Abstract
Antimicrobial peptides (AMPs) from prokaryotic source also known as bacteriocins are ribosomally synthesized by bacteria belonging to different eubacterial taxonomic branches. Most of these AMPs are low molecular weight cationic membrane active peptides that disrupt membrane by forming pores in target cell membranes resulting in cell death. While these peptides known to exhibit broad-spectrum antimicrobial activity, including antibacterial and antifungal, they displayed minimal cytotoxicity to the host cells. Their antimicrobial efficacy has been demonstrated in vivo using diverse animal infection models. Therefore, we have discussed some of the promising peptides for their ability towards potential therapeutic applications. Further, some of these bacteriocins have also been reported to exhibit significant biological activity against various types of cancer cells in different experimental studies. In fact, differential cytotoxicity towards cancer cells as compared to normal cells by certain bacteriocins directs for a much focused research to utilize these compounds as novel therapeutic agents. In this review, bacteriocins that demonstrated antitumor activity against diverse cancer cell lines have been discussed emphasizing their biochemical features, selectivity against extra targets and molecular mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the leading cause of death worldwide (http://seer.cancer.gov/statfacts) and death rate is increasing significantly in the last few decades (Siegel et al. 2015; Howlader et al. 2015). Globally, cancer is the second leading cause of mortality and killed about 8.8 million people according to a recent report by the World Health Organization (WHO) (www.who.int/mediacentre/factsheets/fs297/). In addition to mortality, adverse effects of treatment associated with human cancers pose significant global psychological and economic burden to the affected nations. On the other hand, an exponential advance in biotechnology in the recent past is continuously leading to a greater understanding about many human diseases (Jemal et al. 2009). Altered cellular physiology is the characteristic of cancer cells, which leads to abnormal proliferation of cells. Thus, cancerous cells show certain unique characteristics such as initiating growth signals on their own and do not respond to the mechanisms controlling cellular growth. These cells develop the capacity for limitless replication and stimulate new blood vessel development in order to allow tumor growth. The altered cells enable to invade tissues locally and metastasize distantly all across the body (Hanahan and Weinberg 2000). Typically, cancer patients are treated with surgery, radiotherapy, and chemotherapy to remove growing tumor. However, surgical resection of cancer is a limited approach, often mutilating and mostly to be followed up by chemotherapy and radiotherapy. Surgery and radiotherapy are effective against localized cancers but not suitable for disseminated cancers where chemotherapy remains the sole choice. These anticancer therapies including chemotherapy are only reasonably effective along with the serious side effects due to non-selectivity of target cells, recurrence potentials, and emergence of multidrug-resistant cancerous cells (Lao et al. 2014; Klener 1999; Porta et al. 2015). Considering these constraints, few studies have been undertaken in the recent past relating to the action of AMPs having antitumor activity, with the intent of reducing the number of cases of tumor growth (Joo et al. 2012; Chu et al. 2015). Therefore, there is an extensive and urgent need of developing novel, more selective, effective, less toxic, and safe biologic therapies such as AMPs against rapidly growing cancer cases.
Bacteriocins as anticancer therapeutics
General perspectives
A multitude of metabolites including antimicrobial peptides (AMPs), such as ribosomally synthesized bacteriocins, are produced by various bacterial strains as a strategy to overcome competitive antagonism by other invading bacteria during habituation of a specific niche (Fons and Tuomo Karjalainen, 2000). Although bacteriocins were previously thought to inhibit the growth of only closely related strains or species, but in the recent past they were reported with broad spectrum of antimicrobial activity. In addition, they exhibit selective activity against distantly related bacteria and inhibited the growth of various cancerous cell lines (Joo et al. 2012; Riley and Wertz 2002; Coburn and Gilmore 2003; Dethlefsen et al. 2006). Most of these bacteriocins with anticancer properties were found to be cationic and amphiphilic in nature, that were often produced by bacteria existing in diverse environments. These cationic peptides are also known to be “membrane active” as they interact with a negative surface charge on the cell in contact (Wang et al. 2013; Johnstone et al. 2000; Zhao et al. 2015; Laverty and Gilmore 2014). Killing of cancer cells is usually reported to mediate via cell membrane lytic effect due to the presence of increased number of negatively charged molecules on their surface (Riedl et al. 2011). While a few cationic AMPs are reported to disrupt the integrity of mitochondrial membrane and cause apoptosis in cancer cells (Cho et al. 2012; Chen et al. 2012), others are known to inhibit blood vessel development (angiogenesis) which is essentially required for cancer progression (Mader and Hoskin 2006).
Historic perspectives
Utilization of microbes or their products against cancer is reported dating back to the nineteenth century. For example, culture supernatants of bacteria like Streptococcus pyogenes and Serratia marcescens preparation called Coley’s toxins were given to patients with unresectable tumors. Evidently, patients with regression of malignant tumors treated with this toxin were cured to good health (Coley 1910; Wiemann and Starnes 1994). Subsequently, induction of enhanced secretion of tumor necrosis factor (TNF-α) in the body of a patient was revealed to be the main factor accountable for therapeutic effect of Coley’s toxins. Further, the role of TNF-α factor was also confirmed in animal models (Carswell et al. 1975). On the other hand, it has been reported that the microbial pathogens may proliferate inside the hypoxic cancer lesions, and concurrently, stimulating host immune system against cancer progression during the infection. The vaccine strain BCG (Mycobacterium bovis Calmette-Guerin) is an example that was used to treat superficial bladder cancer (Alexandroff et al. 1999; Gandhi et al. 2013; Herr and Morales 2008; Kawai et al. 2013). Members of the genus Clostridium like C. novyi-NT are also found to be promising in bacteriolytic therapy to treat various tumors (Dang et al. 2001; Maletzki et al. 2010; Agrawal et al. 2004) as they were found to be effective in reducing tumor growth. In the recent past, it has been shown that the selected microbial infections lead to immune activation via macrophages and lymphocytes, resulting in production of anticancer agents like TNF-α (Patyar et al. 2010). Similarly, other microbial metabolites also displayed potential anticancer properties, for example, nisin, the first lantibiotic bacteriocin approved by the Food and Drug Administration, is recently documented as a potential anticancer bacteriocin (Joo et al. 2012; Kamarajan et al. 2015). Currently, several low molecular weight AMPs are emerging as promising novel cancer therapeutics. Therefore, this review aims to provide a detailed insight into the bacteriocins having anticancer properties and their biochemical structures and potential as anticancer therapeutic agents.
Characteristics of bacteriocins relevant to anticancer potential
Most of the bacteriocins are cationic in nature
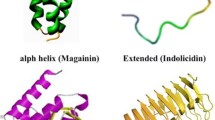
More than 80% of known bacteriocins are cationic in nature owing to an excess number of lysine or arginine amino acid residues (Hammami et al. 2007, 2010). Usually, they are hydrophobic peptides containing between 20 and 60 amino acids in length (Nes and Holo, 2000; Ennahar et al. 2000), though they were found to be unstructured in aqueous solution, but displayed α-helical structure forming tendency when exposed to trifluoroethanol or anionic phospholipids of biological membranes. Additionally, disulfide or a covalent bond formation in certain peptides helps in acquiring loop structure and its maintenance. In particular, the presence of intramolecular ring structures formed as a result of thioether bonds between amino acids is a characteristic feature of the lantibiotics, which is a predominant bacteriocin group (Moll et al. 1999). It was noted that a number of lantibiotics resemble cationic antimicrobial peptides (cAMPs) by virtue of having long linear structures, a cationic charge, and their ability to form pores in cell membranes (Gunther 1991; Smith and Hillman 2008; Sahl 2000). Other bacteriocins largely produced by Gram-positive bacteria resemble antimicrobial peptides produced by eukaryotes, such as defensins (Papagianni 2003; Singh et al. 2014) with cationic and amphiphilic nature, and membrane-permeabilizing properties (Breukink et al. 1999).
Bacteriocins are low cytotoxic in nature
Various bacteriocins have been used or consumed naturally in fermented and non-fermented foods (Settanni and Corsetti 2008; Chen and Hoover 2003) from ages, which includes nisin A (Cutter and Siragusa 1998), enterocin 4 (Nuñez et al. 1997), leucocin A (Leisner et al. 1996), lactocin 705 (Vignolo et al. 1996), and enterocin (Aymerich et al. 2000). Interestingly, these are non-toxic as lack of toxicity for these lantibiotics has been demonstrated in several studies that allowed their widespread clinical applications such as probiotics (bacteriocin-producing strains) and antimicrobials in health care and in food industries (Bastos et al. 2010; Pieterse and Todorov 2010; Murinda and Rashid 2003; Jasniewski et al. 2009). Antimicrobial substances from probiotics have been considered to be “Generally regarded as safe molecules” that confer health benefits to host. Contemporary investigations have highlighted the low cytotoxicity of certain bacteriocins, for example, laterosporulin a class IId defensin-like bacteriocin that provided a missing link between prokaryotic and eukaryotic defensins (Singh et al. 2014). It did not show hemolysis even at significantly higher concentrations of MIC values observed for various indicator microbes (Singh et al. 2014). Similarly, carnobacteriocins BM1 and B2 classified under class IIa bacteriocins also did not exhibit cytotoxicity against Caco-2 (human epithelial colorectal adenocarcinoma) cells, even at 100× higher than MIC values against bacterial strains (Jasniewski et al. 2009). Likewise, penisin, a class Ia lantibiotic, was also demonstrated to be non-cytotoxic against RBCs and Raw (mouse macrophages) and RWPE-1 (human prostate epithelial) cells at 20× higher concentration than MIC values against indicator strains (Baindara et al. 2015). However, peptides like MccE492, a class IId bacteriocin, induced biochemical and morphological changes as observed in apoptosis at low or intermediate concentrations leading to a necrotic phenotype at higher concentrations in cancer cells (Hetz et al. 2002). Therefore, such ability of bacteriocins can be exploited to develop novel anticancer peptides naturally or by recombinant technologies and peptide engineering (Lagos et al. 2009). Their low cytotoxic nature in the context of normal host cells makes them particularly an appealing target for novel anticancer agent development.
In vivo efficacy of bacteriocins
In vitro studies demonstrated the prospective role of bacteriocins as alternative to various therapeutic applications due to their minimal cytotoxic nature. Further, in vivo replication efficacy is essential to potentiate these molecules in clinically relevant situations as novel therapeutic candidates. Many bacteriocins have already been extensively examined from this perspective as shown in Table 1. For example, bacteriocins like Pep5 and epidermin have been reported to prevent Staphylococcus and/or Enterococcus infections in and on catheter tubing (Fontana et al. 2006). In fact, microbisporicin, a novel lantibiotic, demonstrated to have potential inhibitory effect against murine septicemia caused by S. aureus upon intravenous and subcutaneous administration (Castiglione et al. 2008). In vivo efficacy of a lantibiotic NAI-107 has been documented against beta-lactam-resistant S. aureus in a neutropenic murine thigh infection model that revealed it to be effective in comparison to vancomycin and linezolid (Jabés et al. 2011). Similarly, lacticin 3147 has been shown to prevent the systemic infections of S. aureus in a mouse peritonitis model (Piper et al. 2012). Mutacin (B-Ny266), another bacteriocin produced by Streptococcus mutans, was found effective against S. aureus infection in an intra-peritoneal mouse model and was comparable to vancomycin (Mota-Meira et al. 2005). Importantly, lantibiotic mersacidin has been known to eliminate methicillin-resistant S. aureus (MRSA) colonization in a mouse rhinitis model (Kruszewska et al. 2004). Other notable bacteriocins include a novel type B lantibiotic NVB302 effective to manage Clostridium difficile infection (CDI) in an in vitro human gut model and this lantibiotic is now under phase I clinical trials (Crowther et al. 2013). Piscicolin 126, produced by Carnobacterium piscicola, is an antilisterial bacteriocin retained its antimicrobial activity under in vivo conditions when administered intravenously (Ingham et al. 2003). Penisin, a recently characterized lantibiotic, displayed antimicrobial activity against S. aureus in a thigh infection model and thus increased survival rate of mice (Baindara et al. 2015). Nisin, the first lantibiotic used as a food preservative, was extensively studied and results suggested it to be effective against Streptococcus pneumonia when compared to vancomycin in an intravenous regimen (Goldstein et al. 1998). Nisin has also been known to have antibacterial and spermicidal activities in in vivo mice model and proved as a potential vaginal contraceptive (Aranha et al. 2004; Reddy et al. 2004). Similarly, nisin F, a natural variant of nisin found to be effective against S. aureus in vivo while incorporating into bone cement (van Staden et al. 2012), showed protective ability to the respiratory tract against pathogens when administered intra-nasally (De Kwaadsteniet 2009). Nisin A along with Nisin V and Nisin F also showed protection against Listeria monocytogenes in a murine infection model (Campion et al. 2013) and inhibited S. aureus in peritoneal cavity of mice model (Brand et al. 2010). Nisin variants are being used as sanitizers against pathogenic Staphylococcus and Streptococcus species causing mastitis in lactating cows (Cao et al. 2007; Wu et al. 2007; Fernández et al. 2008). Since membrane lytic effects of AMPs are considered as the major mechanism, this mechanism is also attributed for their anticancer activity, thus similar mechanisms may remain efficacious in the case of anticancer actions of AMPs. Though few preliminary in vivo investigations with anticancer peptides (ACPs) are available, but they need further extensive investigations.
Bacteriocins are docile to bioengineering
Bacteriocins are small cationic peptides encoded by genes and due to this peptide nature, they are extremely more acquiescent to engineering to increase activity and specificity towards their target when compared with classical antibiotics (Perez et al. 2014). Bacteriocin bioengineering can be done by manipulating the bacteriocin biosynthetic genes through cloning of these genes and in vitro reconstitution of the biosynthesis process required for antimicrobial peptide production (Cotter 2012). However, bacteriocin without posttranslational modifications can be fully or partially synthesized by chemical synthesis process. These engineered peptides have been proven important for further understanding of their activity and structure–function relationships using site-directed mutagenesis to reveal amino acid residues essentially required for activity (Wang et al. 2014; Oppegård et al. 2007; Sun et al. 2015; Haugen et al. 2008). Further, in silico approach of bacterial genome mining and metagenomic DNA analysis provide information about many unexpressed bacteriocin gene clusters, which may be further used for gene synthesis and engineering (Walsh et al. 2015; Mohimani et al. 2014; Letzel et al. 2014). Such technologic advances can further enhance the targeted activity, efficacy, and safety of natural as well as recombinant ACPs.
Bacteriocin interaction with cancer cell membranes
In eukaryotic cells, membrane phospholipids are distributed unevenly between two layers of the lipid bilayer (Op den Kamp 1979; Verkleija et al., 1973; Fadeel and Xue 2009) with phosphatidylserine localized absolutely in the inner leaflet (Rothman and Lenard 1977; Connor et al. 1989) that plays an important role in cell physiology (Bevers et al. 1982; Manno et al. 2002). Interestingly, cancer cell membranes display overexpression of phosphtidylserine (Dobrzyńska et al. 2005; Utsugi et al. 1991) and O-glycosylated mucins (Yoon et al. 1996; Schwartz et al. 1992; Burdick et al. 1997) on the outer membrane leaflet in comparison to non-transformed cells. Thus, they impart a net negative charge on cell membranes, which enables electrostatic interactions between cancerous cell surface and cationic bacteriocins. In contrast, healthy eukaryotic cells contains zwitter-ionic phosphatidylcholine in the outer membrane leaflet that confers an overall neutral charge on these cells resulting in significant reduction of electrostatic interactions. In addition, change of membrane fluidity in cancer cells when compared with their healthy counterparts affects tumor cell adhesion which is related to cancer metastases (Zeisig et al. 2007; Nakazawa and Iwaizumi 1989; Sok et al. 1999; Kozłowska et al., 1999). Plasma membrane fluidity tends to increase metastatic capability and may further assist cancer cell membrane deterioration by membrane interaction of ACPs. Another significant attribute affecting the targeted/selective activity of ACPs is the presence of abundant and irregular microvilli on cancerous cell surface in comparison to their healthy counterpart, a feature adopted for increasing metastatic potential (Chaudhary and Munshi 1995; Domagala 1980; Ren et al. 1990). The net negative charge along with overall increased surface area in cancer cells may enable ACP-mediated cytotoxicity with greater chances of large number of peptide molecules to interact with cellular surface. Collectively, all these properties of cancer cells may assist the ACPs to interact with cell membrane and subsequent killing of cancer cells selectively without affecting healthy eukaryotic cells. Abundance of anionic lipid cardiolipin in mitochondrial membrane of eukaryotic cells result in negatively charged surface of mitochondria (Schenkel and Bakovic 2014; de Kroon et al., 1997; Wriessnegger et al. 2009). Interestingly, mitochondrial membrane is believed to share common ancestry as it originated from endosymbiotic prokaryotes (Gray et al. 2001; Gray 2012). This further may facilitate the ACPs to disrupt the integrity of mitochondrial membrane and resulting in release of several proteins such as cytochrome C and stimulate the apoptotic cell death pathway (Kim et al. 2006; Smolarczyk et al. 2010). Nevertheless, few studies have suggested that many ACPs with cytotoxic properties may cause cancer cell death by necrosis via cell membrane damage (Ye et al. 2004; Vaucher et al. 2010; Maher and McClean 2006).
Contemporary bacteriocins investigated as potential anticancer peptides
Nisin
Nisin (3.49 kDa) secreted by Lactococcus lactis, is a lantibiotic class of bacteriocins composed 34 amino acids. Recent reports showed that nisin decreases head and neck squamous cell carcinoma (HNSCC) tumorigenesis by increasing cell apoptosis through activation of CHAC1, increased calcium influxes, and induction of cell cycle arrest (Table 2 and Fig. 1). In fact, nisin is safe for human consumption as approved by Food and Drug Administration and currently used in food preservation and is a potential cancer therapeutic (Joo et al. 2012). Nisin ZP (3.47 kDa, 34 amino acids), a natural variant of nisin, induced a high level of apoptosis in HNSCC cells. Indeed, nisin ZP displayed gradual increase in apoptosis with the increase of concentration. Induction of apoptosis was through a calpain-dependent pathway in HNSCC cells. Similarly, apoptosis was also induced in human umbilical vein endothelial cells (HUVEC) and reduced intra-tumoral microvessel density. Long-term treatment with nisin ZP enhanced longevity and maintained the normal histologic structure of the tissue without any evidence of inflammation, fibrosis, or necrosis. It was considered to be a potential novel therapeutic for management of squamous cell carcinoma (Kamarajan et al. 2015). Very recently, the cytotoxic activity of nisin was evaluated against colon cancer SW480 cells where it showed significant antiproliferative impact and raised the apoptotic index (bax/bcl-2 ratio). Further, intrinsic apoptotic pathway was suggested to be responsible for this cytotoxicity induced by nisin (Ahmadi et al. 2017). Moreover, the synergistic effect of nisin with doxorubicin showed significant reduction in tumor volumes when compared to individual treatments of these compounds (Kaur and Kaur 2015). The combined treatment showed apoptosis in tumor tissues as chromatin condensation and marginalization of nuclear material was observed; thus, nisin can be a complement to doxorubicin as chemotherapeutic drug.
Plantaricin A
Plantaricin A (PlnA) is another low molecular weight peptide (2.98 kDa) containing 26 amino acids and secreted by strains of Lactobacillus plantarum C11, WCFS1, and V90 (Table 2). It displayed broad-spectrum antibacterial activity with membrane permeabilizing property (Fig. 1). PlnA could permeabilize eukaryotic cells also with a potency that differed between various cell types. It showed electrostatic attraction to negatively charged phospholipids in the membrane as shown by microfluorometric techniques. Interaction with glycosylated membrane proteins is probably being the first and essential first step for PlnA interaction with membrane. Activities against different cell types including cancerous cells is attributed to different glycosylation patterns (Sand et al. 2010, 2013; Andersland et al. 2010). However, others demonstrated similar sensitivity of PlnA towards cancerous lymphocytes, neuronal cells, kidney cortex, and vero cells from green monkey and human Caki-2 cells that were permeabilized by PlnA (Kristiansen et al. 2005; Fialho et al. 2012). In further support, three-dimensional structure PlnA determined by nuclear magnetic resonance spectroscopy revealed effective structure that can be positioned in membrane interface to engage in chiral interaction with receptor (Jeuken et al. 2000).
Azurin
Azurin from Pseudomonas aeruginosa is a member of the cupredoxin family of redox proteins with molecular weight of about 14 kDa (Fialho et al. 2012) as shown in Table 2. Though different variants of azurins vary in sequence homology (50 and 90%), their structure is highly conserved. They display a rigid β-sandwich core formed by two main β-sheets in their structure (Jeuken et al. 2000). They are reported to exhibit anticancer properties which can preferentially penetrate cancer cells like breast cancer (MCF-7), melanoma (UISO-Mel-2), and osteosarcoma (U2OS) cells to display apoptotic effects, but did not show any effect against normal cells (Punj et al. 2004; Goto et al. 2003; Zaborina et al. 2000; Yamada et al. 2002; Gupta 2002; Yang et al. 2005). A short variant of azurin p28 peptide with 28 amino acids was identified to act as potential protein transduction domain (PTD) in cancer cells (Yamada et al. 2005). This derivative p28 was also reported to inhibit cancer cell growth and prevention of tumor emergence (Bizzarri et al. 2011; Yamada et al. 2013). Interestingly, similar peptides were detected from Lactobacillus salivarius (Shaikh et al. 2012) and other microbes from the human gut (Nguyen 2016) with high binding affinities to cancer targets (Fig. 2).
Induction of apoptosis by these peptides was further confirmed using caspase-mediated mitochondrial pathways as increased p53 intracellular level was observed during the treatment (Yamada et al. 2002). Azurin and peptide p28 displayed selective entry and cytotoxic effects against acute and chronic myeloid leukemia cell line by induction of apoptosis and interfering with angiogenesis of human umbilical vein endothelial cells (Kwan et al., 2009; Mehta et al. 2011). Moreover, they were found to be proficient to interfere in oncogenic transformation as they could halt the formation of precancerous lesions in a dimethyl-1,2-benzanthracene exposed mouse organ culture model (Mehta et al. 2010). Additionally, it was shown to be effective against tumor growth in nude mice that were xenografted with UISO-Mel-2 and MCF-7 cells (Punj et al. 2004; Yang et al. 2005). They also induced apoptosis to inhibit the tumor growth in Dalton’s lymphoma-bearing ascites mice model (Ramachandran and Mandal 2011). Interestingly, p28 displayed significant antitumor activities as confirmed using nude mice xenografted with MCF-7 cells (Yamada et al. 2009). Similar results were shown for p28 as it suppressed tumor growth in HCT-116 (colon cancer), UISO-Mel-23 (melanoma), and MDA-MB-231 (breast cancer) cell xenografts in nude mice (Jia et al. 2011). It was also demonstrated that azurin secretion occurred by producing strains in the presence of cancer cells (Mahfoouz et al. 2007). Overall, azurins are antitumor peptides that display induction of apoptosis through p53 stabilization, inhibition of angiogenesis, and binding with ephrin receptor kinases (Mehta et al. 2011; Chaudhari et al. 2007; Riedl and Pasquale, 2015). Most importantly, p28 did not cause any immune reaction and toxicity in mice as well as in non-human primates, highlighting its potential application as a therapeutic agent (Jia et al. 2011). Thus, azurin differentiates itself from other available drugs and brings about an interesting prospect to investigate other bacteriocins, which might have similar or even better anticancer properties.
Pyocins
Pyocins are high molecular weight (> 10 kDa) AMPs (Fig. 1) secreted by strains of Pseudomonas aeruginosa (Michel-Briand and Baysse 2002). These peptides are constitutive in chromosomes, but environmental factors such as UV radiation ormitomycin C can initiate the biological activity (Kageyama 1964). Pyocins have been categorized into three types: R-, F-, and S-type. R- and F-type pyocins resemble the tails of bacteriophages with differences in flexibility and contractility of their structure. Specifically, R types are non-flexible and contractile entities, whereas F-type pyocins are just the reverse with flexible and non-contractile rod-like structures. R-type pyocins display nuclease and protease resistance with depolarization activity on membranes that subsequently leads to pore formation in membrane. The killing activity carried by large component may be variable for different varieties of pyocins. While pyocins S1, S2, S3, and AP41 act by DNase activity, pyocin S4 acts through tRNase activity; however, pyocin S5 revealed channel-forming activity. Further, a small component for S-type pyocins acts as immunity protein interacting with large component (Michel-Briand and Baysse 2002). For the first time, a partially purified pyocin from P. aeruginosa was shown to inhibit the growth of mouse fibroblast cell line L6OT by Farkas-Himsley and Cheung (1976). Subsequently, anticancer activity of pyocin S2 was observed against diverse cancerous cell lines like HeLa, AS-II, and mKS-A TU-7. However, no such effects were observed against normal mice cells (BALB/3T3), rat kidney cells, and human lung cells. Recently, purified pyocin S2 from P. aeruginosa 42A was reported to exhibit cytotoxic effects against tumor cell lines HepG2 (human hepatocellular carcinoma) and Im9 (human immunoglobulin-secreting cell line derived from multiple myelomas) without affecting normal human fetal foreskin fibroblasts (Abdi-Ali et al. 2004).
Colicins
The plasmid-encoded colicins are usually found to be high molecular weight AMPs with molecular weight > 20 kDa (Fig. 1). These were first observed in Escherichia coli (Table 2) and named as colicin (Feldgarden and Riley 1999; Cascales et al. 2007; Braun et al. 1994; Gratia 1925). Bacterial strains belonging to the family Enterobacteriaceae are known to produce this class of AMPs. Colicin secretion is a result of stress response (Smarda and Smajs 1998). These are three domain proteins and their cellular killing mechanism is accomplished in three distinct steps where the central region acts as a receptor domain, while N-terminal and C-terminals are translocation and cytotoxicity domains, respectively (Helbig and Braun 2011; Arnold et al. 2009). However, unstructured N-terminal was also shown to exhibit antibacterial activity (Johnson et al. 2013). Cytotoxic and cytocidal activities were observed when colicin E3 was used against HeLa cells with specific cleavage of rRNA (Smarda et al., 1978). Studies on colicins E1-E5 and K revealed cellular killing activity against hamster fibroblast V79 cell lines (Smarda 1987). However, selective anticancer activities of colicins such as A, E1, E3, and U were demonstrated to cause cell cycle alterations in human fibroblast cell line (MRC5). Similarly, they were reported against cancerous cell lines like human breast cancer cell lines MCF7 and MDA-MB-231, osteosarcoma cell line HOS, HS913T and MRC5 fibroblasts with predefined p53 gene mutations, but the activity was less towards fibroblasts MRC5 (Chumchalová and Smarda 2003). In another investigation, colicin E3 showed cidal effect against human origin carcinoma cells and HeLa cells (Smarda et al., 1978) with dose-dependent inhibitory response to murine leukemia cells P388 (Fuska et al. 1979). Another evidence was provided in a different study where murine lymphoma cell lines showed decrease in viability with the treatment of colicins A and E2 (Smarda and Oravec 1989). Colicins E1 and E3 destroyed oncogene v-myb transformed chicken monoblasts without affecting the cell cycle events (BM2), indicating the probability of necrosis instead of apoptosis (Smarda et al. 2001). These studies also highlighted the specificity towards anticancer properties of colicins. Several in vivo investigations in mice also supported evidence to the anticancer potential of colicins through intratumor injections that decreased tumor volume (Cursino et al. 2002; Farkas-Himsley et al. 1995) and enhanced longevity in mice with transplanted LP-2 plasmacytoma (Chumchalová and Smarda 2003).
Microcins
Members of the family Enterobacteriaceae are known to produce microcins that are smaller in size (< 10 kDa) in comparison to colicins. So far, there are seven microcins studied in detail and reported (Table 2). The biosynthetic gene clusters of microcins typically contain microcin precursor, ABC transporter for peptide transport and defense, and structural modifications. Genetic machinery such as plasmids and chromosomes harbors most of the secretion factors for the microcin production (Duquesne and Destoumieux-Garzón, 2007). This group includes some low molecular weight molecules with distinct structural modifications, e.g., microcins B17, C7, C51, and J 25 (Agarwal et al. 2011; Metlitskaya et al. 1995; Wilson et al. 2003), others with disulfide bonds. However, microcins like L, V, and 24 did not show any posttranslational modifications (Pons et al. 2004; Jeziorowski and Gordon, 2007; Frana et al. 2004) and microcins E492, M, H47, and I47 have been reported as linear peptides (de Lorenzo 1984; Vassiliadis et al. 2010). Microcin E492 (7.8 kDa) from Klebsiella pneumoniae strain RYC492 is reported to inhibit a large number of pathogenic bacteria belonging to genera including Escherichia, Klebsiella, Salmonella, Citrobacter, and Enterobacter (de Lorenzo 1984). Microcins like E492 were reported to display activity against various human cancer cell lines such as HeLa, Jurkat, RJ2.25, and colorectal carcinoma cells. However, it did not inhibit normal cells such as bone marrow cells, splenocytes, KG-1, human tonsil cells, and nontumor macrophage-derived cells (Hetz et al. 2002). The principal mechanism of action for microcin E492 involved pore formation in the cell membrane and thus disruption of membrane potential (de Lorenzo and Pugsley 1985; Lagos et al. 1993). Studies also emphasized cellular apoptotic features including cell shrinkage, DNA fragmentation, phosphatidylserine release, caspase activity, loss of mitochondrial membrane potential, and release of intracellular calcium ions undermining the significance of apoptosis as the major mechanism of cellular death (Hetz et al. 2002). In fact, microcin E492 was delivered into the host by plasmid-induced bacteriocin production using E. coli VSC257pJEM15 as a safer, non-toxic, non-immunogenic method (Hetz et al. 2002). Moreover, systemic administration of the peptide in mice leads to selective colonization of a probiotic E. coli strain Nissle191 in cancerous cells (Brader et al. 2008). Additionally, E. coli strain Nissle1917 producing both microcin M and microcin H47 is used as a probiotic under the name Mutaflor, that has been extensively used for management of a variety of intestinal diseases (Rembacken et al. 1999; Kruis 2004). These findings suggest E. coli Nissle1917 strain is a suitable carrier for the delivery of microcin E492 in preclinical investigations. Antitumor activities of microcin E492 were shown in a preclinical nude mouse model xenografted with human colorectal carcinoma cells (Lagos et al. 2009).
Pediocins
Pediocins are small, cationic, plasmid-encoded AMPs (Table 2) yielded by members of Pediococcus and other lactic acid-producing genera (Papgianni and Anastasiadoue 2009). Pediocins are highly stable peptides that are effective over a range of temperature and pH. However, they are sensitive to proteolytic enzymes like papain, pepsin, protease, trypsin, and α-chymotrypsin (Kumar et al. 2011). Different types of pediocins are reported to date including pediocin L50, AcH, AcM, CP-2, F, K1, L, L-50, SJ-1, etc., (Papgianni and Anastasiadoue 2009; Ennahar et al. 1996; Elegado et al. 1997; Cintas et al. 1995; Schved et al. 1993). The N-terminal region of pediocins contains a conserved motif Y-G-N-G-V/L also known as “pediocin box” along with two conserved cysteines that are joined by a disulfide bridge to form a three-stranded antiparallel β-sheet structure. While cationic β-sheet domain at N-terminal mediates binding, the hairpin-like C-terminal region involves penetration of the peptide into hydrophobic region of the target cell membrane (Fimland et al. 2005; Drider et al. 2006). Among various pediocins, pediocin PA-1 from P. acidilactici PAC1.0 has been reported to inhibit growth of human lung carcinoma cell line and human colorectal adenocarcinoma cell line (Kaur and Kaur 2015). In a different study, pediocin PA-1 isolated from P. acidilactici K2a2-3 revealed cytotoxicity against human colon adenocarcinoma cell line (HT29) and HeLa cell line (Villarante et al. 2011). Other pediocins like CP2 produced by P. acidilactici CP2 MTCC 5101 and its recombinant variant displayed cytotoxic effects against human cancer cell lines including HepG2, HeLa, and MCF7 (Kumar et al. 2011).
Pep27anal2
Pep27anal2 is an analogue of Pep27 that is effective against S. pneumoniae (Sung et al. 2007). Pep27anal2 contains 27 amino acids with more hydrophobic residues in comparison to Pep27. Pep27anal2 was demonstrated to show cytotoxic effects against leukemic cancer cells such as AML-2, HL-60, Jurkat, gastric cancer cells, SNU-601 and MCF-7 cells. Simultaneously, Pep27 anal2 revealed penetration of cell membrane as a mechanism of action and cell-destroying activity was independent of caspase and cytochrome-C. Results also suggested that the hydrophobic nature of the peptide played an important role in membrane interactions and anticancer activity with potential of being a candidate for anticancer therapeutic agents (Sung et al. 2007; Lee et al. 2005; Huang et al. 2011).
Bovicin
Bovicin is a low molecular weight (2.4 kDa), broad-spectrum AMP produced by Streptococcus bovis HC5 (Table 2). Bovicin resembles nisin in both structure and function with stability towards high temperature and low pH (Fig. 1). Though it was resistant to proteinase K and α-chymotrypsin, enzymes like pronase E and trypsin show effect on bioactivity. The mechanism of activity is mainly by disrupting the integrity of cell membrane through pore formation resulting in ionic imbalance, specifically, affecting potassium efflux in target cells (Mantovani et al. 2002). Bovicin is found to be effective against MCF7 and HepG2 cancer cell lines (Paiva et al. 2012).
Laterosporulins (LS)
Laterosporulin is a defensin-like peptide from Brevibacillus spp. strains GI-9 and SKDU10. They have displayed human defensin-like structure and broad-spectrum activity against bacteria (Singh et al. 2014). However, LS10 showed antimycobacterial activity as it inhibited pathogenic strains of Mycobacterium tuberculosis Rv strain (Table 2). The amino acid composition analysis of LS10 showed predominance of hydrophobic amino acids (Baindara et al., 2017a, b) and it is capable of killing Mtb H37Rv strain residing inside the phagosomes of murine macrophages. It was found to be non-toxic to macrophage cells even at higher concentrations (Baindara et al. 2016). This was also involved in membrane disruption as demonstrated by alterations in ATP levels and the NAD(P)/NAD(P)H ratios. There was no effect on RBCs as no hemolysis was observed even at increased concentration in comparison to their MIC values. LS10 displayed cytotoxicity against diverse cancer cells like MCF-7, HEK293T, HT1080, HeLa, and H1299 at significantly low concentrations (10 μM), except prostate epithelium cells RWPE-1. Release of lactate dehydrogenase from cancer cell lines at 15 μM concentration indicates the lytic ability of LS10. Furthermore, flow cytometry analysis revealed that LS10 induced apoptosis in cancer cell lines even at 2.5 μM concentration. Nevertheless, RWPE-1 cells remained viable even at 20 μM concentration (Baindara et al., 2017a, b).
Conclusions
Bacteriocins are AMPs with unique biologic properties, which make them quite appealing and promising therapeutic compounds for a variety of disease conditions. Particularly, the anticancer properties of bacteriocins have been studied, but they are applied only to a limited extent yet. Purified bacteriocins including plantaricin, nisin, pyocin, colicin, pediocin, and microcin (Lagos et al. 2009) have shown inhibitory properties against different cancer cell lines as few of them have been examined in xenograft mouse models also (Shaikh et al. 2012; Cornut et al. 2008; Saito and Watanabe 1979). Bacteriocins are membrane active peptides and altered genetic expression of surface charge on cancer cell surface makes them more specific and targeted to interact with bacteriocins (Zhao et al. 2006; Riedl et al. 2011; Martín et al., 2015). Few other salient structural characteristics of bacteriocins are positively charged amino acid residues, hydrophobicity, amphipathic structures and oligomerization that enhance their potential anticancer activities. The anticancer mechanism of bacteriocins largely includes apoptosis, inhibition of cell proliferation, depolarization of cell membrane, blockage of angiogenesis, and inhibition of tumorigenesis as observed in vivo. However, well controlled in vivo investigations must be carried out to gain better insights in mechanism of action against cancerous cell lines. Biophysical studies, structure analysis, dynamics, topology, and molecular mechanisms of membrane disruption and the specific membrane component activities are essentially required to provide new insights to understand the anticancer phenomenon of potential anticancer bacteriocins. However, susceptibility towards the serum components like proteases is one of the major challenges of using bacteriocins in vivo. Chemical syntheses of bacteriocins by incorporating D-amino acids that are less susceptible to proteolytic cleavage in the gut have been tried. For example, synthesis of lactococcin G with replaced D-amino acids in N- and C-terminals for improved stability against peptidases (Oppegård et al., 2010) and site-directed mutations of trypsin recognition sites in salivaricin P (O’Shea et al. 2010) reveal the efforts focused on improving stability of bacteriocins in gut environment. Moreover, the functional vehicles for the controlled focused delivery of bacteriocins could also improve their in vivo stabilities and applicability.
As bacteriocins are amenable to bioengineering, they provide an opportunity to improve the efficacy of naturally occurring bacteriocins by creating the hybrid bacteriocins with desired properties. Molecular screening of three novel bacteriocins Lsl_003, Lsl_0510, and Lsl_0554 from Lactobacillus salivarius by Shaikh et al. (2012) revealed binding affinities towards common cancer targets p53, Rb1, and AR. Among these, Lsl_0510 showed the highest binding affinity towards all three receptors (p53, Rb1, and AR) that suggested it as an ideal candidate for future cancer therapeutics (Shaikh et al. 2012). Recent studies provided convincing evidence that oral and gut bacteria may be implicated in carcinogenesis in humans (Ahn et al. 2012; Michaud and Izard 2014; Grover et al. 2016). In these scenarios, it is plausible that bacteriocins having dual activities as antimicrobial as well as anticancer properties like nisin may have greater composite benefits of reestablishing a healthy microbiome and disrupting the carcinogenesis (Shin et al. 2016). Such agents essentially require a rigorous well-designed quality-focused research to develop them as promising clinical therapeutic agents for human use.
References
Abdi-Ali A, Worobec EA, Deezagi A, Malekzadeh F (2004) Cytotoxic effects of pyocin S2 produced by Pseudomonas aeruginosa on the growth of three human cell lines. Can J Microbiol 50:375–381
Agarwal V, Metlitskaya A, Severinov K, Nair SK (2011) Structural basis for microcin C7 inactivation by the MccE acetyltransferase. J Biol Chem 286:21295–21303
Agrawal N, Bettegowda C, Cheong I, Geschwind J-F, Drake CG, Hipkiss EL, Tatsumi M, Dang LH, Diaz LA, Pomper M, Abusedera M, Wahl RL, Kinzler KW, Zhou S, Huso DL, Vogelstein B (2004) Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A 101:15172–15177
Ahmadi S, Ghollasi M, Hosseini HM (2017) The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb Pathog 111:193–197
Ahn J, Chen CY, Hayes RB (2012) Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control 23:399–404
Alexandroff AB, Jackson AM, O’Donnell M, James K (1999) BCG immunotherapy of bladder cancer: 20 years on. Lancet 353:1689–1694
Andersland K, Jølle GF, Sand O, Haug TM (2010) Peptide pheromone plantaricin a produced by Lactobacillus plantarum permeabilizes liver and kidney cells. J Membr Biol 235:121–129
Aranha C, Gupta S, Reddy KVR (2004) Contraceptive efficacy of antimicrobial peptide Nisin: in vitro and in vivo studies. Contraception 69:333–338
Arnold T, Zeth K, Linke D (2009) Structure and function of colicin S4, a colicin with a duplicated receptor-binding domain. J Biol Chem 284:6403–6413
Aymerich T, Garriga M, Ylla J (2000) Application of enterocins as biopreservatives against Listeria innocua in meat products. J Food Prot 63:721–726
Baindara P, Chaudhry V, Mittal G, Liao LM, Matos CO, Khathri N, Franco OL, Patil PB, Korpole S (2015) Characterization of the antimicrobial peptide Penisin, a class Ia novel Lantibiotic from Paenibacillus sp. strain A3. Antimicob Agents Chemother 60:580–591
Baindara P, Singh N, Ranjan M, Nallabelli N, Chaudhry V, Pathania GL, Sharma N, Kumar A, Patil PB, Korpole S (2016) Laterosporulin10: a novel defensin like class IId bacteriocin from Brevibacillus sp. strain SKDU10 with inhibitory activity against microbial pathogens. Microbiol 162:1286–1299
Baindara P, Gautam A, Raghava GPS, Korpole S (2017a) Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci Rep 19;7:46541. https://doi.org/10.1038/srep46541
Baindara P, Kapoor A, Korpole S, Grover V (2017b) Cysteine-rich low molecular weight antimicrobial peptides from Brevibacillus and related genera for biotechnological applications. World J Microbiol Biotechnol 33:124
Bastos MDCDF, Coutinho BG, Coelho MLV (2010) Lysostaphin: a staphylococcal bacteriolysin with potential clinical applications. Pharmaceuticals 3:1139–1161
Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF (1982) Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem 122:429–436
Bizzarri AR, Santini S, Coppari E, Bucciantini M, Di Agostino S, Yamada T, Beattie CW, Cannistraro S (2011) Interaction of an anticancer peptide fragment of azurin with p53 and its isolated domains studied by atomic force spectroscopy. Int J Nanomedicine 6:3011–3019
Brader P, Stritzker J, Riedl CC, Zanzonico P, Cai S, Burnazi EM, Ghani ER, Hricak H, Szalay AA, Fong Y, Blasberg R (2008) Escherichia coli Nissle 1917 facilitates tumor detection by positron emission tomography and optical imaging. Clin Cancer Res 14:2295–2302
Brand M, de Kwaadsteniet M, Dicks LMT (2010) The ability of nisin F to control Staphylococcus aureus infection in the peritoneal cavity, as studied in mice. Lett Appl Microbiol 51:645–649
Braun V, Pilsl H, Groß P (1994) Colicins: structures, modes of action, transfer through membranes, and evolution. Arch Microbiol 161:199–206
Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl H, de Kruijff B (1999) Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364
Burdick MD, Harris A, Reid CJ, Iwamura T, M A H (1997) Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem 272:24198–24202
Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR (2011) Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol 49:2356–2364
Campion A, Casey PG, Field D, Cotter PD, Hill C, Ross RP (2013) In vivo activity of nisin A and nisin V against Listeria monocytogenes in mice. BMC Microbiol 13:23
Cao LT, Wu JQ, Xie F, Hu SH, Mo Y (2007) Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J Dairy Sci 90:3980–3985
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 72:3666–3670
Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubès R, Postle K, Riley M, Slatin S, Cavard D (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229
Castiglione F, Lazzarini A, Carrano L, Corti E, Ciciliato I, Gastaldo L, Candiani P, Losi D, Marinelli F, Selva E, Parenti F (2008) Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multi-resistant pathogens. Chem Biol 15:22–31
Chaudhari A, Mahfouz M, Fialho AM, Yamada T, Granja AT, Zhu Y, Hashimoto W, Schlarb-Ridley B, Cho W, Das Gupta TK, Chakrabarty AM (2007) Cupredoxin-cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochem 46:1799–1810
Chaudhary J, Munshi M (1995) Scanning electron microscopic analysis of breast aspirates. Cytopathology 6:162–167
Chen H, Hoover DG (2003) Bacteriocins and their food applications. Compr Rev Food Sci Food Saf 2:82–100
Chen Y-LS, Li J-H, Yu C-Y, Lin C-J, Chiu P-H, Chen P-W, Lin C-C, Chen W-J (2012) Novel cationic antimicrobial peptide GW-H1 induced caspase-dependent apoptosis of hepatocellular carcinoma cell lines. Peptides 36:257–265
Cho J, Hwang IS, Choi H, Hwang JH, Hwang JS, Lee DG (2012) The novel biological action of antimicrobial peptides via apoptosis induction. J Microbiol Biotechnol 22:1457–1466
Chu H-L, Yip B-S, Chen K-H, Yu H-Y, Chih Y-H, Cheng H-T, Chou Y-T, Cheng J-W (2015) Novel antimicrobial peptides with high anticancer activity and selectivity. PLoS One 10:e0126390
Chumchalová J, Smarda J (2003) Human tumor cells are selectively inhibited by colicins. Folia Microbiol (Praha) 48:111–115
Cintas LM, Rodriguez JM, Fernandez MF, Sletten K, Nes IF, Hernandez PE, Holo H (1995) Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl Environ Microbiol 61:2643–2648
Coburn PS, Gilmore MS (2003) The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell Microbiol 5(10):661–669
Coley WB (1910) The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med 3:1–48
Connor J, Bucana C, Fidler IJ, Schroit J (1989) Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc Natl Acad Sci U S A 86:3184–3188
Cornut G, Fortin C, Soulières D (2008) Antineoplastic properties of bacteriocins: revisiting potential active agents. Am J Clin Oncol 31:399–404
Cotter PD (2012) A bacteriocin perspective. Bioengineered 3:313–319
Crowther GS, Baines SD, Todhunter SL, Freeman J, Chilton CH, Wilcox MH (2013) Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J Antimicrob Chemother 68:168–176
Cursino L, Šmarda J, Chartone-Souza E, Nascimento AMA (2002) Recent updated aspects of colicins of enterobacteriaceae. Braz J Microbiol 33:185–195
Cursino L, Smajs D, Smarda J, Nardi RMD, Nicoli JR, Chartone-Souza E, Nascimento AMA (2006) Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo. J Appl Microbiol 100:821–829
Cutter CN, Siragusa GR (1998) Incorporation of nisin into a meat binding system to inhibit bacteria on beef surfaces. Lett Appl Microbiol 27:19–23
Dabour N, Zihler A, Kheadr E, Lacroix C, Fliss I (2009) In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int J Food Microbiol 133:225–233
Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B (2001) Combination bacteriolytic therapy for the treatment of experimental tumors. Proc NatlAcad Sci USA 98:15155–15160
Dethlefsen L, Eckburg PB, Bik EM, Relman D (2006) Assembly of the human intestinal microbiota. Trends Ecol Evol 21:517–523
Dobrzyńska I, Szachowicz-Petelska B, Sulkowski S, Figaszewski Z (2005) Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol Cell Biochem 276:113–119
Domagala WKL (1980) Surface configuration of human tumor cells obtained by fine needle aspiration biopsy. Scan Electron Microsc 3:101–108
Drider D, Fimland G, Héchard Y, McMullen LM, Prévost H (2006) The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev 70:564–582
Duquesne S, Destoumieux-Garzón D (2007) Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep 24:75005
Elegado FB, Kim WJ, Kwon DY (1997) Rapid purification, partial characterization, and antimicrobial spectrum of the bacteriocin, Pediocin AcM, from Pediococcus acidilactici M. Int J Food Microbiol 37:1–11
Ennahar S, Aoude-Werner D, Sorokine O, Van Dorsselaer A, Bringel F, Hubert JC, Hasselmann C (1996) Production of pediocin AcH by Lactobacillus plantarum WHE 92 isolated from cheese. Appl Environ Microbiol 62:4381–4387
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106
Fadeel B, Xue D (2009) The ins and outs of phospholid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol 44:264–277
Farkas-Himsley H, Cheung R (1976) Bacterial proteinaceous products (bacteriocins) as cytotoxic agents of neoplasia. Cancer Res 36:3561–3567
Farkas-Himsley H, Hill R, Rosen B, Arab S, Lingwood C a (1995) The bacterial colicin active against tumor cells in vitro and in vivo is verotoxin 1. Proc Natl Acad Sci U S A 92:6996–7000
Feldgarden M, Riley MA (1999) The phenotypic and fitness effects of colicin resistance in Escherichia coli K-12. Evolution (N Y) 53:1019–1027
Fernández L, Delgado S, Herrero H, Maldonado A, Rodríguez JM (2008) The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J Hum Lact 24:311–316
Fialho AM, Salunkhe P, Manna S, Mahali S, Chakrabarty AM (2012) Glioblastoma multiforme: novel therapeutic approaches. ISRN Neurol 2012:1–10
Fimland G, Johnsen L, Dalhus B, Nissen-Meyer J (2005) Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. J Pept Sci 11:688–696
Fons AG, Tuomo Karjalainen M (2000) Mechanisms of colonisation and colonisation resistance of the digestive tract part 2: bacteria/bacteria interactions. Microb Ecol Health Dis 12:240–246
Fontana MBC, de Bastos MDCF, Brandelli A, Freire De Bastos MDC, Brandelli A (2006) Bacteriocins Pep5 and epidermin inhibit Staphylococcus epidermidis adhesion to catheters. Curr Microbiol 52:350–353
Frana TS, Carlson SA, Rauser DC, Jones BD, Fergen BJ, Griffith RW (2004) Effects of microcin 24-producing Escherichia coli on shedding and multiple-antimicrobial resistance of Salmonella enterica serotype Typhimurium in pigs. Am J Vet Res 65:1616–1620
Fuska J, Fuskova A, Smarda JMJ (1979) Effect of colicin E3 on leukemia cells P388 in vitro. Experientia 35:406–407
Gandhi NM, Morales A, Lamm DL (2013) Bacillus Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int 112:288–297
Goldstein BP, Wei J, Greenberg K, Novick R (1998) Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J Antimicrob Chemother 42:277–278
Goto M, Yamada T, Kimbara K, Horner J, Newcomb M, Das Gupta TK, Chakrabarty a M (2003) Induction of apoptosis in macrophages by Pseudomonas aeruginosa azurin: tumour-suppressor protein p53 and reactive oxygen species, but not redox activity, as critical elements in cytotoxicity. Mol Microbiol 47:549–559
Grasemann H, Stehling F, Brunar H, Widmann R, Laliberte TW, Molina L, Döring G, Ratjen F (2007) Inhalation of Moli1901 in patients with cystic fibrosis. Chest 131:1461–1466
Gratia A (1925) Sur un remarquable example d’antagonisme entre deux souches de colibacille. Compt Rend Soc Biol 93(Cross reference):1040–1042
Gray MW (2012) Mitochondrial evolution. Cold Spring Harb Perspect Biol 4:a011403
Gray M, Burger G, Lang BF (2001) The origin and early evolution of mitochondria. Genome Biol 2: reviews 1018:1–1018
Grover V, Kapoor A, Sehgal K, Kaur G (2016) Chronic inflammation and carcinogenesis—emerging role of chronic inflammatory periodontal disease. Cancer Res Front 2:200–225
Gunther J (1991) Lantibiotics—ribosomally synthesized biologically active polypeptides containing sulfide bridges and α,β-didehydroamino acids. Angew Chem Int Ed 30:1051–1068
Gupta DT (2002) Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci U S A:14098–14103
Hammami R, Zouhir A, Ben Hamida J, Fliss I (2007) BACTIBASE: a new web-accessible database for bacteriocin characterization. BMC Microbiol 7:89
Hammami R, Zouhir A, Le Lay C, Ben Hamida J, Fliss I (2010) BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol 10:22
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Haugen HS, Kristiansen PE, Fimland G, Nissen-Meyer J (2008) Mutational analysis of the class IIa bacteriocin curvacin A and its orientation in target cell membranes. Appl Environ Microbiol 74:6766–6773
Helbig S, Braun V (2011) Mapping functional domains of colicin M. J Bacteriol 193:815–821
Herr HW, Morales A (2008) History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol 179:53–56
Hetz C, Bono MR, Barros LF, Lagos R (2002) Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci U S A 99:2696–2701
Hillman JD, Mo J, McDonell E, Cvitkovitch D, Hillman CH (2007) Modification of an effector strain for replacement therapy of dental caries to enable clinical safety trials. J Appl Microbiol 102:1209–1219
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/archive/csr/1975_2012/ Accessed 20 Aug 2018
Huang Y-B, Wang X-F, Wang H-Y, Liu Y, Chen Y (2011) Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Ther 10:416–426
Ingham A, Ford M, Moore RJ, Tizard M (2003) The bacteriocin piscicolin 126 retains antilisterial activity in vivo. J Antimicrob Chemother 51:1365–1371
Jabés D, Brunati C, Candiani G, Riva S, Romanó G, Donadio S (2011) Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant gram-positive pathogens. Antimicrob Agents Chemother 55:1671–1676
Jasniewski J, Cailliez-Grimal C, Chevalot I, Millière J-B, Revol-Junelles A-M (2009) Interactions between two carnobacteriocins Cbn BM1 and Cbn B2 from Carnobacterium maltaromaticum CP5 on target bacteria and Caco-2 cells. Food Chem Toxicol 47:893–897
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Jeuken LJ, Ubbink M, Bitter JH, van Vliet P, Meyer-Klaucke W, Canters GW (2000) The structural role of the copper-coordinating and surface-exposed histidine residue in the blue copper protein azurin. J Mol Biol 299:737–755
Jeziorowski A, Gordon DM (2007) Evolution of microcin V and colicin Ia plasmids in Escherichia coli. J Bacteriol 189:7045–7052
Jia L, Gorman GS, Coward LU, Noker PE, McCormick D, Horn TL, Harder JB, Muzzio M, Prabhakar B, Ganesh B, Das Gupta TK, Beattie CW (2011) Preclinical pharmacokinetics, metabolism, and toxicity of azurin-p28 (NSC745104) a peptide inhibitor of p53 ubiquitination. Cancer Chemother Pharmacol 68:513–524
Johnson CL, Ridley H, Pengelly RJ, Salleh MZ, Lakey JH (2013) The unstructured domain of colicin N kills Escherichia coli. Mol Microbiol 89:84–95
Johnstone SA, Gelmon K, Mayer LD, Hancock RE, Bally MB (2000) In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des 15:151–160
Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL (2012) Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med 1:295–305
Kageyama M (1964) Studies of a pyocin. J Biochem 55:49–53
Kamarajan P, Hayami T, Matte B, Liu Y, Danciu T, Ramamoorthy A, Worden F, Kapila S, Kapila Y (2015) Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS One 10:e0131008
Kang BS, Seo JG, Lee GS, Kim JH, Kim SY, Han YW, Kang H, Kim HO, Rhee JH, Chung MJ, Park YM (2009) Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acne, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol 47:101–109
Kaur S, Kaur S (2015) Bacteriocins as potential anticancer agents. Front Pharmacol 6:272
Kawai K, Miyazaki J, Joraku A, Nishiyama H, Akaza H (2013) Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci 104:22–27
Kim R, Emi M, Tanabe K (2006) Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol 57:545–553
Klener P (1999) Chemotherapy side effects and their management. Basic Clin Oncol 19:279–295 ST
Kozłowska K, Nowak J, Kwiatkowski B, Cichorek M (1999) ESR study of plasmatic membrane of the transplantable melanoma cells in relation to their biological properties. Exp Toxicol Pathol 51:89–92
Kristiansen PE, Fimland G, Mantzilas D, Nissen-Meyer J (2005) Structure and mode of action of the membrane-permeabilizing antimicrobial peptide pheromone plantaricin A. J Biol Chem 280:22945–22950
de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B (1997) Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim Biophys Acta 1325:108–116
Kruis W (2004) Antibiotics and probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 20(Suppl 4):75–78
Kruszewska D, Sahl H-G, Bierbaum G, Pag U, Hynes SO, Ljungh A (2004) Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J Antimicrob Chemother 54:648–653
Kumar B, Balgir PP, Kaur B, Garg N (2011) Cloning and expression of bacteriocins of Pediococcus spp.: a review. Arch Clin Microbiol 2:1–18
de Kwaadsteniet M, Doeschate KT, Dicks LMT (2009) Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett Appl Microbiol 48(1):65–70
Kwan JM, Fialho AM, Kundu M, Thomas J, Hong CS, Das Gupta TK, Chakrabarty AM (2009) Bacterial proteins as potential drugs in the treatment of leukemia. Leuk Res 33:1392–1399
Lagos R, Wilkens M, Vergara C, Cecchi X, Monasterio O (1993) Microcin E492 forms ion channels in phospholipid bilayer membranes. FEBS Lett 321:145–148
Lagos R, Tello M, Mercado G, García V, Monasterio O (2009) Antibacterial and antitumorigenic properties of microcin E492, a pore-forming bacteriocin. Curr Pharm Biotechnol 10:74–85
Lao Y, Wang X, Xu N, Zhang H, Xu H (2014) Application of proteomics to determine the mechanism of action of traditional Chinese medicine remedies. J Ethnopharmacol 155(1):1–8
Laverty G, Gilmore B (2014) Cationic antimicrobial peptide cytotoxicity. SOJ Microbiol Infect Dis 2:1
Lee DG, Hahm K-S, Park Y, Kim H-Y, Lee W, Lim S-C, Seo Y-K, Choi C-H (2005) Functional and structural characteristics of anticancer peptide Pep27 analogues. Cancer Cell Int 5:21
Leisner JJ, Greer GG, Stiles ME (1996) Control of beef spoilage by a sulfide-producing Lactobacillus sake strain with bacteriocinogenic Leuconostoc gelidum UAL187 during anaerobic storage at 2 degrees C. Appl Environ Microbiol 62:2610–2614
Letzel A, Pidot SJ, Hertweck C (2014) Genome mining for ribosomally synthesized and post-translationally modified peptides (RiPPs) in anaerobic bacteria. BMC Genomics 15:983
Lopez FE, Vincent PA, Zenoff AM, Salomón RA, Farías RN (2007) Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J Antimicrob Chemother 59:676–680
de Lorenzo V (1984) Isolation and characterization of microcin E492 from Klebsiella pneumoniae. Arch Microbiol 139:72–75
de Lorenzo V, Pugsley AP (1985) Microcin E492, a low-molecular-weight peptide antibiotic which causes depolarization of the Escherichia coli cytoplasmic membrane. Antimicrob Agents Chemother 27:666–669
Mader JS, Hoskin DW (2006) Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs 15:933–946
Maher S, McClean S (2006) Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol 71:1289–1298
Maletzki C, Gock M, Klier U, Klar E, Linnebacher M (2010) Bacteriolytic therapy of experimental pancreatic carcinoma. World J Gastroenterol 16:3546–3552
Manno S, Takakuwa Y, Mohandas N (2002) Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci U S A 99:1943–1948
Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Gtz F, Manosroi W, Manosroi J (2010) Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm 392:304–310
Mantovani HC, Hu H, Worobo RW, Russell JB (2002) Bovicin HC5, a bacteriocin from Streptococcus bovis HC5. Microbiology 148:3347–3352
Martín R, Escobedo S, Martín C, Crespo A, Quiros LMSJ (2015) Surface glycosaminoglycans protect eukaryotic cells against membrane-driven peptide bacteriocins. Antimicrob Agents Chemother 59:677–681
Mehta RR, Hawthorne M, Peng X, Shilkaitis A, Mehta RG, Beattie CW, Das Gupta TK (2010) A 28-amino-acid peptide fragment of the cupredoxin azurin prevents carcinogen-induced mouse mammary lesions. Cancer Prev Res 3:1351–1360
Mehta RR, Yamada T, Taylor BN, Christov K, King ML, Majumdar D, Lekmine F, Tiruppathi C, Shilkaitis A, Bratescu L, Green A, Beattie CW, Das Gupta TK (2011) A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis 14:355–369
Metlitskaya AZ, Katrukha GS, Shashkov AS, Zaitsev DA, Egorov TA, Khmel IA (1995) Structure of microcin C51, a new antibiotic with a broad spectrum of activity. FEBS Lett 357:235–238
Michaud DS, Izard J (2014) Microbiota, oral microbiome, and pancreatic cancer. Cancer J 20:203–206
Michel-Briand Y, Baysse C (2002) The pyocins of Pseudomonas aeruginosa. Biochimie 84:499–510
Mohimani H, Kersten RD, Liu WT, Wang M, Purvine SO, Wu S, Brewer HM, Pasa-Tolic L, Bandeira N, Moore BS, Pevzner PA, Dorrestein PC (2014) Automated genome mining of ribosomal peptide natural products. ACS Chem Biol 9:1545–1551
Moll GN, Konings WN, Driessena JM (1999) Bacteriocins: mechanism of membrane insertion and pore formation. Antonie van Leeuwenhoek Int J Gen Mol Microbiol 76:185–198
Mota-Meira M, Morency H, Lavoie MC (2005) In vivo activity of mutacin B-Ny266. J Antimicrob Chemother 56:869–871
Murinda SE, Rashid KARR (2003) In vitro assessment of the cytotoxicity of nisin, pediocin, and selected colicins on simian virus 40-transfected human colon and Vero monkey kidney cells with trypan blue staining viability assays. J Food Prot 66:847–853
Nakazawa I, Iwaizumi M (1989) A role of the cancer cell membrane fluidity in the cancer metastases: an ESR study. Tohoku J Exp Med 157:193
Nes IF, Holo H (2000) Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50–61
Nguyen CNVD (2016) Discovery of azurin-like anticancer bacteriocins from human gut microbiome through homology modeling and molecular docking against the tumor suppressor p53. Biomed Res Int 2016:12
Nuñez M, Rodríguez JL, García E, Gaya P, Medina M (1997) Inhibition of Listeria monocytogenes by enterocin 4 during the manufacture and ripening of Manchego cheese. J Appl Microbiol 83:671–677
O’Shea EF, O’Connor PM, Cotter PD, Ross RP, Hill C (2010) Synthesis of trypsin-resistant variants of the listeria-active bacteriocin salivaricin P. Appl Environ Microbiol 76:5356–5362
Op den Kamp J (1979) Lipid asymmetry in membranes. Annu Rev Biochem 48:47–71
Oppegård C, Fimland G, Thorbaek L, Nissen-Meyer J (2007) Analysis of the two-peptide bacteriocins lactococcin G and enterocin 1071 by site-directed mutagenesis. Appl Environ Microbiol 73:2931–2938
Oppegård C, Rogne P, Kristiansen PE, Nissen-Meyer J (2010) Structure analysis of the two-peptide bacteriocin lactococcin G by introducing D-amino acid residues. Microbiology 156:1883–1889
Paiva AD, de Oliveira MD, de Paula SO, Baracat-Pereira MC, Breukink E, Mantovani HC (2012) Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiol (United Kingdom) 158:2851–2858
Papagianni M (2003) Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv 21:465–499
Patyar S, Joshi R, Byrav DSP, Prakash MB, Das BK (2010) Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci 17:21
Perez RH, Zendo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Factories 13(Suppl 1):S3
Pieterse R, Todorov SD (2010) Bacteriocins: exploring alternatives to antibiotics in mastitis treatment. Braz J Microbiol 41:542–562
Piper C, Hill C, Cotter PD, Ross RP (2011) Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microb Biotechnol 4:375–382
Piper C, Casey PG, Hill C, Cotter PD, Ross RP (2012) The lantibiotic Lacticin 3147 prevents systemic spread of Staphylococcus aureus in a murine infection model. Int J Microbiol 2012:806230. https://doi.org/10.1155/2012/806230
Pons AM, Delalande F, Duarte M, Benoit S, Lanneluc I, Sablé S, Van Dorsselaer A, Cottenceau G (2004) Genetic analysis and complete primary structure of microcin L. Antimicrob Agents Chemother 48:505–513
Porta C, Cosmai L, Gallieni M, Pedrazzoli P, Malberti F (2015) Renal effects of targeted anticancer therapies. Nat Rev Nephrol 11:354–370
Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, E a C, Graves J, Yamada T, Constantinou AI, Christov K, White B, Li G, Majumdar D, Chakrabarty AM, Das Gupta TK (2004) Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene 23:2367–2378
Ramachandran S, Mandal M (2011) Induction of apoptosis of azurin synthesized from P. aeruginosa MTCC 2453 against Dalton’s lymphoma ascites model. Biomed Pharmacother 65:461–466
Reddy KVR, Aranha C, Gupta SM, Yedery RD (2004) Evaluation of antimicrobial peptide nisin as a safe vaginal contraceptive agent in rabbits: in vitro and in vivo studies. Reprod 128:117–126
Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon ATR (1999) Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635–639
Ren J, Hamada J, Okada F, Takeichi N, Morikawa K, Hosokawa MKH (1990) Correlation between the presence of microvilli and the growth or metastatic potential of tumor cells. Jpn J Cancer Res 81:920–926
Riedl SJ, Pasquale EB (2015) Targeting the Eph system with peptides and peptide conjugates. Curr Drug Targets 16:1031–1047
Riedl S, Zweytick D, Lohner K (2011) Membrane-active host defense peptides—challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids 164:766–781
Riley MA, Wertz JE (2002) Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol 56:117–137
Rothman JE, Lenard J (1977) Membrane asymmetry. Science 195:743–753
Ryan MP, Meaney WJ, Ross RP, Hill C (1998) Evaluation of lacticin 3147 and a teat seal containing this bacteriocin for inhibition of mastitis pathogens. Appl Environ Microbiol 64:2287–2290
Sahl H (2000) New insights into the mechanism of action of lantibiotics—diverse. J Antimicrob Chemother 46:1–6
Saito H, Watanabe T (1979) Effect of a bacteriocin produced by Mycobacterium smegmatis on growth of cultured tumor and normal cells. Cancer Res 39:5114–5117
Salvucci E, Saavedra L, Hebert EM, Haro C, Sesma F (2012) Enterocin CRL35 inhibits Listeria monocytogenes in a murine model. Foodborne Pathog Dis 9:68–74
Sand SL, Oppegård C, Ohara S, Iijima T, Naderi S, Blomhoff HK, Nissen-Meyer J, Sand O (2010) Plantaricin A, a peptide pheromone produced by Lactobacillus plantarum, permeabilizes the cell membrane of both normal and cancerous lymphocytes and neuronal cells. Peptides 31:1237–1244
Sand SL, Nissen-Meyer J, Sand O, Haug TM (2013) Plantaricin A, a cationic peptide produced by Lactobacillus plantarum, permeabilizes eukaryotic cell membranes by a mechanism dependent on negative surface charge linked to glycosylated membrane proteins. Biochim Biophys Acta Biomembr 1828:249–259
Schenkel LC, Bakovic M (2014) Formation and regulation of mitochondrial membranes. Int J Cell Biol 2014:709828
Schved F, Lalazar A, Henis Y, Juven BJ (1993) Purification, partial characterization and plasmid linkage of pediocin SJ1, a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol 74:67–77
Schwartz B, Bresalier RS, Kim YS (1992) The role of mucin in colon-cancer metastasis. Int J Cancer 52:60–65
Settanni L, Corsetti A (2008) Application of bacteriocins in vegetable food biopreservation. Int J Food Microbiol 121:123–138
Shaikh F, Abhinand P, Ragunath P (2012) Identification & characterization of Lactobacillus salavarius bacteriocins and its relevance in cancer therapeutics. Bioinformation 8:589–594
Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL (2016) Biomedical applications of nisin. J Appl Microbiol 120:1449–14465
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Silkin L, Hamza S, Kaufman S, Cobb SL, Vederas JC (2008) Spermicidal bacteriocins: lacticin 3147 and subtilosin A. Bioorg Med Chem Lett 18:3103–3106
Singh PK, Solanki V, Sharma S, Thakur KG, Krishnan B, Korpole S (2014) The intramolecular disulfide-stapled structure of laterosporulin, a class IId bacteriocin, conceals a human defensin-like structural module. FEBS J 282:203–214
Smarda JKJ (1987) Cytotoxic effects of colicins E1-E5 and K on hamster fibroblasts. Folia Microbiol (Praha) 32:133–136
Smarda J, Smajs D (1998) Colicins—exocellular lethal proteins of Escherichia coli. Folia Microbiol (Praha) 43:563–582
Smarda J, Obdrzalek V, Taborsky I, Mach J (1978) The cytotoxic and cytocidal effect of colicin E3 on mammalian tissue cells. Folia Microbiol (Praha) 23:272–277
Smarda J, Oravec C (1989) Cytocidal effect of bacteriocin on lymphoma cells. Akt Klin Onkol 21:209–212
Smarda J, Fialova M, Šmarda J (2001) Cytotoxic effects of colicins E1 and E3 on v-myb-transformed chicken monoblasts. Folia Biol (Praha) 47:11–13
Smith L, Hillman JD (2008) Therapeutic potential of type A (I) lantibiotics, a group of cationic peptide antibiotics. Curr Opin Microbiol 11:401–408
Smolarczyk R, Cichoń T, Kamysz W, Głowala-Kosińska M, Szydło A, Szultka L, Sieroń AL, Szala S (2010) Anticancer effects of CAMEL peptide. Lab Investig 90:940–952
Sok M, Sentjurc M, Schara M (1999) Membrane fluidity characteristics of human lung cancer. Cancer Lett 139:215–220
van Staden D, Brand M, Dicks LMT (2012) Nisin F-loaded brushite bone cement prevented the growth of Staphylococcus aureus in vivo. J Appl Microbiol 112:831–840
Steiner I, Errhalt P, Kubesch K, Hubner M, Holy M, Bauer M, Muller M, Hinterberger S, Widmann R, Mascher D, Freissmuth M, Kneussl M (2008) Pulmonary pharmacokinetics and safety of nebulized duramycin in healthy male volunteers. Naunyn Schmiedeberg's Arch Pharmacol 378:323–333
Stern NJ, E a S, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE, Seal BS (2006) Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother 50:3111–3116
Sun L, Song H, Zheng W (2015) Improvement of antimicrobial activity of pediocin PA-1 by site-directed mutagenesis in C-terminal domain. Protein Pept Lett (11):1007–1012
Sung WS, Park Y, Choi C-H, Hahm K-S, Lee DG (2007) Mode of antibacterial action of a signal peptide, Pep27 from Streptococcus pneumoniae. Biochem Biophys Res Commun 363:806–810
Sutyak KE, Anderson RA, Dover SE, Feathergill KA, Aroutcheva AA, Faro S, Chikindas ML (2008) Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol 2008
Turovskiy Y, Ludescher RD, Aroutcheva AA, Faro S, Chikindas ML (2009) Lactocin 160, a bacteriocin produced by vaginal Lactobacillus rhamnosus, targets cytoplasmic membranes of the vaginal pathogen, Gardnerella vaginalis. Probiotics Antimicrob Proteins 1:67–74
Utsugi T, Schroita J, Connor J, Bucana CD, Fidler IJ (1991) Elevated expression of phosphatidylserine in the outer-membrane leaflet of human tumor-cells and recognition by activated human blood monocytes. Cancer Res 51:3062–3066
Vassiliadis G, Destoumieux-Garzón D, Lombard C, Rebuffat S, Peduzzi J (2010) Isolation and characterization of two members of the siderophore-microcin family, microcins M and H47. Antimicrob Agents Chemother 54:288–297
Vaucher R, Teixeira ML, Brandelli A (2010) Investigation of the cytotoxicity of antimicrobial peptide P40 on eukaryotic cells. Curr Microbiol 60:1–5
Verkleija J, Zwaal RF, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LL (1973) The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta 323:178–193
Vignolo G, Fadda S, de Kairuz MN, Holgado d R, Oliver G (1996) Control of Listeria monocytogenes in ground beef by “Lactocin 705”, a bacteriocin produced by Lactobacillus casei CRL 705. Int J Food Microbiol 29:397–402
Villarante KI, Elegado FB, Iwatani S, Zendo T, Sonomoto K, de Guzman EE (2011) Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J Microbiol Biotechnol 27:975–980
Walsh CJ, Guinane CM, Hill C, Ross RP, O’Toole PW, Cotter PD (2015) In silico identification of bacteriocin gene clusters in the gastrointestinal tract, based on the Human Microbiome Project’s reference genome database. BMC Microbiol 15:183
Wang C, Tian L-L, Li S, Li H-B, Zhou Y, Wang H, Yang Q-Z, Ma L-J, Shang D-J (2013) Rapid cytotoxicity of antimicrobial peptide tempoprin-1CEa in breast cancer cells through membrane destruction and intracellular calcium mechanism. PLoS One 8:e60462
Wang G, Manns DC, Churey JJ, Worobo RW (2014) Development of a homologous expression system for and systematic site-directed mutagenesis analysis of thurincin H, a bacteriocin produced by Bacillus thuringiensis SF361. Appl Environ Microbiol 80:3576–3584
Wiemann B, Starnes CO (1994) Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 64:529–564
Wilson KA, Kalkum M, Ottesen J, Yuzenkova J, Chait BT, Landick R, Muir T, Severinov K, Darst SA (2003) Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail. J Am Chem Soc 125:12475–12483
Wriessnegger T, Leitner E, Belegratis MR, Ingolic E, Daum G (2009) Lipid analysis of mitochondrial membranes from the yeast Pichia pastoris. Biochim Biophys Acta 1791:166–172
Wu J, Hu S, Cao L (2007) Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob Agents Chemother 51:3131–3135
Yamada T, Goto M, Punj V, Zaborina O, Kimbara K, Das Gupta TK, Chakrabarty AM (2002) The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect Immun 70:7054–7062
Yamada T, Fialho AM, Punj V, Bratescu L, Das Gupta TK, Chakrabarty AM (2005) Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol 7:1418–1431
Yamada T, Mehta RR, Lekmine F, Christov K, King ML, Majumdar D, Shilkaitis A, Green A, Bratescu L, Beattie CW, Das Gupta TK (2009) A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol Cancer Ther 8:2947–2958
Yamada T, Christov K, Shilkaitis a, Bratescu L, Green a, Santini S, Bizzarri a R, Cannistraro S, Gupta TKD, Beattie CW (2013) P28, a first in class peptide inhibitor of Cop1 binding to P53. Br J Cancer 108:2495–2504
Yang DS, Miao XD, Ye ZM, Feng J, Xu RZ, Huang X, Ge FF (2005) Bacterial redox protein azurin induce apoptosis in human osteosarcoma U2OS cells. Pharmacol Res 52:413–421
Ye JS, Zheng XJ, Leung KW, Chen HM, Sheu FS (2004) Induction of transient ion channel-like pores in a cancer cell by antibiotic peptide. J Biochem 136:255–259
Yoon WH, Park HD, Lim K, Hwang BD (1996) Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem Biophys Res Commun 222:694–699
Zaborina O, Dhiman N, Chen ML, Kostal J, Holder IA, Chakrabarty AM (2000) Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiol 146:2521–2530
Zeisig R, Koklic T, Wiesner B, Fichtner I, Sentjurc M (2007) Increase in fluidity in the membrane of MT3 breast cancer cells correlates with enhanced cell adhesion in vitro and increased lung metastasis in NOD/SCID mice. Arch Biochem Biophys 459:98–106
Zhao H, Sood R, Jutila A, Bose S, Fimland G, Nissen-Meyer J, Kinnunen PKJ (2006) Interaction of the antimicrobial peptide pheromone plantaricin A with model membranes: implications for a novel mechanism of action. Biochim Biophys Acta Biomembr 1758:1461–1474
Zhao J, Hao X, Liu D, Huang Y, Chen Y (2015) In vitro characterization of the rapid cytotoxicity of anticancer peptide HPRP-A2 through membrane destruction and intracellular mechanism against gastric cancer cell lines. PLoS One 10:e0139578
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baindara, P., Korpole, S. & Grover, V. Bacteriocins: perspective for the development of novel anticancer drugs. Appl Microbiol Biotechnol 102, 10393–10408 (2018). https://doi.org/10.1007/s00253-018-9420-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9420-8