD to improve specificity, penetration, and tumor-tissue accumulation. Mol Pharm 16(2):561–572
Huang Y (2016) Anticancer activity and mechanism of alpha-helical antimicrobial peptide. Drug Des 5:e133
Johnstone SA, Gelmon K, Mayer LD, Hancock RE, Bally MB (2000) In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des 15(2):151–160
Kang H-L, Lee W-K, Song J-Y, Choi S-H, Park S-G, Ryu B-D, Lee E-J, Kim J-S, Park J-U, Baik S-C (2005) Helicobacter pylori Strain 51 (Korean Isolate): Ordered Overlapping BAC Library, Combined Physical and Genetic Map, and Comparative Analysis with H. pylori Strain 26695 and Strain J99. J Microbiol Biotechnol 15(4):844–854
Karpiński T (2012) New peptide (Entap) with anti-proliferative activity produced by bacteria of Enterococcus genus, Habilitation thesis. Scientific Publisher of Poznań University of Medical
Karpiński T, Szkaradkiewicz A, Gamian A (2013) New enterococcal anticancer peptide. In 23rd European Congress of Clinical Microbiology and Infectious Diseases. Berlin, Germany
Lee DG, Park Y, Kim HN, Kim HK, Kim PI, Choi BH, Hahm K-S (2002b) Antifungal mechanism of an antimicrobial peptide, HP (2–20), derived from N-terminus of Helicobacter pylori ribosomal protein L1 against Candida albicans. Biochem Biophys Res Commun 291(4):1006–1013
Lee DG, Kim HN, Park Y, Kim HK, Choi BH, Choi C-H, Hahm K-S (2002a) Design of novel analogue peptides with potent antibiotic activity based on the antimicrobial peptide, HP (2–20), derived from N-terminus of Helicobacter pylori ribosomal protein L1. Biochim Biophys Acta 1598(1–2):185–194
Lee DG, Hahm K-S, Park Y, Kim H-Y, Lee W, Lim S-C, Seo Y-K, Choi C-H (2005) Functional and structural characteristics of anticancer peptide Pep27 analogues. Cancer Cell Int 5(1):21
Lee JK, Gopal R, Park S-C, Ko HS, Kim Y, Hahm K-S, Park Y (2013) A proline-hinge alters the characteristics of the amphipathic α-helical AMPs. PLoS ONE 8(7):e67597
Lee J-K, Park S-C, Hahm K-S, Park Y (2014) A helix-PXXP-helix peptide with antibacterial activity without cytotoxicity against MDRPA-infected mice. Biomaterials 35(3):1025–1039
Liu R, Ni Y, Song J, Xu Z, Qiu J, Wang L, Zhu Y, Huang Y, Ji M, Chen Y (2019) Research on the effect and mechanism of antimicrobial peptides HPRP-A1/A2 work against Toxoplasma gondii infection. Parasite Immunol 41(5):e12619
Maher S, McClean S (2008) Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem Pharmacol 75(5):1104–1114
Mai X, Huang J, Tan J, Huang Y, Chen Y (2015) Effects and mechanisms of the secondary structure on the antimicrobial activity and specificity of antimicrobial peptides. J Pept Sci 21(7):561–568
Marqus S, Pirogova E, Piva TJ (2017) Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci 24(1):21
Matsumoto Y, Miwa S, Zhang Y, Zhao M, Yano S, Uehara F, Yamamoto M, Hiroshima Y, Toneri M, Bouvet M (2015) Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget 6(13):11369
McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H (2009) Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz 104(2):320–344
Nauts HC, Swift WE, Coley BL (1946) The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, MD, reviewed in the light of modern research. Can Res 6(4):205–216
Park Y, Hahm K-S (2005) Effects of N-and C-terminal truncation of HP (2–20) from Helicobacter pylori ribosomal protein L1 (RPL1) on its anti-microbial activity. Biotechnol Lett 27(3):193–199
Park Y, Lee DG, Kim HN, Kim HK, Woo E-R, Choi C-H, Hahm K-S (2002) Importance of the length of the N-and C-terminal regions of Helicobacter pylori ribosomal protein L1 (RPL1) on its antimicrobial activity. Biotechnol Lett 24(14):1209–1215
Park Y, Park SC, Park HK, Shin SY, Kim Y, Hahm KS (2007) Structure-activity relationship of HP (2–20) analog peptide: enhanced antimicrobial activity by N-terminal random coil region deletion. Pept Sci 88(2):199–207
Park S-C, Kim M-H, Hossain MA, Shin SY, Kim Y, Stella L, Wade JD, Park Y, Hahm K-S (2008) Amphipathic α-helical peptide, HP (2–20), and its analogues derived from Helicobacter pylori: pore formation mechanism in various lipid compositions. Biochim Biophys Acta 1778(1):229–241
Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry 31(49):12416–12423
Pütsep K, Normark S, Boman HG (1999b) The origin of cecropins; implications from synthetic peptides derived from ribosomal protein L1. FEBS Lett 451(3):249–252
Pütsep K, Brändén C-I, Boman HG, Normark S (1999a) Antibacterial peptide from H. pylori. Nature 398(6729):671
Rathinam VA, Zhao Y, Shao F (2019) Innate immunity to intracellular LPS. Nat Immunol 20(5):527–533
Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, Rusk AW, Tung D, Miller M, Roix J (2014) Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med 6(249):249ra111
Schweizer F (2009) Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol 625(1–3):190–194
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462(1–2):55–70
Sieber SA, Marahiel MA (2003) Learning from nature’s drug factories: nonribosomal synthesis of macrocyclic peptides. J Bacteriol 185(24):7036–7043
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Song S, Vuai MS, Zhong M (2018) The role of bacteria in cancer therapy–enemies in the past, but allies at present. Infect Agents Cancer 13(1):9
Tan TG, Mui E, Cong H, Witola WH, Montpetit A, Muench SP, Sidney J, Alexander J, Sette A, Grigg ME (2010) Identification of T. gondii epitopes, adjuvants, and host genetic factors that influence protection of mice and humans. Vaccine 28(23):3977–3989
Wiemann B, Starnes CO (1994) Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 64(3):529–564
Woo E-R, Lee DG, Chang Y, Park Y, Hahm K (2002) Virus-cell fusion inhibitory activity of novel analogue peptides based on the HP (2–20) derived from N-terminus of Helicobacter pylori Ribosomal Protein L1. Protein Pept Lett 9(6):477–486
Yamada T, Goto M, Punj V, Zaborina O, Chen ML, Kimbara K, Majumdar D, Cunningham E, Gupta TKD, Chakrabarty AM (2002) Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci 99(22):14098–14103
Zare-Bidaki M, Assar S, Hakimi H, Abdollahi SH, Nosratabadi R, Kennedy D, Arababadi MK (2016) TGF-β in toxoplasmosis: friend or foe? Cytokine 86:29–35
Zhang D, Fan L, Ma L, Liu J, Zhou K, Song X, Sun M, Mo X, He C, Chen Y (2019) Helicobacter pylori ribosomal protein-A2 peptide/silk fibroin nanofibrous composites as potential wound dressing. J Biomed Nanotechnol 15(3):507–517
Zhao L, Huang Y, Gao S, Cui Y, He D, Wang L, Chen Y (2013) Comparison on effect of hydrophobicity on the antibacterial and antifungal activities of α-helical antimicrobial peptides. Sci China Chem 56(9):1307–1314
Zhao J, Huang Y, Liu D, Chen Y (2015a) Two hits are better than one: Synergistic anticancer activity of α-helical peptides and doxorubicin/epirubicin. Oncotarget 6(3):1769
Zhao J, Hao X, Liu D, Huang Y, Chen Y (2015b) In vitro characterization of the rapid cytotoxicity of anticancer peptide HPRP-A2 through membrane destruction and intracellular mechanism against gastric cancer cell lines. PLoS ONE 10(9):e0139578
Zhu J, Huang Y, Chen M, Hu C, Chen Y (2019) Functional synergy of antimicrobial peptides and chlorhexidine acetate against gram-negative/gram-positive bacteria and a fungus in vitro and in vivo. Infect Drug Resist 12:3227
Author information
Authors and Affiliations
Contributions
Performed conceptualization, investigation, and writing [AY and SS], reviewed and edited the manuscript [MK, KG, AM, AA, SMH].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yaghoubi, A., Khazaei, M., Ghazvini, K. et al. Peptides with Dual Antimicrobial-Anticancer Activity Derived from the N-terminal Region of H. pylori Ribosomal Protein L1 (RpL1). Int J Pept Res Ther 27, 1057–1067 (2021). https://doi.org/10.1007/s10989-020-10150-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10150-3