Abstract
Immunotoxins are chimeric proteins that combine antibodies and toxins in targeted cancer therapy. Despite the promising application prospects, immunogenicity and nonspecific cytotoxicity of immunotoxins with toxins from bacterial or plant origin have hindered their clinical application. In this study, an anti-HER2 single-chain antibody fragment 4D5, was fused to a human-origin antitumor drug, TRAIL to produce a humanized immunotoxin in Escherichia coli BL21 (DE3). An appropriate linker between the domains was selected using the software Antheprot 5.0. The 4D5 scFv-TRAIL was obtained using a SUMO tag for solubility-enhancing and purified by Ni–NTA affinity chromatography after tag removing. The purity and yield of 4D5 scFv-TRAIL reached 92% and 11.6 mg/L in shake flasks, respectively. Additionally, MTT assays showed 4D5 scFv-TRAIL exhibited selective in vitro cytotoxicity on the breast cancer cell line MDA-MB-231 without cytotoxic effect on the normal cell FBHE. Therefore, 4D5 scFv-TRAIL is a potential humanized immunotoxin for treatment of HER2-overexpressing tumor cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Immunotoxins are chimeric proteins consisting of an antibody fragment linked to a toxin moiety (Pastan et al. 2007) and show promise in targeted cancer therapy. To circumvent the immunogenicity and nonspecific toxicity caused by toxin moieties derived from bacteria and plants, researchers have developed humanized immunotoxins in which the toxin moiety is of human origin (Mathew and Verma 2009). The selective targeting and the apoptosis inducing of endogenous proteins to cancer cells are propitious to the design of chimeric proteins (Ichim and Tait 2016). Endogenous proteins that mediate apoptotic pathways could be fused with cytokines, peptide hormones, or antibody fragments (Mazor et al. 2014). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can selectively induce apoptosis in tumor cells, while not affecting the normal cells (de Miguel et al. 2016). Thus, TRAIL is a promising candidate for human-origin toxins. A combination of CD40-specific antibody derived single-chain variable fragment (scFv) G28-5 and TRAIL domain showed capacity for stimulation of antigen presenting cells and apoptosis induction (Liu et al. 2017).
Herceptin is an approved antibody for the treatment of human epidermal growth factor receptor 2 (HER2) overexpressing tumors. This antibody binds to tumor cells overexpressing HER2 and inhibits cell growth (Baselga et al. 1998). The derived humanized single-chain antibody 4D5 is constructed by combining variable domains of light with heavy chains of Herceptin. The 4D5 scFv is thermodynamically stable and shows specificity for the HER2 antigen with high affinity (Zhang et al. 2013). Previously, 4D5 scFv was proven to be suitable for bi-functional cytotoxic protein generation (Lv et al. 2016; Willuda et al. 1999). Therefore, the single-chain antibody was a potential candidate for the antibody moiety of immunotoxin.
To generate potential immunotoxin targeting at HER2/neu overexpressing tumors, the 4D5 scFv moiety and TRAIL moiety were conjugated genetically. However, soluble expression and protein function were common problems when fusion protein was expressed in E.coli. The linker insertion between fusion parts was reported to be useful for fusion protein expression and folding (Huang. et al. 2013). The structure and activity of fusion proteins can be affected by linkers. Thus, the design and analysis of suitable linkers has been a research focus. The linker sequence should be rationally designed and carefully verified leading to complicated and time-consuming process for linker selection (Seras-Franzoso et al. 2014). The protein analysis software, Antheport, developed for predicting the physical and chemical properties of proteins and further analyzing the differences between proteins (Deléage 2012) may allow an easier linker selection process for researchers.
In this study, we genetically fused TRAIL to 4D5 scFv using a flexible linker selected by Antheport to produce a desired immunotoxin for treating HER2/neu-overexpressing tumor cells. The 4D5 scFv-TRAIL was successfully expressed in E. coli. The in vitro cytotoxicity of 4D5 scFv-TRAIL indicated that it was a potential recombinant immunotoxin.
Materials and Methods
Strains, Plasmids and Reagents
The E. coli strains DH5α and BL21(DE3) and the plasmid pET28a(+) were used for recombinant strain and plasmid construction. The cell lines NCI-H460 and MCF-7 and MDA-MB-231 cells were preserved in our laboratory.
The genes were cloned with primer pairs to create the plasmids pET-SUMO-4D5 scFv-TRAIL and pET-SUMO-TRAIL. The expression of recombinant protein was controlled by the T7 promoter. The molecular weights of SUMO-4D5 scFv-TRAIL and SUMO-TRAIL were approximately 59 kDa and 32 kDa, respectively. The immunotoxin expression strain E. coli BL21(DE3)/pET-SUMO-4D5 scFv-TRAIL and E. coli BL21(DE3)/pET-SUMO-TRAIL was constructed by transforming pET-SUMO-4D5 scFv-TRAIL and pET-SUMO-TRAIL into E. coli BL21(DE3), respectively. The details of the strains and plasmids are shown in Table 1. The primers used are listed in Table 2.
Induction of Recombinant Immunotoxin
Bacteria were incubated at 30 °C until they reached an OD600 of approximately 0.6, and the temperature subsequently decreased to 18 °C for induction. The recombinant protein production was induced by adding 1 mM IPTG to the culture. Cells were collected by centrifugation after an overnight induction, washed twice with wash buffer (PBS, pH 7.4) and resuspended in PBS with 10 mM imidazole (pH 7.4). The harvested cell lysis was performed by sonication at 200 W. Afterwards, the supernatant and precipitation were separated by centrifugation at 12,000 rpm for 20 min.
Separation and Purification of Soluble Immunotoxin
Then, the supernatant of cell lysate was applied to a Ni–NTA column for purification. Samples were eluted and collected in sequence with PBS containing 200 mM, 300 mM and 500 mM imidazole (pH 7.4). The solution obtained by 300 mM imidazole was adequately dialyzed twice for 4 h each time with PBS. Then, 4D5 scFv-TRAIL was obtained by removing the His-SUMO tag using SUMO protease after incubation with the dialyzed solution overnight. The supernatant was then applied to a Ni–NTA column for a second time. Next, 4D5 scFv-TRAIL was eluted by PBS with 50 mM imidazole (pH 7.4), while the other components bound to the column due to the His tag. Subsequently, 4D5 scFv-TRAIL was adequately dialyzed twice for 4 h in PBS at 4 °C.
Protein Analysis
The protein samples were collected and loaded in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The obtained SDS–PAGE gels were analyzed using a Gel Analyzer. The concentration of samples was measured using a BCA protein assay kit (Biomega, USA). At least three independent experiments were repeated for further analysis.
In Vitro Cytotoxicity Assay
The in vitro cytotoxicity of immunotoxin was determined by MTT assays. A total of 1 × 104 tumor cells were inoculated in each well. The immunotoxins were added to the wells after the cells were incubated at 37 °C for 24 h. One hundred microliters of sample per well was added and further incubated for another 48 h (Zhu et al. 2006). The same amount of RPMI-1640 medium was used as a control. Then, 20 μL per well of MTT was added and coincubated with the cells for another 4 h. The cytotoxicity of the protein samples was determined in triplicate.
Statistical Analysis
All data were collected from at least three independent experiments. The data were statistically analyzed by Student’s t test. The difference was statistically significant when P < 0.05.
Results and Discussion
Design of the Recombinant Immunotoxin 4D5 scFv-TRAIL
The gene for the 4D5 scFv, targeting HER2-overexpressing tumor cells, and the human TRAIL (114–281) gene were used as templates for the construction of the recombinant immunotoxin. The overall functionality and performance of immunotoxins can be significantly affected by their orientation (Cao et al. 2013). As for some TNF-family variants, receptor binding and activation could be interfered by protein domains fused C-terminally to the TNF ligands (Müller et al. 2010). Therefore, 4D5 scFv was designed to fuse to the N-terminus of TRAIL. The template genes were first fused together and expressed under the T7 promoter of pET-28a. However, the recombinant immunotoxin was expressed almost in the form of inclusion bodies as shown in Fig. 1. Experiments to optimize the in vitro renaturation process failed to remarkably strengthen the soluble expression level. Thus, the immunotoxin need optimizing to obtain soluble and active proteins. The SUMO tag was useful to assist protein folding into the correct steric conformation in the E. coli host system (Butt et al. 2005). The solubility-promoting tag SUMO fused to the N-terminus of the recombinant immunotoxin may achieve soluble expression. Furthermore, a linker was needed between the two domains to avoid the effects due to the limited space when expressed.
The expression of recombinant 4D5 scFv-TRAIL and SUMO-4D5 scFv-TRAIL and. Lane 1 refers to the precipitate of cell lysate of E. coli BL21(DE3)/pET-4D5 scFv-TRAIL; lane 2: the supernatant of cell lysate of E. coli BL21(DE3)/pET-4D5 scFv-TRAIL; lane 3: induced whole cell lysate of E. coli BL21(DE3)/pET-4D5 scFv-TRAIL; lane 4: the precipitate of cell lysate of E. coli BL21(DE3)/pET-SUMO-4D5 scFv-TRAIL; lane 5: the supernatant of cell lysate of E. coli BL21(DE3)/pET-SUMO-4D5 scFv-TRAIL; lane 6: induced whole cell lysate of E. coli BL21(DE3)/pET-SUMO-4D5 scFv-TRAIL
Selection of the Linker Between 4D5 scFv and TRAIL
The structure and bioactivity of recombinant immunotoxins can be significantly influenced by the component domains (Lebendiker and Danieli 2016). Linker insertion was reported to not only improve the expression efficiency of the fusion protein but also assist polypeptide folding and maintain the biological activity of component domains.
Therefore, software with searching algorithms was developed to screen suitable linker sequences for different fusion parts (George and Heringa 2002). These databases and software have allowed researchers to perform high-throughput linker selection. The software Antheprot 5.0 was used to select a suitable linker for 4D5 scFv-TRAIL in this study (Deléage 2012; Geourjon et al. 1991).
Based on the characteristics of linking and fusing, linkers selected in this study were designed by primarily considering flexibility and length. The majority of empirical linkers have been divided into flexible, rigid and in vivo cleavable linkers (Chen et al. 2013). Six linkers were predicted using this software (Table 3). The flexible linker was (GGGGS)n, and the rigid linker was PKPSTPPGGS. The six linkers with different lengths and flexibilities were designed as following: (GGGGS)2, (GGGGS)4, PKPSTPPGGS, (PKPSTPPGGS)2, GGGSPKPSTPPGGGS and GGGS(PKPSTPP)2GGGS. Because only the linker sequence was different in each protein, differences between the images analyzed using Antheport 5.0 were only shown around the position of the linker.
The flexibility of each protein was shown in Fig. 2. The higher the value was, the more flexible the protein was. According to Fig. 2, the predicted flexibility of each linker was consistent with the flexibility designed. The protein with linker (GGGGS)4 was the longest and the most flexible. The proteins containing the rigid linker (No. 3 to No. 6) was less flexible than the other proteins (No. 1 and No. 2).
The solvent accessibility mainly indicates whether amino acids are exposed to the solvent, which is an important means to predict the hydrophobicity of proteins. As shown in Fig. 3, the solvent accessibility of protein with the rigid linker (No. 4) was the highest, and thus, the amino acids around the rigid linker are predicted to be mostly exposed to the solvent. According to the prediction, the solvent accessibility of proteins increased with the rigidity of the linker. The solvent accessibility of amino acids around the flexible linker (No. 1 and No. 2) was lower than that of the other linkers. The amino acids were inferred to be mainly hidden inside the protein, suggesting that the immunotoxin was less hydrophobic than the others. Moreover, as the length of linker and the distance between the two domains increased, the steric hindrance was minimized so that the two domains were less affected by the fusion linked with (GGGGS)4 than (GGGGS)2. Thus, (GGGGS)4 was chosen as the linker between the two domains. For fusion proteins expressed as inclusion bodies, in vitro refolding is a complicated and time-consuming process. This linker selection method was proposed for its accuracy and convenience.
To confirm the 3D structure of fusion protein by different linkers, Phyre2 was used to predict and analyze the structural information. According to Fig. 4, the third structure of 4D5 scFv-TRAIL with linker 4 was more extend than the others. This may due to its strong rigid property. The structure of 4D5 scFv-TRAIL with other linkers were more compact. Nine ligand binding site residues were predicted with linker 2, while the number of binding site were two-four with the other linkers. The binding sites predicted were GLY 100, ASP 102, ASP 108, SER 122, GLY 123, PHE 189, LEU 190, TYR 191 and SER192. The more binding sites have the benefit of binding with HER-2 over-expressing cells. On the other hand, homo-trimer formed by recombinant TRAIL or its fusion proteins was reported to be crucial for their biological activity (Luo et al. 2006). The TRAIL domain was less interfered by the fused 4D5 scFv domain since the binding sites were mainly on the 4D5 scFv domain.
Expression and Purification of 4D5 scFv-TRAIL
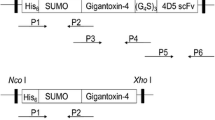
The recombinant plasmids were constructed as shown in Fig. 5. Three pairs of primers were designed to clone the gene encoding the immunotoxin. The amplified fragments were subsequently inserted into pET28a(+) to construct the expression vector. Plasmid pET28a/SUMO-TRAIL was constructed as a control. As shown in Fig. 1, SUMO-4D5 scFv-TRAIL (approximately 59 kD) was successfully expressed in E. coli BL21(DE3) by inducing with 0.1 mM IPTG. According to the result, the 4D5 scFv-TRAIL expressed without tag was almost in form of inclusion body. However, the SUMO-4D5 scFv-TRAIL was expressed in both soluble and insoluble form. The percentage of SUMO-4D5 scFv-TRAIL in total soluble proteins (29.5%) were higher than that of 4D5 scFv-TRAIL (8.86%).
The supernatant of the cell lysate was collected after recombinant protein expression and loaded onto a Ni–NTA Sepharose column to obtain the SUMO-tagged 4D5 scFv-TRAIL. The tagged immunotoxin was eluted with 200 mM, 300 mM and 500 mM imidazole in sequence. Elution with 300 mM imidazole resulted in the least impurities. The eluate with 300 mM imidazole was dialyzed to remove the imidazole for further purification. SUMO tag was accurately recognized and efficiently cleaved by SUMO protease (Butt et al. 2005). The mixture was then applied to the Ni–NTA column again to obtain purified target protein. The 4D5 scFv-TRAIL was almost not adsorbed by the column and separated with the other proteins in the mixture. The purities of SUMO-4D5 scFv-TRAIL and 4D5 scFv-TRAIL were 86.2% and 92%, respectively (Fig. 6).
Purification of SUMO-4D5 scFv-TRAIL and 4D5 scFv-TRAIL. Lane 1: cell lysate of E. coli BL21(DE3)/pET-4D5 scFv-TRAIL; lane 2: unbound proteins; lane 3: samples eluted by 200 mM imidazole; lane 4: samples eluted by 300 mM imidazole; lane 5: samples eluted by 500 mM imidazole; lane 6: supernatant of samples digested by SUMO protease after dialysis; lane 7: precipitate of samples digested by SUMO protease after dialysis; lane 8: purified 4D5 scFv-TRAIL; lane 9: samples eluted by 500 mM imidazole; M: molecular weight marker
In Vitro Cytotoxic Activity of 4D5 scFv-TRAIL
To detect the inhibitory effect of different concentrations of 4D5 scFv-TRAIL on different tumor cells, we studied the inhibition of cell proliferation by MTT assays. In this experiment, TRAIL was used as a control, and the MTT assay was conducted using different cell lines, namely, the non-small cell lung cancer cell line NCI-H460, the breast cancer cell lines MDA-MB-231 and MCF-7 and the fetal bovine heart endothelial cell line FBHE.
In previous study NCI-H460 cells were shown to be sensitive to TRAIL (Fan et al. 2016; Wang et al. 2018). Thus, TRAIL was used as a control to examine the inhibitory effect of the fusion protein on this cell line to assess whether the activity of the TRAIL domain in the immunotoxin was affected by fusion. The two HER-2-overexpressing cells MCF-7 and MDA-MB-231 cell lines were insensitive and sensitive to TRAIL, respectively (Park et al. 2008; Shankar et al. 2008). They were used to determine the cytotoxicity of 4D5 scFv-TRAIL towards breast cancer cells for contrast.
The cell lines were incubated with 4D5 scFv-TRAIL and TRAIL protein for 24 h. According to the results of the MTT experiments shown in Fig. 7a and b, both the experimental group and the control group on of NCI-H460 cells showed a significant dose-dependent cytotoxic effect. We found that 4D5 scFv-TRAIL suppressed the proliferation of NCI-460 cells (Fig. 7a), while the cell growth inhibition effect on MDA-MB-231 cells was more pronounced than that on NCI-460 cells (P < 0.05) (Fig. 7b). The IC50 value of 4D5 scFv-TRAIL towards NCI-460 cells and MDA-MB-231 cells were 9.5 nM and 5.8 nM. The fusion of 4D5 scFv and TRAIL could increase its cytotoxicity towards MDA-MB-231 cells by one-fold compared with TRAIL. These results indicated that the immunotoxin retained activity of the TRAIL domain. As shown in Fig. 7a, the 4D5 scFv-TRAIL fusion protein had a weaker inhibitory effect on the proliferation of NCI-H460 cells than TRAIL. This indicated that the function of TRAIL domain in the immunotoxin was reduced, which was probably due to the interference between the two domains of the fusion protein (Lebendiker and Danieli 2016). In contrast, the immunotoxin inhibited the proliferation of the Her-2-overexpressing breast cancer cell line MDA-MB-231 more efficiently than TRAIL. The enhanced inhibitory activity was speculated to be associated with the targeting function of the 4D5 scFv domain in the immunotoxin.
From the experimental results shown in Fig. 7c, no significant difference was observed in cell viability between the experimental group and the control group (P > 0.05) of MCF-7 cells. Therefore, the fusion protein immunotoxin 4D5 scFv-TRAIL did not enhance the inhibition of MCF-7 cell proliferation. Deficient caspase-8 protein and caspase-3 resulted in defective stimulation of caspase-8 processing and correspondingly less cleavage of caspase-3 in MCF7 cells.(Zhang and Zhang 2008). The mechanism of MCF-7 cells resistance to TRAIL may be a major factor affecting the effect of immunotoxins.
The results shown in Fig. 7d indicated that the 4D5 scFv-TRAIL had little effect on the normal cell line FBHE. This may be due to the targeting property of 4D5 scFv to HER2-overexpression cells and selective property of TRAIL on cancer cell. The fusion may also affect the activity of TRAIL domain.
Conclusion
Immunotoxins have been widely studied and successfully generated (Brinkmann 2000). Targeted therapeutics have been a focus of cancer therapy research due to their high selectivity and efficacy compared to conventional therapy.
In this study, by utilizing a single chain antibody of human origin, a monovalent recombinant immunotoxin, TRAIL, fused to 4D5 scFv with a flexible linker, (GGGGS)4, was obtained. The humanized immunotoxin 4D5 scFv-TRAIL can specifically target to the surface of HER2-overexpressing tumor cells and inhibit cell growth while not affecting the normal cells. Therefore, 4D5 scFv-TRAIL has the potential to treat HER2/neu-positive tumors.
Abbreviations
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- HER2:
-
Human epidermal growth factor receptor 2
- scFv:
-
Single-chain antibody fragment
- SUMO:
-
Small ubiquitin-related modifier
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J (1998) Recombinant humanized Anti-HER2 antibody (Herceptin™) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58:2825–2831
Brinkmann U (2000) Recombinant antibody fragments and immunotoxin fusions for cancer therapy. Vivo 14:21
Butt TR, Edavettal SC, Hall JP, Mattern MR (2005) SUMO fusion technology for difficult-to-express proteins. Protein Expres Purif 43:1–9
Cao Y, Marks JW, Liu Z, Cheung LH, Hittelman WN, Rosenblum MG (2013) Design optimization and characterization of Her2/neu-targeted immunotoxins: comparative in vitro and in vivo efficacy studies. Oncogene 33:429–439
Chen X, Zaro JL, Shen W-C (2013) Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev 65:1357–1369
de Miguel D, Lemke J, Anel A, Walczak H, Martinez-Lostao L (2016) Onto better TRAILs for cancer treatment. Cell Death Differ 23:733–747
Deléage G (2012) An interactive 3D viewer of molecules compatible with the suite of ANTHEPROT programs. J Biophys Chem 3:35–38
Fan J, Wang Z, Huang L, Shen Y (2016) Efficient refolding of the bifunctional therapeutic fusion protein VAS-TRAIL by a triple agent solution. Protein Expr Purif 125:68–73
George RA, Heringa J (2002) An analysis of protein domain linkers: their classification and role in protein folding. Protein Eng 15:871
Geourjon C, Deléage G, Roux B (1991) Antheprot: an interactive graphics software for analyzing protein structures from sequences. J Mol Graph 9:188–190 167
Huang Z, Zhang C, Chen S, Ye F, Xing X (2013) Active inclusion bodies of acid phosphatase PhoC: aggregation induced by GFP fusion and activities modulated by linker flexibility. Microb Cell Fact 12(1):25
Ichim G, Tait SW (2016) A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer 16:539–548
Lebendiker M, Danieli T (2016) Production of prone-to-aggregate proteins. FEBS Lett 588:236–246
Liu PC et al (2017) Inhibition of NF-kappaB pathway and modulation of MAPK signaling pathways in glioblastoma and implications for lovastatin and tumor necrosis factor-related apoptosis inducing ligand (TRAIL) combination therapy. PLoS ONE 12:e0171157
Luo Y-Q, Wang L-H, Ma X-L, Kong J-X, Jiao B-H (2006) Construction, expression, and characterization of a new targeted bifunctional fusion protein: tumstatin45-132-TNF. IUBMB Life 58:647–653
Lv X, Zhang J, Xu R, Dong Y, Sun A, Shen Y, Wei D (2016) Gigantoxin-4-4D5 scFv is a novel recombinant immunotoxin with specific toxicity against HER2/neu-positive ovarian carcinoma cells. Appl Microbiol Biot 100:6403–6413
Mathew M, Verma RS (2009) Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer Sci 100:1359–1365
Mazor R et al (2014) Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc Natl Acad Sci USA 111:8571–8576
Müller N, Schneider B, Pfizenmaier K, Wajant H (2010) Superior serum half life of albumin tagged TNF ligands. Biochem Bioph Res Commun 396:793–799
Park C, Moon D-o, Ryu C-h, Bt Choi, Lee Wh, Kim G-y, Choi Yh (2008) β-Sitosterol sensitizes MDA-MB-231 cells to TRAIL-induced apoptosis. Acta Pharmacol Sin 29:341
Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ (2007) Immunotoxin treatment of cancer. Annu Rev Med 58:221–237
Seras-Franzoso J, Peebo K, García-Fruitós E, Vázquez E, Rinas U, Villaverde A (2014) Improving protein delivery of fibroblast growth factor-2 from bacterial inclusion bodies used as cell culture substrates. Acta Biomater 10:1354–1359
Shankar E, Sivaprasad U, Basu A (2008) Protein kinase Cɛ confers resistance of MCF-7 cells to TRAIL by Akt-dependent activation of Hdm2 and downregulation of p53. Oncogene 27:3957
Wang Z, Zhang M, Lv X, Fan J, Zhang J, Sun J, Shen Y (2018) GroEL/ES mediated the in vivo recovery of TRAIL inclusion bodies in Escherichia coli. Sci Rep 8:15766
Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeisterwittke U, Plückthun A (1999) High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res 59:5758
Zhang Y, Zhang B (2008) TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res 6:1861–1871
Zhang M et al (2013) Construction and characterization of a recombinant human beta defensin 2 fusion protein targeting the epidermal growth factor receptor: in vitro study. Appl Microbiol Biotechnol 97:3913–3923
Zhu H, Pan RJ, Wang TW, Shen YL, Wei DZ (2006) Functional solubilization of aggregation-prone TRAIL protein facilitated by coexpressing with protein isoaspartate methyltranferase. Appl Microbiol Biot 72:1033–1038
Acknowledgements
This work was supported by the Shanghai Leading Academic Discipline Project (Project: B505); the Research Program of State Key Laboratory of Bioreactor Engineering; and Grants from the National Natural Science Foundation of China (81673969).
Author information
Authors and Affiliations
Contributions
ZW conducted the experiments, analyzed the results, and wrote the paper. YS and LC conceived the idea for the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Chi, L. & Shen, Y. Design, Expression, Purification and Characterization of the Recombinant Immunotoxin 4D5 scFv-TRAIL. Int J Pept Res Ther 26, 889–897 (2020). https://doi.org/10.1007/s10989-019-09894-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09894-4