Abstract
Food consumption and body weight regulations are done in the hypothalamus. Indeed, hypothalamus is the brain’s main area in controlling food intake. Hypothalamus controls food intake via special nuclei such as: ARC, PVN, DMH, VMH and LHA. PVN are second order neurons. This nucleus received multiple inputs from different areas of the brain via specific and non-specific receptors. NPYR and α2-adrenoceptors are orexigenic receptors, but Melanocortin receptors are anorexigenic receptors. PVN integrated inputs from multiple areas, and then transmitted various outputs for central regulation of food intake. To identify neural pathways and the role of neurotransmitters on central control of food intake in PVN, multiple researches have been done via injection of various neurotransmitters on the laboratory animals. Leptin applies its effect on regulation of feeding behaviours through arcuate nucleus-paraventricular nucleus axis and MC4R receptor. Also, Insulin is a hypophagic neurotransmitter, and its hypophagic effect applied in PVN via NPY1 and MC4R receptors. Ghrelin has a biphasic effect on food intake in birds and mammals. In birds, ghrelin inhibits food intake, but in mammals stimulated food intake. In birds, ghrelin via 5HT2A receptor on CRF neurons located on PVN reduced food intake. In mammals via GHSR (ghrelin receptor) and NPY1 receptors increased food intake. Somatostatin acts in the brain as an inhibitory and hyperphagic neurotransmitter. Hyperphagic effect of Somatostatin on PVN exert via SST2, OX1 and NPY1 receptors respectively. Therefore, PVN has an important effect on central regulation of food intake via different neurotransmitters and pathway.
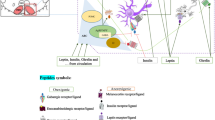
Graphic Abstract
The role of PVN in central regulation of food intake. ARC arcuate nucleus, PVN paraventricular nucleus, LHA lateral hypothalamus area, CRF Corticotropin-realising factor, POMC pro-opiomelanocortin, CART Cocaine and amphetamine-regulated transcript, NPY/AgRP neuropeptide Y/agouti-related protein, Y1R NPY1 receptor, GHSR growth hormone secretagogues receptor, LEPR Leptin receptor, INSR Insulin receptor, OX1R orexin receptor, MC4R Melanocortin4 receptor, SST2R Somatostatin2 receptor.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Regulation of food intake and body weight due to the ability of the brain, especially the hypothalamus to integrate endocrine, behavioural, and autonomic functions via afferent and efferent pathways from brainstem and peripheral organs done (Elmquist et al. 2005; Jalali et al. 2019). It has been shown that the hypothalamus is the brain’s main area in controlling food intake. The peripheral and central signals are integrated together in the hypothalamus, and they regulate the level of central neuropeptides for managing energy expenditure and food intake (Wynne et al. 2005; Denbow and Cline 2014). Various studies have been shown that, ventromedial hypothalamus (VMH) is an anorexigenic center and lateral hypothalamus area (LHA) is an orexigenic center (Sohn 2015).
The hypothalamus control of food intake via special nuclei such as: arcuate nucleus (ARC), paraventricular nucleus (PVN), dorsomedial hypothalamus (DMH), VMH, and LHA (Fig. 1) (Wynne et al. 2005). ARC is a hypothalamic nucleus involved in controlling food intake. It is located in the vicinity of the third ventricle of the brain. This nucleus receives appetite-related signals from the peripheral circulation system (through the incomplete blood–brain-barrier) and other areas of the brain, such as nucleus of tract solitary (NTS). The ARC integrates these signals, and transmitted them as neural projections such as NPY, AgRP, melanocortin, and etc. to the second order neurons. The second order neurons are PVN, VMH, DMH, and LHA (Fig. 2). These projections and other information from the brain stem and cortex integrated in second order neurons (especially in the PVN), and via changes in other neuroendocrine systems, effect on energy homeostasis (Fekete et al. 2000; Jensen 2001; Wynne et al. 2005; Hamidi and Yousefvand 2017).
Different hypothalamic nuclei involved in controlling food intake. ARC arcuate nucleus, AM amygdala, CC corpus callosum, CCX cerebral cortex, DMN dorsomedial nucleus, FX fornix, HI hippocampus, LHA lateral hypothalamic area, ME medi-an eminence, OC optic chiasm, PFA perifornical area, PVN paraventricular nucleus, SE septum, 3 V third ventricle, TH thalamus, VMN ventromedial nucleus (Yu and Kim 2012)
NPY projections in arcuate nucleus to second order neurons. 3rd third ventricle of the brain, ARC arcuate nucleus, PVN paraventricular nucleus, DMH dorsomedial hypothalamus, LH lateral hypothalamus, VMH ventromedial hypothalamus, NPY/AgRP neuropeptide Y/agouti-related protein, POMC pro-opiomelanocortin, Y1 NPY1 receptor, Y2 NPY2 receptor, Y5 NPY5 receptor (Joost 2012)
PVN, the main place of the hypothalamus to send the multiple outputs to control of food intake, and it is located in the dorso rostral hypothalamus, and adjust to the third brain ventricular (Cone 2005; Ferguson et al. 2008; Sohn 2015). PVN is an anorexigenic center. Indeed, researchers have shown that the damage to this nucleus causes overeating and obesity in rodents (Duplan et al. 2009). This nucleus receives the series of axons from the subcortical regions of the areas involved in motor behaviour including hypothalamus, hippocampus, amygdala, locus coeruleus, periaqueductal gravy matter and the raphe nucleus (Van der werf et al. 2002). As well, receive various inputs (such as insulin, leptin, and etc.) from other parts of the hypothalamus, and through this effect on food intake (Sutton et al. 2016). Therefore, PVN is a key area in the control of appetite. Projections from NPY-containing neurons in the ARC, are sent to the PVN, and through these regulate energy intake via other pathways such as Thyrotropin- releasing hormone (TRH) secretion (Nillni 2010). In addition, PVN is involved in the regulation of osmotic balance and water intake regulation. Numerous neurotransmitters effects water intake through this nucleus (De Arruda et al. 2003; De Souza Villa et al. 2008; Karasawa et al. 2014).
Considering the importance of PVN as a place for integrate multiple inputs to the hypothalamus and exporting appropriate hypothalamic outputs for central regulation of feeding behaviours, this study examined the role of PVN in the central regulation of nutritional behaviours.
Therefore, this paper will discusses about the non-specific receptors involved in PVN function in food intake, the signalling process related to central control of food intake, and finally the role of this nucleus in central control of feeding behaviours.
Study Methodology
In this overview, various valid papers from electronic sources used, which in them the role of paraventricular nucleus in regulation of feeding behaviours investigated. Authentic articles indexed in the Web of Science, Scopus, PubMed, SID, Google scholar, and ISI databases by using of Key words: Central regulation of food intake, Hypothalamus, Paraventricular nucleus, Brain ventriculars, Brain neurotransmitters, and Feeding behaviours studied (Fig. 3). The review articles entered in this study are presented in Table 1.
The Role OF Some Non-specific Receptors on PVN in Central Regulation of Food Intake
Central regulation of food intake is carried out in different nuclei of the hypothalamus by multiple inputs from various regions of the brain. Among the different nuclei of the hypothalamus, PVN plays a critical role in receiving and integrating these inputs. These inputs apply their effects on PVN through their specific and non-specific receptors located on the PVN (Lenard and Berthoud 2008; Sorrentino and Ragozzino 2017). Therefore, the receptors located on PVN play the important roles in the function of this nucleus in central regulation of food intake. Then, briefly discuss the roles of some of non-specific receptors.
-
1.1.
Neuropeptide Y (NPY) receptors: NPY receptors are important receptors in the central regulation of food intake. These receptors are distributed throughout the central nervous system (CNS). Among the NPY receptors involved in central food intake regulation (NPY1, NPY2, and NPY5), NPY1 and NPY5 receptors are located in the PVN. NPY receptors are G-protein coupled receptors. By attaching the ligand to these receptors, the output of PVN is a central increase in food intake. In fact, these receptors are orexigenic receptors (Henry et al. 2005; Yousefvand et al. 2018a, b, 2019).
-
1.2.
Melanocortin (MC) receptors: Hypothalamic melanocortin system consists of pro-opiomelanocortin (POMC), and central melanocortin receptors include: MCR3 and MCR4. These two receptors are important mediator of the effects of melanocortin ligands such as: alpha-melanocyte-stimulating hormone (α-MSH) and agouti related protein (AGRP) on nutritional behaviour and energy balance. In birds, only MC4R plays an important role in controlling energy balance (Strader et al. 2003; Lee et al. 2008). These receptors are located on PVN, and by their activation, reduce food intake and increase energy consumption. So these receptors are anorexigenic receptors, which located in the PVN (Kim et al. 2014).
-
1.3.
α2-adrenoceptors: α2-adrenoreceptors are involved in the central control of food intake, and from the G-protein coupled receptors family. These receptors are located on the PVN. α2-adrenoreceptors mediated the hyperphagic effects of noradrenaline, and other α2-adrenoreceptors mimics. Activation of these receptors leads to exit of orexigenic messages from PVN (Taksande et al. 2011). Therefore, these receptors are orexigenic receptors.
Signalling Mechanisms in the Paraventricular Nucleus
The signalling pathway of the neurotransmitters related to regulation of food intake in the PVN maybe is AMP-activated protein kinase (AMPK) signal. This signal is a heterodimer which including catalytic and regulatory subunits. Some factors such as leptin, insulin and MC3/MC4 agonist inhibits 2AMPK activity in the ARC nucleus and PVN, while another factors such as AgRP, stimulate 2AMPK. Indicated that, increasing in AMPK level in the PVN leads to accretion food intake. Activity of 2AMPK perhaps controlled with MC4R (Andersson et al. 2004). PVN with integration of multiple signals, initiates changes in other neuroendocrine systems.
Thyrotropin releasing hormone neurons (TRHN) in the PVN are innervated with NPY/AgRP and melanocortin projections from the ARC. NPY via inhibiting of phosphorylation of cAMP response element binding Protein (CREB), has an inhibitory effect on gene expression of pro-thyrotropin-releasing hormone (pro-TRH) in PVN (Fig. 4), while MSH projections apply motivatory effcet on gene expression of pro-TRH (Fekete et al. 2000). In fact MSH via increased phosphorylation of CREB, has a stimulatory effect on gene expression of pro-TRH. Therefore, there is an interaction between NPY and MSH neurons in regulating gene expression of pro-TRH in PVN. Projections of NPY to the PVN operate on corticotrophin releasing hormone-expressing neurons which effect on energy homeostasis (Sarkar and Lechan 2003).
The key appetite signal which integration in the special nuclei of the hypothalamus. ARC arcuate nucleus, PVN paraventricular nucleus, LHA lateral hypothalamus area, POMC pro-opiomelanocortin, CART Cocaine and amphetamine-regulated transcript, Y2R NPY2 receptor, GHSR growth hormone secretagogues receptor, LepR Leptin receptor, InsR Insulin receptor, ORX orexin receptor, TRH Thyrotropin-releasing hormone, CRH Corticotropin-releasing hormone, MCH Melanin-concentrating hormone (Schellekens et al. 2012)
The Role of Paraventricular Nucleus in Regulation of Feeding Behaviour
Many factors in the CNS are affected in regulating food intake in mammals and birds. In these species central regulation of food intake is complicated and impressed by many neurotransmitters (Richards et al. 2007; Hussain and Bloom 2013; Zendehdel et al. 2013a, c); although there are differences between these two species in regulation of feeding behaviour (Zendehdel and Hassanpour 2014). Mostly, feeding control neurons are located in the hypothalamus. It’s obvious that, the hypothalamus plays the major role in central regulation of food intake (Jensen 2001). Among the hypothalamus nuclei, PVN is very sensitive to injection of various neurohormones or neurotransmitters involved in feeding behaviour (Lawrence et al. 2002; Wynne et al. 2005). To identify neural pathways and the role of neurotransmitters on central control of food intake, various studies have been conducted on the laboratory animals (Table 2). These studies performed on regulation of food intake by direct injection into the nucleus, intracerebroventricular (ICV) injection, and injection to the third ventricular of the brain (Hamidi and Yousefvand 2017; Zendehdel et al. 2013b, 2015). Water intake and energy balance are important topics in physiology. Water is a critical component for living organisms and plays an important role in metabolic processes and temperature regulation (Biranvand et al. 2014; Yousefvand et al. 2017). In central control of water intake and osmotic pressure, different brain areas such as PVN are involved. Several studies in this field confirm the role of PVN in regulating water intake (Silverstein and Plisetskaya 2000; Hajdu et al. 2000; Hashimoto et al. 2007; Mietlicki et al. 2009; Karasawa et al. 2014b). The current review study was designed to summarize various studies performed on the PVN. The following is an explanation of the effect of some important neurotransmitters, which very effective in the processes involved in regulation of food intake, and numerous studies have been done them (Table 2) on central regulation of feeding behaviour and their mechanism of action in the PVN. However, in some cases the contradictory effects of these neurotransmitters in central control of food intake have been reported.
Leptin is the most important mediators involved in controlling energy homeostasis. This mediator acts on central control of energy expenditure through arcuate nucleus-paraventricular nucleus axis (Ahima et al. 2000). Leptin directly controls the circuits involved in controlling energy balance which from the arcuate nucleus to the paraventricular nucleus. This Probabilistic control is done via MC4R receptor and in three ways: (1) direct postsynaptic modulation by leptin. (2) Regulation of MC4R mRNA expression and α-MSH responsiveness by leptin. (3) Regulation by the constitutive activity of the MC4R signalling (Ghamari-Langroudi and Cone 2011).
Insulin as a hormone secreted from pancreas and controls blood sugar, and it is an important adiposetic signal to the brain. Insulin synthesized in the brain nuclei and has direct effects on central control of food intake and energy consumption (Plum et al. 2005). Several researches have been conducted on the effect of central insulin on regulation of food intake (Benoit et al. 2002; Honda et al. 2007; Shiraishi et al. 2008, Shiraishi et al. 2011; Yousefvand et al. 2018a, 2019). Central insulin reduces food intake and its hypophagic effect as follows: Insulin receptors are located on the POMC and NPY neurons in ARC nucleus, and as well POMC (MC3/4R) and NPY1 receptors are located on the PVN. Administration of insulin to the ventricles of the brain resulted in POMC neurons stimulated and NPY neurons inhibited, so increased in POMC and decreased in NPY gene expression. After that, POMC level raised and NPY level decreased. Increased in POMC level resulted in stimulated the POMC receptors and decreased in NPY level in synaptic space cussed reduced activity of NPY1 receptor (Benoit et al. 2002; Yousefvand et al. 2018a). Therefore PVN exported hypophagic output, and cusses reduction in food intake.
Ghrelin neurotransmitter is involved in the central control of food intake. Ghrelin inhibits food intake in birds, but in mammals is a strong stimulant for food intake. This contradiction shows the difference in central regulation of food intake between mammals and birds (Zendehdel and Hassanpour 2014; Denbow and Cline 2014; Thomas et al. 2015). The hypothetical mechanism of inhibition of food intake in birds by ghrelin neurotransmitter: ghrelin (by ICV injection) stimulates the 5Hydroxytryptamin2A (5HT2A) receptor in Corticotropin-releasing factor (CRF) neurons located on PVN. CRF neurons are anorexigenic neurons in PVN, and receive messages from ARC nucleus through receptors that are located on it. Ghrelin stimulated these neurons, and increase CRF expression. By increasing CRF expression, the PVN exerts an inhibitory message for food intake. Therefore reduced food intake (Saito et al. 2005; Honda et al. 2007; Zendehdel et al. 2013c; Dos-Santos et al. 2018). The assumptive mechanism for increase food intake via ghrelin in mammals: the growth hormone secretagogues receptor (GHSR) (ghrelin receptor) located on AGRP/NPY neurons on ARC nucleus. ICV injection of ghrelin resulted in stimulated AGRP/NPY neurons via GHSR, and increased NPY level and excited NPY1 receptor on PVN (Thomas et al. 2015). Therefore via stimulation of NPY1 receptor on PVN, increased food intake. Ghrelin in mammals exert hyperphagic effect on food intake via NPY/AGRP neurons in ARC nucleus and NPY1 receptor in PVN (Kamegai et al. 2000; Wren et al. 2001; Nakazato et al. 2001).
Somatostatin is known to be a hypothalamic inhibitor of the secretion of growth hormone from pituitary gland. Somatostatin acts in the brain as an inhibitory neurotransmitter. This neurotransmitter distributed in multiple areas of the brain, particularly in ARC, PVN, and VMH in hypothalamus. Since these brain regions involved in central control of food intake, the role of somatostatin in central regulation of food intake is not surprising (Viollet et al. 2008; Schneeberger et al. 2014; Stengel et al. 2015). Somatostatin has an increased food intake via a different neural pathway in the PVN. From different somatostatin receptors (SST1-SST5), SST2/SST3 receptors are located on the PVN (Stengel et al. 2015). Indicated that, SST2 receptor is the primary role in the orexigenic effect of somatostatin. SST2 receptor activated Orexin1 receptor (OX1R) in LHA, and this receptor stimulated NPY1 receptor on PVN. Therefore PVN increased central food intake (Stengel et al. 2010a, b; Karasawa et al. 2014; Yousefvand et al. 2018b).
Conclusion
The PVN is an important nucleus for central regulation of food intake in the hypothalamus. This nucleus receives many inputs from different areas of the brain such as: hypothalamus, hippocampus, amygdala, locus coeruleus, periaqueductal gravy matter and the raphe nucleus and peripheral circulation include: insulin, leptin, and etc. then, integrated these inputs and transmitted final outputs for other reigns for effect on food intake. On this nucleus, there are several receptors through which they receive different signals and send the required response. Many neurotransmitters, either inside or outside of this nucleus (due to neuromodulators), have their effects on food intake. Because the number of neurotransmitters was high, in this review article just to mention of the most important neurotransmitters, including leptin, insulin, ghrelin, and somatostatin. Leptin and insulin is an anorexigenic, somatostatin is orexigenic neurotransmitters, and ghrelin has biphasic effect: in birds, anorexigenic and in mammals, orexigenic neurotransmitter. These neurotransmitters apply their effects on central regulation of food intake via PVN.
Abbreviations
- VMH:
-
Ventromedial hypothalamus
- LHA:
-
Lateral hypothalamus area
- ARC:
-
Arcuate nucleus
- PVN:
-
Paraventricular nucleus
- DMH:
-
Dorsomedial hypothalamus
- NTS:
-
Nucleus of solitary tract
- TRH:
-
Thyrotropin- releasing hormone
- NPY:
-
Neuropeptide Y
- CNS:
-
Central nervous system
- MC:
-
Melanocortin
- POMC:
-
Pro-opiomelanocortin
- α-MSH:
-
α-Melanocyte-Stimulating Hormone
- AgRP:
-
Agouti related protein
- AMPK:
-
AMP-activated protein kinase
- TRHN:
-
Thyrotropin releasing hormone neurons
- CREB:
-
cAMP response element binding Protein
- Pro-TRH:
-
Pro-thyrotropin-releasing hormone
- ICV:
-
Intracerebroventricular
- 5HT2A :
-
5Hydroxytryptamin2A
- CRF:
-
Corticotropin-releasing factor
- GHSR:
-
Growth hormone secretagogues receptor
- SST:
-
Somatostatin receptor
- OX1R:
-
Orexin receptor
- AVP:
-
Arginine vasopressin
- N/OFQ:
-
Nociceptin/orphanin FQ
- CART:
-
Cocaine and amphetamine-regulated transcript
- mRNA:
-
Messenger ribo nucleic acid
- NEP:
-
Norepinephrine
- DA/Ach:
-
Dopaminergic/Cholinergic/Nucleus accumbens
- CRH:
-
Corticotropin releasing hormone
- HR:
-
Histaminergic receptor
References
Ahima RS, Saper CB, Flier JS, Elmquist JK (2000) Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21(3):263–307
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behaviour by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun 39(2):105–113
Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ (2004) AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279(13):12005–12008
Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC (2002) The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 22(20):9048–9052. https://doi.org/10.1523/JNEUROSCI.22-20-09048.2002
Biranvand ZS, Mousavi SG, Shamsollahi MO, Cheraghi JA, Taherpour KA (2014) Effects of chlorpheniramine (histamine H1 receptor antagonist) on food and water intake in broiler chickens in hunger and satiety. IJABBR 2(2):321–327
Bungo T, Shiraishi JI, Yanagita K, Ohta Y, Fujita M (2009) Effect of nociceptin/orphanin FQ on feeding behaviour and hypothalamic neuropeptide expression in layer-type chicks. Gen Comp Endocrinol 163(1–2):47–51
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8(5):571–578
De Arruda Camargo LA, Saad WA, Cerri PS (2003) Effects of V1 and angiotensin receptor subtypes of the paraventricular nucleus on the water intake induced by vasopressin injected into the lateral septal area. Brain Res Bull 61(5):481–487
De Souza Villa P, Menani JV, de Arruda Camargo GM, de Arruda Camargo LA, Saad WA (2008) Activation of the serotonergic 5-HT1A receptor in the paraventricular nucleus of the hypothalamus inhibits water intake and increases urinary excretion in water-deprived rats. Regul Pept 150(1–3):14–20
Denbow DM, Cline MA (2014) Food intake regulation. In: Scanes CG (ed) Sturkie’s avian physiology. Academic Press, Ireland, pp 469–485
Dos-Santos RC, Grover HM, Reis LC, Ferguson AV, Mecawi AS (2018) Electrophysiological effects of ghrelin in the hypothalamic paraventricular nucleus neurons. Front Cell Neurosci 12(275):1–15
Duplan SM, Boucher F, Alexandrov L, Michaud JL (2009) Impact of Sim1 gene dosage on the development of the paraventricular and supraoptic nuclei of the hypothalamus. Eur J Neurosci 30(12):2239–2249
Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB (2005) Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493(1):63–71
Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM (2000) α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci 20(4):1550–1558
Ferguson AV, Latchford KJ, Samson WK (2008) The paraventricular nucleus of the hypothalamus—a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12(6):717–727
Ghamari-Langroudi M, Cone RD (2011) Shining a light on energy homeostasis. Cell Metab 13(3):235–236
Hajdu I, Obal F Jr, Gardi J, Laczi F, Krueger JM (2000) Octreotide-induced drinking, vasopressin, and pressure responses: role of central angiotensin and ACh. Am J Physiol Regul Integr Comp Physiol 279(1):271–277
Hajnal A, Mark GP, Rada PV, Lénárd L, Hoebel BG (1997) Norepinephrine microinjections in the hypothalamic paraventricular nucleus increase extracellular dopamine and decrease acetylcholine in the nucleus accumbens: relevance to feeding reinforcement. J Neurochem 68(2):667–674
Hamidi F, Yousefvand S (2017) Role of the hypothalamic arcuate nucleus in regulation of food intake (review study). J Neyshabur Univ Med Sci 5(1):52–65 (Persian)
Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, Takei Y, Ueta Y (2007) Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology 148(4):1638–1647
Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR (2000) A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96(1–2):45–51
Henry M, Ghibaudi L, Gao J, Hwa JJ (2005) Energy metabolic profile of mice after chronic activation of central NPY Y1, Y2, or Y5 receptors. Obes Res 13(1):36–47
Honda K, Kamisoyama H, Saneyasu T, Sugahara K, Hasegawa S (2007) Central administration of insulin suppresses food intake in chicks. Neurosci Lett 423(2):153–157. https://doi.org/10.1016/j.neulet.2007.07.004
Hussain SS, Bloom SR (2013) The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes 37(5):625–633. https://doi.org/10.1038/ijo.2012.93
Jalali M, Zendehdel M, Babapour V, Gilanpour H (2019) Interaction between central oxytocinergic and glutamatergic systems on food intake in neonatal chicks: role of NMDA and AMPA receptors. Int J Pept Res Ther 25(1):195–203
Jensen J (2001) Regulatory peptides and control of food intake in non-mammalian vertebrates. Comp Biochem Physiol A: Mol Integr Physiol 128(3):469–477. https://doi.org/10.1016/S1095-6433(00)00329-9
Joost HG (2012) Appetite control. Springer, Berlin
Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I (2000) Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 141(12):4797–4800
Karasawa H, Yakabi S, Wang L, Taché Y (2014) Orexin-1 receptor mediates the increased food and water intake induced by intracerebroventricular injection of the stable somatostatin pan-agonist, ODT8-SST in rats. Neurosci Lett 576:88–92
Kim JD, Leyva S, Diano S (2014) Hormonal regulation of the hypothalamic melanocortin system. Front Physiol 5:480
Lawrence CB, Snape AC, Baudoin FM, Luckman SM (2002) Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143(1):155–162
Lee M, Kim A, Conwell IM, Hruby V, Mayorov A, Cai M, Wardlaw SL (2008) Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides 29(3):440–447
Lenard NR, Berthoud HR (2008) Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity 16(3):11–22
Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T (2009) Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10(5):355–365
Mahzouni M, Zendehdel M, Babapour V, Charkhkar S (2016) Methylamine induced hypophagia is mediated via dopamine D1 and D2 receptors in neonatal meat chicks. Vet Res Commun 40(1):21–27
Mietlicki EG, Nowak EL, Daniels D (2009) The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav 96(1):37–43
Miller GD (2017) Appetite regulation: hormones, peptides, and neurotransmitters and their role in obesity. Am J Lifestyle Med. https://doi.org/10.1177/1559827617716376
Mirnaghizadeh SV, Zendehdel M, Babapour V (2017) Involvement of histaminergic and noradrenergic receptors in the oxytocin-induced food intake in neonatal meat-type chicks. Vet Res Commun 41(1):57–66
Mortezaei SS, Zendehdel M, Babapour V, Hasani K (2013) The role of glutamatergic and GABAergic systems on serotonin-induced feeding behaviour in chicken. Vet Res Commun 37(4):303–310
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198
Nillni EA (2010) Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol 31(2):134–156
Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP (2014) AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab 3(2):209–215
Plum L, Schubert M, Brüning JC (2005) The role of insulin receptor signalling in the brain. Trends Endocrinol Metab 16(2):59–65
Rayatpour A, Ghasemi M, Radahmadi M, Izadi MS (2017) Effect of intraparaventricular administration of corticotropin releasing hormone on food intake in food-deprived rats. IUMS 35(436):770–775
Richards MP, Proszkowiec-Weglarz M (2007) Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poul Sci 86(7):1478–1490. https://doi.org/10.1093/ps/86.7.1478
Rodgers RJ, Halford JC, De Souza RN, De Souza AC, Piper DC, Arch JR, Blundell JE (2000) Dose—response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept 96(1–2):71–84
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125(1–3):201–208
Sarkar S, Lechan RM (2003) Central administration of neuropeptide Y reduces α-melanocyte-stimulating hormone-induced cyclic adenosine 5′-monophosphate response element binding protein (CREB) phosphorylation in pro-thyrotropin-releasing hormone neurons and increases CREB phosphorylation in corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 144(1):281–291
Schellekens H, Finger BC, Dinan TG, Cryan JF (2012) Ghrelin signalling and obesity: at the interface of stress, mood and food reward. Pharmacol Ther 135(3):316–326
Schneeberger M, Gomis R, Claret M (2014) Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J Endocrinol 220(2):25–46
Shiraishi JI, Yanagita K, Fujita M, Bungo T (2008) Central insulin suppresses feeding behaviour via melanocortins in chicks. Domest Anim Endocrinol 34(3):223–228. https://doi.org/10.1016/j.domaniend.2007.05.002
Shiraishi JI, Yanagita K, Fukumori R, Sugino T, Fujita M, Kawakami SI, McMurtry JP, Bungo T (2011) Comparisons of insulin related parameters in commercial-type chicks: evidence for insulin resistance in broiler chicks. Physiol Behav 103(2):233–239
Silverstein JT, Plisetskaya EM (2000) The effects of NPY and insulin on food intake regulation in fish. Am Zool 40(2):296–308
Sohn JW (2015) Network of hypothalamic neurons that control appetite. BMB Rep 48(4):229
Sorrentino M, Ragozzino G (2017) The regulation of food intake: the brain-endocrine network. Int J Clin Endocrinol Metab 1(1):041–048
Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y (2010a) Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology 151(9):4224–4235
Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y (2010b) Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav 101(5):614–622
Stengel A, Karasawa H, Taché Y (2015) The role of brain somatostatin receptor 2 in the regulation of feeding and drinking behaviour. Horm Behav 73:15–22
Strader AD, Schiöth HB, Buntin JD (2003) The role of the melanocortin system and the melanocortin-4 receptor in ring dove (Streptopelia risoria) feeding behaviour. Brain Res 960(1–2):112–121
Sutton AK, Myers MG Jr, Olson DP (2016) The role of PVH circuits in leptin action and energy balance. Annu Rev Physiol 78:207–221
Tajalli S, Jonaidi H, Abbasnejad M, Denbow DM (2006) Interaction between nociceptin/orphanin FQ (N/OFQ) and GABA in response to feeding. Physiol Behav 89(3):410–413
Taksande BG, Kotagale NR, Nakhate KT, Mali PD, Kokare DM, Hirani K, Subhedar NK, Chopde CT, Ugale RR (2011) Agmatine in the hypothalamic paraventricular nucleus stimulates feeding in rats: involvement of neuropeptide Y. Br J Pharmacol 164(2b):704–718
Thomas MA, Ryu V, Bartness TJ (2015) Central ghrelin increases food foraging/hoarding that is blocked by GHSR antagonism and attenuates hypothalamic paraventricular nucleus neuronal activation. Am J Physiol Heart Circ Physiol 310:275–285
Van der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39(2–3):107–140
Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J (2008) Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol 286(1–2):75–87
Wirth MM, Olszewski PK, Yu C, Levine AS, Giraudo SQ (2001) Paraventricular hypothalamic α-melanocyte-stimulating hormone and MTII reduce feeding without causing aversive effects. Peptides 22(1):129–134
Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR (2001) Ghrelin causes hyperphagia and obesity in rats. Diabetes 50(11):2540–2547
Wynne K, Stanley S, McGowan B, Bloom S (2005) Appetite control. J Endocrinol 184(2):291–318. https://doi.org/10.1677/joe.1.05866
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2017) Effects of insulin and somatostatin on water intake in neonatal chickens. Iran JPP 2(3):166–173
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2018a) Hypophagic effects of insulin are mediated via NPY1/NPY2 receptors in broiler cockerels. Can J Physiol Pharmacol 96(12):1301–1307
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2018b) Interaction of neuropeptide Y receptors (NPY1, NPY2 and NPY5) with somatostatin on somatostatin-induced feeding behaviour in neonatal chicken. Br Poult Sci 60(1):71–78
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2019) Survey the effect of insulin on modulating feed intake via NPY receptors in 5-day-old chickens. Int J Pept Res Ther 12:1–10
Yu JH, Kim MS (2012) Molecular mechanisms of appetite regulation. Diabetes Metab J 36(6):391–398
Zendehdel M, Hassanpour S (2014) Central regulation of food intake in mammals and birds: a review. Neurotransmitter 12(1):1–7. https://doi.org/10.14800/nt.251
Zendehdel M, Hamidi F, Babapour V, Mokhtarpouriani K, Fard RM (2012a) The effect of melanocortin (Mc3 and Mc4) antagonists on serotonin-induced food and water intake of broiler cockerels. J Vet Sci 13(3):229–234
Zendehdel M, Hamidi F, Babapour V, Taghavian F (2012b) The effect of intracerebroventricular injection of serotonin, parachlorophenylalanine and reserpine on food and water intake in food-deprived broiler cockerels. Iran Vet J 8(1):51–60
Zendehdel M, Mokhtarpouriani K, Babapour V, Baghbanzadeh A, Pourrahimi M, Hassanpour S (2013a) The effect of serotonergic system on nociceptin/orphanin FQ induced food intake in chicken. J Physiol Sci 63(4):271–277. https://doi.org/10.1007/s12576-013-0263-x
Zendehdel M, Mokhtarpouriani K, Babapour V, Pourrahimi M, Hamidi F (2013b) The role of 5-HT2A and 5-HT2C receptors on harmaline induced eating behaviour in 24-h food-deprived broiler cockerels. Iran J Vet Res 14(2):94–99
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2013c) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Cummun 37(1):37–41
Zendehdel M, Hasani K, Babapour V, Mortezaei SS, Khoshbakht Y, Hassanpour S (2014) Dopamine-induced hypophagia is mediated by D1 and 5HT-2c receptors in chicken. Vet Res Cummun 38(1):11–19
Zendehdel M, Hamidi F, Hassanpour S (2015) The effect of histaminergic system on nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther 21(2):179–186
Zendehdel M, Parvizi Z, Hassanpour S, Baghbanzadeh A, Hamidi F (2017) Interaction between nociceptin/orphanin FQ and adrenergic system on food intake in neonatal chicken. Int J Pept Res Ther 23(1):155–161
Acknowledgements
The authors would like to thank the Ferdowsi University of Mashhad for their support.
Author information
Authors and Affiliations
Contributions
All authors have agreed to be named as authors on this manuscript. Any work (data, text, or theories) of others besides the authors has been properly acknowledged. The work is original and not previously published. All data are true and accurate to the knowledge of the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest with the contents of this article.
Research Involving Human Participants and/or Animals
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
Farshid Hamidi: Idea of the article, performed literature search, graphic designer, revised the paper. Shiba Yousefvand: Performed literature search, writing the paper, graphic designer.
Rights and permissions
About this article
Cite this article
Yousefvand, S., Hamidi, F. Role of Paraventricular Nucleus in Regulation of Feeding Behaviour and the Design of Intranuclear Neuronal Pathway Communications. Int J Pept Res Ther 26, 1231–1242 (2020). https://doi.org/10.1007/s10989-019-09928-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09928-x