Abstract
The epithelial cell adhesion molecule (EpCAM) is a membrane glycoprotein overexpressed in epithelial-derived neoplasms and therefore is a highly interesting target for antibody therapy in a wide range of carcinomas. Single chain variable fragment (ScFv) antibodies, generated by the association of the variable heavy (VH) and light chains (VL) of immunoglobulins through a short polypeptide linker, retain the binding properties of classical antibodies. Due to its characteristics, Escherichia coli (E. coli) has inspired a great deal of interest for production of antibody fragments with high concentrations. Here, ScFv against EpCAM extracellular domain (EpEX) was expressed in E. coli BL21™(DE3) strain. The effect of different expression conditions on the total protein level was also investigated. Moreover, an attempt was made to overcome the problem of insolubility of the recombinant protein with alterations of expression condition like inducer concentration and temperature as well as addition of the solubility-enhancing agents. Our results showed that the maximum total protein expression was attained 7 h after induction at 37 °C with 0.5 mM IPTG (663.53 ± 7.33 mg/l). Moreover, the expressed antiEpEX-ScFv protein was 39.8% or 29.1% soluble in the presence of 50% glycerol, and Tween20 plus 50% glycerol respectively. Although the solubility of recombinant protein was significantly increased from 39.8% at 37 °C to 43.7% at 16 °C, the maximum level of soluble recombinant protein was attained at 37 °C. Consequently, we report a strategy combining different culture conditions and the solubility-enhancing additives such as glycerol for improving protein solubility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The epithelial cell adhesion molecule (EpCAM) is a type-I transmembrane protein involved in cell signaling, proliferation, differentiation, and migration via Ca2 + -independent homotypic cell–cell adhesion activity (Martowicz et al. 2016). EpCAM is expressed only on the basolateral cell surface of simple epithelia in normal tissues. So it is apically inaccessible, whereas in a wide variety of epithelial-derived cancers, including, stomach, adenocarcinomas of colon, pancreas, ovarian, lung, and breast it is not only upregulated but also its subcellular distribution is changed. It is homogeneously dispersed on cancer cell surface thus being easily accessible to antibody-based immunotherapy (Patriarca et al. 2012; Simon et al.2013; Mohammadgholizad and Hashemi 2019).
EpCAM-targeted antibody based therapy was frequently investigated since the 1980s and the therapeutic potential of these antibodies has been proved in cancers overexpressing the EpCAM receptor (Brischwein et al.2006; Kirchner et al. 2002). Furthermore, advances in recombinant antibody technology could facilitate designing and producing a wide variety of engineered antibody molecules including Fab fragments, Fv fragments and ScFv (single chain fragment variable) fragments in which the genes coding for VH and VL (the variable regions of the heavy (VH) and light (VL) chains of an immunoglobulin molecule) has been linked with a disulfide bond or a short flexible peptide linker. Due to low molecular masses and simple structures, ScFvs have better tumor penetration, lower retention times in nontarget tissue, more rapid blood clearance, and reduced immunogenicity (Kholodenko et al. 2019; Wang et al. 2015). Although recombinant ScFv fragments, whether obtained from phage libraries or cloned from hybridomas, have high specificity and affinity, but their in vivo applications may be limited because of low thermodynamic stability. CDR grafting of the fragments with suboptimal stability onto the framework of another ScFv with higher stability is an approach to repair. Using this strategy, the stability of high-affinity antiEpEX-ScFv cloned from the hybridoma MOC31 was improved by Willuda et al. In their study the binding residues of antiEpEX-ScFv were grafted onto the framework of the ScFv 4D5. Based on this modification, an unstable ScFv MOC31 was converted to a very stable 4D5MOC-B ScFv fragment with the same antigen specificity (Willuda et al. 1999).
Due to fast growth in inexpensive and simple media, low cost for protein production and easy manipulation, Escherichia coli (E. coli) has been promoted for production of antibody fragments. However, recombinantly-expressed proteins usually face solubility problem and tend to form inclusion bodies in E. coli. However, in vitro refolding of inclusion body proteins into biologically active forms is mostly time consuming and unpredictable. Moreover, in many cases the yields are low (Malekian et al. 2019). Numerous strategies have been developed for overcoming the solubility problem of expressed proteins including signal peptides mediated secretion of proteins to the E. coli periplasm, the use of solubility-enhancing tags, using weaker promoters, coexpression with chaperones and foldases, and optimization of the cultivation conditions (Farajnia et al. 2019). For example, the solubility of heterologous proteins has been shown to be increased by prolonged induction with decreased amounts of isopropyl-β-D-thiogalactoside (IPTG) at low temperatures (Rasooli et al. 2019). Unfortunately these strategies must be evaluated on a case-by-case basis. Moreover, based on previously published data, when the expressed protein was detected in the insoluble fraction, it might be solublely expressed in E. coli and possibly be trapped within inclusion bodies. Modification of the lysis buffer condition by the presence of an additive may cause these proteins to be observed in the soluble fraction. This method is applicable for increase in solubility of any recombinant protein (Leibly et al.2012).
Here, for the first time, 4D5MOC-B ScFv fragment was expressed in E. coli BL21™(DE3) strain. We also investigated the solubility of expressed protein in the presence of solubility-enhancing additives in the lysis buffer. Moreover, the effect of temperature and IPTG concentration on the expression level of recombinant protein and its solubility was evaluated.
Materials and Methods
Bacterial Strains, Plasmids, and Reagents
E. coli (DH5α) was used for plasmid preparation and BL21 (DE3) was used as a host for recombinant ScFv expression (kindly provided by Dr. keramati, Pasteur institute of IRAN, Tehran, Iran). The pET22b ( +) vector (kindly provided by Dr. keramati, Pasteur institute of IRAN, Tehran, Iran) was used to clone the ScFv gene. Luria–Bertani (LB) medium [1% (w/v) tryptone, 0.5% (w/v) yeast extract, and 1% (w/v) NaCl, pH 7.0] was used for growing the E. coli strains. The growth medium was supplemented with the ampicillin (100 μg/mL) when required. Restriction endonucleases and T4 DNA ligase were purchased from Thermo Fisher Scientific (USA). All chemicals and reagents used were obtained from standard commercial sources.
Construction of pET22b ( +) Harboring AntiEpEX-ScFv Gene
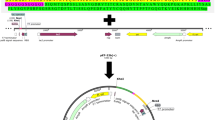
The pGH vector harbouring the synthesized codon optimized antiEpEX-ScFv gene (provided by Generay Biotech Co, China) was confirmed by restriction enzyme digestion (NcoI and NdeI). After confirmation, it was digested with XhoI and NdeI enzymes, gel-extracted using the High Pure PCR Product Purification kit (Roche Diagnostics GmbH, Germany). Utilizing the corresponding restriction sites, purified fragment was cloned into the confirmed pET22b ( +) vector to construct the pET22b ( +)-antiEpEX-ScFv recombinant expression plasmid (Fig. 1). The recombinant plasmid was transformed into E. coli (DH5α) using ampicillin for selection. The pET22b ( +)-antiEpEX-ScFv was confirmed by digesting the plasmid with restriction enzymes NcoI and XbaI also PstI. Via sequencing, the correct frame of the cloned antiEpEX-ScFv gene was confirmed (Macrogen, Korea). A C-terminal hexa-histidine tag was employed for detection and purification of the expressed EpEX.
The AntiEpEX-ScFv Expression
The expression plasmid pET22b ( +)-antiEpEX-ScFv was transformed into E. coli BL21 (DE3). A single colony of BL21 (DE3) harboring pET22b ( +)-antiEpEX-ScFv was inoculated into LB broth containing ampicillin (100 μg/ml). In cell density between 0.7–0.9, 1 mM IPTG (Sinaclon, Iran) was added to the culture to induce the expression of antiEpEX-ScFv. Under the same condition, the induced culture was shaken for another 3, 5 and 7 h. For optimized expression of antiEpEX-ScFv, cultivations were accomplished under different temperatures (25, 30 or 37 °C) and IPTG concentrations (0.25, 0.5, 1 or 2 mM). Optical density (OD) of each sample was assessed at the final expression time. The cell pellets were harvested by centrifugation at 10000 g for 5 min. Cells were lysed by incubation in the presence of lysis buffer (100 mM NaH2PO4, 10 mM Tris–cl, 8 mM Urea).
AntiEpEX-ScFv Expression Analysis
SDS-PAGE and western blot analysis were performed for detection of protein expression. Induced cells were harvested and resuspended in loading buffer containing 3% SDS and 0.2 mg/ml bromophenol blue, 5% glycerol, 5% 2-mercaptoethanol, 0.25 M Tris–HCl, pH 6.8. The samples were heated to 95 °C for 5 min followed by SDS-PAGE separation (80 V for 5% gel and 150 V for 12% gel) and stained with Coomassie Brilliant Blue R-250. The protein expression level was quantified by densitometry analysis of SDS-PAGE bands using TotalLab TL120 software (nonlinear Inc, Durham nc, USA). For western blotting, bacterial lysate was separated by 12% SDS-PAGE and electrophoretically transferred to PVDF membrane. The transferred membrane was blocked overnight with 2.5% bovine serum albumin (BSA) in phosphate-buffered saline with 0.1% Tween-20 (PBST) and incubated for 1 h with 1/10,000 dilution of Anti-His Tag polyclonal antibody as the primary antibody (Sigma, UK). After washing, membrane was incubated in a 1.5/10,000 dilution of anti-mouse HRP conjugated immunoglobulin as the secondary one (Sigma, UK). The blot was developed using 3, 3′-diaminobenzidine (DAB) substrate.
Solubility Assessment of AntiEpEX-ScFv
The solubility of a protein strongly depends on the composition of the lysis buffer. Here, the solubility of protein was tested under different conditions. To solubility assessment of recombinant antiEpEX-ScFv, pellets were harvested by centrifugation at 8000 g for 15 min and resuspended in10 ml of the buffers shown in Table 1. Cells were vortexed. This lysate was further lysed by sonication (400 W for 18 min 20 s ON, 10 s OFF). The sonicated samples were centrifuged at 10,000 g for 25 min at 4 °C. The soluble fraction was collected after the insoluble debris was pelleted. The prepared soluble and insoluble fractions were subjected to SDS-PAGE and protein bands were visualized by Coomassie Brilliant Blue R-250 staining. To identify the effect of temperature and IPTG concentration on solubility, cultivations were carried out under different temperatures (16, 25, 30 or 37 °C) as well as different IPTG concentrations (0.25, 0.5, 1 or 2 mM) and the soluble and insoluble fractions were prepared as described before.
Determination of Protein Concentration
Protein concentration was quantitatively analysed using BCA and bovine serum albumin as standard (Takara, Japan). The standard curve was generated based on concentrations of BSA standard samples. The concentration of the total protein was determined using the standard curve as a reference. Total protein samples were also electrophoresed on a 12% SDS-PAGE gel. The band intensity of recombinant protein was analyzed using TL120 software (Nonlinear Inc, Durham NC, USA). Based on estimated intensity, TL120 calculates the quantity of recombinant protein as a percentage of total protein. According to total protein concentrations obtained from BCA assay and percentage of recombinant protein obtained from TL120 analysis, the concentration of recombinant protein can be calculated.
Results
Cloning and Expression of AntiEpEX-ScFv
The successful recombinant plasmid (pET22b ( +)-antiEpEX-ScFv) was confirmed by restriction enzyme analysis. The electrophoresis results showed clear bands of ~ 5344 bp and 884 bp when recombinant plasmid was digested with NcoI and XbaI (Fig. 2a) as well as bands of 408, 1443, and 4377 bp when recombinant plasmid was digested with PstI (Fig. 2b). In addition, Nucleotide sequencing verified correct assembly of the fusion gene. The pET22b ( +) plasmid containing 751 bp antiEpEX-ScFv coding sequence was transformed into E. coli strain BL21 (DE3) competent cells. Analysis of the bacterial lysate via SDS PAGE after cultivation for antiEpEX-ScFv expression at the 1 mM IPTG at 37 °C resulted in detection of the protein band at theoretically expected molecular weight (30 kDa) for BL21 (DE3) E. coli strain (Fig. 3a). By screening various incubation times (3, 5, 7 h), the high protein expression level was obtained at 7 h after induction (data not shown). Based on densitometry and BCA analysis, a good expression was detected up to 33.85% of the total protein (486.8 ± 11 μg/mL). Using the anti-his (C-terminal) antibody in western blotting analysis, the expression of antiEpEX-ScFv as a his-tagged fusion protein was confirmed (Fig. 3b).
Restriction enzyme analysis of recombinant pET22b ( +) expression vector harbouring gene encoding antiEpEX-ScFv protein. The recombinant vector was confirmed by digestion. The electrophoresis results showed a) clear bands of ~ 5344 bp and 884 bp when recombinant plasmid was digested with NcoI and XbaI (Lane 1) as well as b) bands of 408, 1443, and 4377 bp when recombinant plasmid was digested with PstI (Lane 1). Lane M: DNA marker 100 bp, Lane N: DNA marker 1 kb
Expression analysis of the antiEpEX-ScFv protein using SDS-PAGE and western blotting. a proteins were separated on a 12% SDS-PAGE gel and visualized by coomassie brilliant blue R250 staining. Total protein from E. coli BL21 (DE3) containing pET22b (antiEpEX-ScFv) plasmid before induction (lane 1) and after induction with 1 mM IPTG for 7 h (lane 2) at 37 °C (M: marker). b Western blotting analysis of the recombinant antiEpEX-ScFv protein. BL21 (DE3) bacterial lysate before (lane 1) and after (lane 2) induction was treated with the anti His monoclonal antibodies (M: pre-stained protein marker). The antiEpEX-ScFv (30-kDa) is denoted by an arrow
Cultivation Temperature and IPTG Concentration Effects on Anti- antiEpEX-ScFv Expression Level
According to previous studies, the expression performance of recombinant proteins is affected by two major factors including inducer concentration and temperature. In the T7 expression system, IPTG is generally an effective inducer of the expression of recombinant proteins. However, the concentration of IPTG must be optimized on a case by case basis due to its toxicity to E. coli and high cost (Akbari et al. 2015). Here, cultivation was carried out under different temperatures (25, 30 or 37 °C) and IPTG concentrations (0.25, 0.5, 1 or 2 mM) (Fig. 4). Densitometry analysis showed that the highest expression level of fusion protein was achieved by 0.5 mM IPTG induction (Fig. 5). The highest expression level of EpEX was obtained at 37 °C for 7 h after induction (663.53 ± 7.33 mg/l) (Fig. 5). At this temperature, the productivity of antiEpEX-ScFv protein was decreased using higher IPTG concentrations. Lowering the expression temperature to 30 °C as well as 25 °C decreased the expression level of recombinant protein after 7 h induction by various concentrations of IPTG (Fig. 5). Under the above optimal conditions (cultivation at 37 °C and 0.5 mM IPTG), a high percentage of the target protein was expressed in BL21 (DE3) E. coli strain (39.6% of the total protein). The volumetric productivity of recombinant protein reached 663.53 ± 7.33 mg/l in this strain (Fig. 5).
The effects of IPTG concentration (mM) and incubation temperature on protein expression. After separation on a 12% SDSPAGE gel, protein bands were visualized by coomassie brilliant blue R250 staining. Protein expression was induced with different concentration of IPTG (0.25, 0.5, 1 or 2 mM) at a) 25 °C, b) 30 °C and c) 37 °C for 7 h. d comparison between maximum expression levels obtained from a, b and c using TL120. Lane M: marker. Lane 1: whole cell lysis from E. coli BL21 (DE3) containing pET22b without insert, Lane 2: bacterial culture before induction
Quantitative representation of cultivation temperature and IPTG concentration effects on antiEpEX-ScFv expression level. Cultivation was carried out under different temperatures (25, 30 or 37 °C) and IPTG concentrations (0.25, 0.5, 1 or 2 mM). Protein concentration was quantitatively analysed using BCA and bovine serum albumin as standard. Based on estimated intensity, TL120 software calculates the quantity of recombinant protein as a percentage of total protein. According to total protein concentrations obtained from BCA assay and percentage of recombinant protein obtained from TL120 analysis, the concentration of recombinant protein can be calculated. Results showed that the volumetric productivity of recombinant protein was decreased in all cultivation conditions compared with optimal condition (cultivation at 37 °C and 0.5 mM IPTG). Data are expressed as the mean ± SD of two experiments (**p < 0.01, ***p < 0.001)
Effect of Buffer on Protein Solubility
The composition of the lysis buffer can strongly affect the solubility of a protein. Here, the solubility of antiEpEX-ScFv protein was tested under different conditions. Using SDS-PAGE, soluble and insoluble fractions were analyzed after cell disruption in samples of total cell. Densitometry analysis using TL120 software was performed to protein quantification. As shown in Fig. 6, over-expression of antiEpEX-ScFv protein in the cytoplasm of E. coli BL21 (DE3) strain in an optimized condition contributed to a high level accumulation of the recombinant protein in the insoluble cell extract, and no protein band was detected in the soluble fraction. In an attempt to overcome the insolubility problem in lysis processes, Tween-20 and glycerol were added in the digestion buffer as solubility-enhancing additives. Results showed that the expressed antiEpEX-ScFv protein was 39.8% or 29.1% soluble in the presence of 50% glycerol, and Tween-20 plus 50% glycerol respectively. In the presence of Tween-20, 10% glycerol or Tween-20 plus 10% glycerol the recombinant protein was almost observed in insoluble fraction. Furthermore, compared with 7.5 N buffer, an increase in the concentration of NaCl (from 50 mM to 2 M) (2S buffer) had no effect on protein solubility in our study.
Effect of buffer on antiEpEX-ScFv protein solubility. a The soluble and insoluble fractions of expressed protein were prepared using lysis buffer with different composition and subjected to SDS-PAGE. Protein bands were visualized by coomassie brilliant blue staining. b Comparison between the means and standard deviations of the solubility percentages of antiEpEX-ScFv protein expressed after induction with 1 mM IPTG at 37 °C for 7 h and lysed with different lysis buffer. Results showed that the solubility of expressed antiEpEX-ScFv protein was increased in the presence of 50% glycerol, and Tween-20 plus 50% glycerol compared with 7.5 N buffer. The solubility was evaluated as soluble/ (insoluble and insoluble) ratio. Data are expressed as the mean ± SD of two experiments (***p < 0.001)
Effect of Temperature and IPTG Concentration on AntiEpEX-ScFv Solubility Improvement
To test whether temperature and IPTG concentration can affect the solubility of the expressed protein, we examined the solubility level of antiEpEX-ScFv at several temperatures (16, 25, 30 and 37 °C) and IPTG concentrations (0.25, 0.5, 1 and 2 mM) (Fig. 7). The distribution of the recombinant antiEpEX-ScFv protein in pellet, and supernatant samples was studied using SDS-PAGE. The effect of reducing IPTG concentration on soluble expression of antiEpEX-ScFv was assessed at 37 °C. At this temperature, although the recombinant protein was detected in both soluble and insoluble fractions in all tested IPTG concentrations but the maximum solubility for antiEpEX-ScFv was attained at 0.5 mM IPTG concentration. To find out the optimal temperature to produce soluble antiEpEX-ScFv, the solubility of the protein was compared at several temperatures at 0.5 mM IPTG. As illustrated in Fig. 7, although the maximum solubility was attained at 16 °C (43.7%) but the maximum level of soluble recombinant protein was attained at 37 °C.
Effect of temperature and IPTG concentration on antiEpEX-ScFv solubility improvement. The solubility level of antiEpEX-ScFv at several temperatures (16, 25, 30 and 37 °C) and IPTG concentrations (0.25, 0.5, 1 and 2 mM) was examined. a The soluble and insoluble fractions of expressed protein were prepared using lysis buffer containing 50% glycerol and separated on a 12% SDS-PAGE gel and visualized by coomassie brilliant blue R250 staining. b Comparison between the means and standard deviations of the solubility percentages of antiEpEX-ScFv protein expressed after induction with different temperatures and IPTG concentrations, and lysed with lysis buffer containing 50% glycerol. The solubility was evaluated as soluble/ (insoluble and insoluble) ratio. Data are expressed as the mean ± SD of two experiments
Discussion
Overcoming the lack of specificity, tumor targeted therapy has been emerged as a successful strategy. The E. coli ability in recombinantly production of ScFvs makes these targeting agents good alternatives to traditional whole antibodies. In the present study, the antiEpEX-ScFv gene was expressed in pET22b ( +) vector under the strong T7 promoter system in E. coli BL21 (DE3). First, a fairly good expression (486.8 ± 11 μg/mL) was achieved at 37 °C in the presence of 1 mM IPTG. By screening various incubation times (3, 5, 7 h), the high protein expression. level was obtained at 7 h after induction. In good agreement with our results, up to now, many researchers utilized BL21 (DE3) to improve expression of recombinant proteins in E. coli. Absence of two main proteases including ompT and lon makes the BL21 strain one of the preferred choice for recombinant protein expression (Cornelis 2000). As an example, ScFv derived from cetuximab which is an anti-epidermal growth factor receptor(EGFR) monoclonal antibody, has been successfully expressed in BL21 (DE3) bacterial system (Kim et al. 2014).
In E. coli, the high-level cytoplasmic expression of recombinant proteins mainly has led to the accumulation of insoluble inclusion bodies, requiring extensive in vitro subsequent downstream processing to access their biological activities. However, bioactive proteins are mostly extracted with a very low yield. Thus, to obtain high yields of expressed proteins, different strategies have been employed to improve solubility. Here, our experimental approaches to improve the total and soluble expression of antiEpEX-ScFv include lowering the growth temperature of induced cultures as well as inducing the cultures with lower concentrations of the inducer. Here, cultivation was carried out under different temperatures (25, 30 or 37 °C) and IPTG concentrations (0.2, 0.5, 1, 2 mM) (Fig. 4). Based on obtained results, the antiEpEX-ScFv was optimally produced in 37 °C in the presence of low concentration of IPTG (o.5 mM) (663.53 ± 7.33 mg/l) (Figs. 4 and 5). The situation was similar when acetolactate synthase was expressed in E. coli. In Zhang study, as the IPTG concentration increased over 0.6 mM, the amount of expressed protein was significantly decreased (Zheng et al. 2015). Similarly, a large amount of rMan i 1 was obtained in as low as 0.1 mM concentration of IPTG (Tsai et al. 2017). The results obtained here also are in accordance with data reported by Zhang and coworkers which showed an improved expression of extracellular human anti-HBsAg ScFv in recombinant E. coli with a low IPTG concentration (0.5 mM) for induction (Zhang et al. 2009). Lim et al. also have reported that IPTG concentrations higher than 1 mM could inhibit the expression of anti-exotoxin ScFv (Lim et al. 2004). The toxicity of high concentration of IPTG may be due to an increased metabolic load and induction of bacterial proteases which may degrade heterologous proteins (Akbari et al. 2015). Temperature can also affect the final yield of the expressed recombinant protein via influencing the rate of mRNA expression, protein aggregation, folding, and secretion. Here, we could access to an optimal expression of antiEpEX ScFv at a growth temperature of 37 °C (Fig. 5). Similarly, in study designed by Volontè and colleagues, the recombinant protein was optimally expressed at a growth temperature of 37 °C (Volontè et al. 2011). In good agreement with our results, the maximal anti-Her2 ScFv expression was also previously observed to be at 37 °C (Akbari et al. 2015).
Proteins apparent in the insoluble fraction may be in a partially folded state. Using a non-ideal buffer can cause protein unfolding resulting in protein aggregation after centrifugation. The presence of additives during cell lysis can either aid as chemical chaperones or stabilize the proteins from partially unfolding rescuing proteins observed in insoluble fraction (Leibly et al. 2012). Here, in order to prevent the production of protein aggregates during lysis processes, two solubility-enhancing additives were added in lysis buffers. The obtained results indicated that glycerol could enhance protein solubility (Fig. 6). Consistently, Leiby et al. showed that glycerol was an ideal solubility additive for all proteins studied. Considered proteins in Leiby study were either fully insoluble or small percentage of them was in the soluble fraction. They reported that the presence of additives could lead to an improvement in the solubility of 33 out of 41 proteins screened (Leibly et al. 2012). Our results also demonstrated that the recombinant protein was almost insoluble in the presence of Tween-20 (Fig. 6). These data are in contrast to Sun et al. study. To overcome the problem of insolubility in tag removal processes, they utilized several solubility-enhancing additives, including 0.5% Tween-20 in the digestion buffer to promote the solubility of target proteins. Tween-20 as a nonionic surfactant can bind to hydrophobic regions of the proteins and protect them from the surface-induced damage and aggregation. They showed that the addition of 0.5% Tween-20 is able to suppress the aggregation of scFvs against human IL-17A in vitro in tag removal processes (Sun et al. 2012). Although there is no clear explanation for the results we obtained, based on sun et al. report, the concentration of tween-20 might be one of the most important issues that should be considered here. So, increasing the concentration of tween-20 can be considered in future experiments. Furthermore, compared with 7.5 N buffer, an increase in salt concentration (2S buffer) had no effect on protein solubility in our study (Fig. 6). This is consistent with the Leiby et al. study in which they showed no increase in protein solubility when the 24 buffer combinations with varied salt concentrations were used (Leibly et al. 2012).
As mentioned before, temperature and inducer concentration are two factors can be considered to reduce the production of inclusion bodies (Francis and Page 2010). Lowering the expression temperature mostly leads to improve soluble expression of recombinant proteins. Reduction in rates of cell processes including transcription, translation, and cell division at a lower temperature, may cause the foreign proteins to have enough time to fold into their native conformations and be expressed in soluble forms. Based on this strategy, a number of recombinant proteins including subtilisin E, human interferon α-2 ricin A chain, β-lactamase, and Fab fragments were shown to have a higher solubility at low temperatures (Vasina and Baneyx 1997). The solubility of expressed antiEpEX-ScFv was analyzed under 0.5 mM IPTG concentration at different induction temperatures; the highest solubility was obtained at 16 °C. Although the solubility of recombinant protein was significantly increased from 39.8% at 37 °C to 43.7% at 16 °C, but the maximum level of soluble recombinant protein was attained at 37 °C (Fig. 7). In line with our results, a significant increase in solubility of TB10.4—Trx fusion protein was observed when it was overexpressed at 18 °C (Piubelli et al. 2013).
Conclusion
In this study, the gene encoding a ScFv against epithelial cell adhesion molecule extracellular domain has been successfully cloned and expressed in E. coli BL21 (DE3). Optimization of conditions during recombinant protein production for improved yield and solubility is a major goal for protein scientists. Here, the highest concentration of protein expression was obtained with 0.5 mM IPTG, post-induction incubation at 37 °C and seven-hour post-induction incubation. When induction temperature was lowered to 16 °C, the solubility of recombinant protein was significantly increased from 39.8% at 37 °C to 43.7% at 16 °C.
A significant fraction of proteins maybe expressed in soluble form in E. coli but produces aggregates after cell lysis. Adjusting the cell lysis buffer conditions could lead to protein rescue and avoid the need to design new constructs or change expression systems. So, here we evaluated two solubility-enhancing additives to determine conditions that can increase the solubility of the recombinant protein. The obtained results indicated that glycerol could enhance protein solubility. In summary, the strategy combining the varied temperatures and inducer concentrations, and the solubility-enhancing additives can be effective for soluble expression of antiEpEX-ScFv.
References
Akbari V, Sadeghi HMM, Jafarian-Dehkordi A, Chou CP, Abedi D (2015) Optimization of a single-chain antibody fragment overexpression in Escherichia coli using response surface methodology. Res Pharm Sci 10:75
Brischwein K et al (2006) MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol 43:1129–1143
Cornelis P (2000) Expressing genes in different Escherichia coli compartments. Curr Opin Biotechnol 11:450–454
Farajnia S, Ghorbanzadeh V, Dariushnejad H (2019) effect of molecular chaperone on the soluble expression of recombinant fab fragment in E. coli international. J Pept Res Ther:1–8
Francis DM, Page R (2010) Strategies to optimize protein expression in E. coli. Curr Protoc Protein Sci 61:5–21–25, 29
Kholodenko V et al (2019) Multimerization through Pegylation improves pharmacokinetic properties of scFv fragments of GD2-specific antibodies. Molecules 24:3835
Kim YP et al (2014) Effective therapeutic approach for head and neck cancer by an engineered minibody targeting the EGFR receptor. PLoS ONE 9:e113442
Kirchner E, Gerhards R, Voigtmann R (2002) Sequential immunochemotherapy and edrecolomab in the adjuvant therapy of breast cancer: reduction of 17–1A-positive disseminated tumour cells. Ann Oncol 13:1044–1048
Leibly DJ, Nguyen TN, Kao LT, Hewitt SN, Barrett LK, Van Voorhis WC (2012) Stabilizing additives added during cell lysis aid in the solubilization of recombinant proteins. PLoS ONE 7:e52482
Lim K-P, Li H, Nathan S (2004) Expression and purification of a recombinant scFv towards the exotoxin of the pathogen, Burkholderia pseudomallei. J Microbiol 42:126–132
Malekian R, Jahanian-Najafabadi A, Moazen F, Ghavimi R, Mohammadi E, Akbari V (2019) High-yield production of granulocyte-macrophage colony-stimulating factor in E. coli BL21 (DE3) by an auto-induction strategy. Iran J Pharma Res: IJPR 18:469
Martowicz A, Seeber A, Untergasser G (2016) The role of EpCAM in physiology and pathology of the epithelium. Histol Histopathol 31:349–355
Mohammadgholizad F, Hashemi A (2019) Construction of recombinant Pichia pastoris expressing single-chain antibody fragment against extracellular domain of EpCAM. Koomesh 21:743–750
Patriarca C, Macchi RM, Marschner AK, Mellstedt H (2012) Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev 38:68–75
Piubelli L et al (2013) Optimizing Escherichia coli as a protein expression platform to produce Mycobacterium tuberculosis immunogenic proteins. Microb Cell Fact 12:115
Rasooli F, Hashemi A (2019) Efficient expression of EpEX in the cytoplasm of Escherichia coli using thioredoxin fusion protein. Res Pharm Sci 14:554–565
Simon M, Stefan N, Plückthun A, Zangemeister-Wittke U (2013) Epithelial cell adhesion molecule-targeted drug delivery for cancer therapy. Expert Opin Drug Deliv 10:451–468
Sun W, Xie J, Lin H, Mi S, Li Z, Hua F, Hu Z (2012) A combined strategy improves the solubility of aggregation-prone single-chain variable fragment antibodies. Protein Expr Purif 83:21–29
Tsai W-C, Wu T-C, Chiang B-L, Wen H-W (2017) Cloning, expression, and purification of recombinant major mango allergen Man i 1 in Escherichia coli. Protein Exp Purif 130:35–43
Vasina JA, Baneyx F (1997) Expression of aggregation-prone recombinant proteins at low temperatures: a comparative study of the Escherichia coli cspA and tacPromoter systems. Protein Exp Purif 9:211–218
Volontè F, Piubelli L, Pollegioni L (2011) Optimizing HIV-1 protease production in Escherichia coli as fusion protein. Microb Cell Fact 10:53
Wang M, Zhang Y, Li B, Zhu J (2015) Construction of scFv that bind both fibronectin-binding protein A and clumping factor A of Stapylococcus aureus. Res Vet Sci 100:109–114
Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, Plückthun A (1999) High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res 59:5758–5767
Zhang J, Wang J, Zhong R, Niu B (2009) Optimization of human anti-HBsAg scFv secretary expression in Escherichia coli Zhonghua shi yan he lin chuang bing du xue za zhi= Zhonghua shiyan he linchuang bingduxue zazhi= Chin J Exp Clin Virol 23:50–52
Zheng P, Sun X, Guo L, Shen J (2015) Cloning, expression, and characterization of an acetolactate synthase (ALS) gene from Anabaena azotica. Process Biochem 50:1349–1356
Funding
This study was funded by the research deputy of Shahid Beheshti University of Medical Sciences in Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Atieh Hashemi and Masoumeh Rajabibazl contributed to the study conception and design. Material preparation, data collection and analysis were performed by Reyhaneh Najafi Soulari, Majid Basafa and Atieh Hashemi. The first draft of the manuscript was written by Atieh Hashemi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soulari, R.N., Basafa, M., Rajabibazl, M. et al. Effective Strategies to Overcome the Insolubility of Recombinant ScFv Antibody against EpCAM Extracellular Domain in E. coli. Int J Pept Res Ther 26, 2465–2474 (2020). https://doi.org/10.1007/s10989-020-10044-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10044-4