Abstract
Immunotoxins are a new class of antibody-targeted therapy in clinical development. Traditional immunotoxins that are constructed from the toxins of plants or bacteria need to be internalized to the cytoplasm and thus have limited antitumor efficacy. In the present study, we combined a recently reported sea anemone cytolysin Gigantoxin-4 with an anti-HER2/neu single-chain variable fragment 4D5 scFv to construct a novel immunotoxin. We fused a SUMO tag to the N-terminus of Gigantoxin-4-4D5 scFv and it was successfully expressed in Escherichia coli strain BL21 (DE3) in a soluble form. After purification, the purity of Gigantoxin-4-4D5 scFv reached 96 % and the yield was 14.3 mg/L. Our results demonstrated that Gigantoxin-4-4D5 scFv exerted a highly cytotoxic effect on the HER2/neu-positive ovarian carcinoma SK-OV-3 cell line. And the hemolytic activity was weaker, making it safe for normal cells. The results of immunofluorescence analysis showed that this novel immunotoxin could specifically bind to SK-OV-3 cells with no recognition of human embryonic kidney 293 cells. Scanning electron microscope observations and extracellular lactate dehydrogenase activity indicated that it could induce necrosis in SK-OV-3 cells by disrupting the cell membrane. Moreover, it could also mediate apoptosis of SK-OV-3 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunotoxins are fusion proteins composed of a toxin fused to an antibody or growth factor with distinct target specificity (Kreitman 2009). By binding to a specific receptor antigen on tumor cells, immunotoxins get internalized into the target cells and kill the cancer cells by inhibiting protein synthesis and activating important apoptosis cascades (Antignani and Fitzgerald 2013). Compared with other antitumor drugs, immunotoxins exhibit excellent specificity and potent antitumor ability that make them excellent candidate for cancer therapeutics. The toxins from plants or bacteria, such as gelonin, trichosanthin, diphtheria toxin, and Pseudomonas exotoxin, have been previously used to construct immunotoxins for the treatment of various cancers and some are currently in clinical trials (Zhou et al. 2012; Veenendaal et al. 2002; Kwok et al. 1999; Smaldone and Davies 2010; Olsen et al. 2001; Weldon and Pastan 2011). However, one of the features that immunotoxins should possess to effectively kill cancer cells is that they must be internalized and translocated into the cytoplasm; this is a rate-limiting step. In addition, immunotoxins in the cytoplasm can be easily degraded by lysosomes (Farah et al. 1998; Kreitman 1999). To overcome these limitations, more potential immunotoxins must be explored.
Sea anemone cytolysin is a strong candidate for the creation of novel immunotoxins. The mechanism of killing target cells using cytolysin differs from traditional immunotoxins in that it forms a pore on the cell surface and increases cell membrane permeability, causing the leakage of cellular contents (Mayra et al. 2009; Bárbara et al. 2012; Miguel et al. 2011). Moreover, it has an added benefit of not inducing drug tolerance. Avila et al. reported using a hemolytic toxin from the sea anemone Stichodactyla helianthus to link an antibody that recognized a specific antigen expressed on immature T-lymphocytes as well as to a monoclonal antibody directed against a carcinoembryonic antigen via chemical crosslinking agents (Avila et al. 1988, 1989). This is the first study report on sea anemone cytolysin conjugated to a monoclonal antibody. More recently, equinatoxin II (EqtII) from Actinia equina was linked to a monoclonal antibody via an avidin-biotin interaction (Potrich et al. 2000). Additionally, sticholysin I from Stichodactyla helianthus was linked to a monoclonal antibody recognizing the colon tumor associated antigen ior C2 through SMCC (Tejucaa et al. 2004). Both of these studies conjugated toxins to antibodies by chemical approaches that are cumbersome and have limited reproducibility; moreover, immunotoxins acquired have a low yield and are difficult to purify (Pastan et al. 2007). Therefore, some improvements are required.
Recently, a new cytolysin, Gigantoxin-4, was separated from Stichodactyla gigantea and is highly homologous to sticholysin I and equinatoxin II. Similar to other sea anemone cytolysin, Gigantoxin-4 can also induce pore formation and efficiently damage the cell membrane (Hu et al. 2011). However, Gigantoxin-4 is nonspecific and therefore also destroys the normal cells along with the cancer cells. This obstacle can be overcome by conjugating Gigantoxin-4 to a monoclonal antibody or other means by which specific recognition of the target cells can be achieved.
Herceptin is widely used in the clinical treatment for HER2/neu overexpressing tumors. A recombinant single-chain fragment of this monoclonal antibody, 4D5 scFv, has been previously shown to selectively bind to the HER2 antigen with a high affinity, is thermodynamically stable, and folds well (Willuda et al. 1999). Compared with the full monoclonal antibody, 4D5 scFv can penetrate tissues easily, does not exhibit adverse effects involving the constant domains, and readily fuses to other proteins, such as toxins and enzymes (Kubetzko et al. 2006).
To overcome these concerns and create more potential immunotoxin, we conjugated Gigantoxin-4 to 4D5 scFv using a flexible linker (G4S)3 by genetic engineering methods. Soluble expression of Gigantoxin-4-4D5 scFv in Escherichia coli was successfully achieved by fusing SUMO tag to its N-terminus. The selectivity and cytotoxicity of Gigantoxin-4-4D5 scFv demonstrate its potential as a novel candidate for treating HER2/neu overexpressing SK-OV-3 cells.
Materials and methods
Materials
Restriction endonucleases, DNA polymerase, were purchased from Takara Company (Dalian, China). Plasmid Mini Kit, PCR Purification Kit, and Gel Extraction Kit were purchased from Promega (Madison, WI, USA). The Escherichia coli strain BL21 (DE3), plasmid pET28a, were preserved in our laboratory. The SUMO protease was bought from LifeSensors (Madison, WI, USA). The Ni-NTA Sepharose FF and CM Sepharose FF were obtained from GE Healthcare Company (Piscataway, NJ, USA). Human embryonic kidney 293 (HEK-293) cells, ovarian carcinoma SK-OV-3 cells, ovarian carcinoma OVCAR-3 cells, and breast cancer SK-BR-3 cells were obtained from cell bank of Chinese Academy of Science (Shanghai, China). All other chemicals and regents were purchased from Sigma (St.Louis, MO, USA).
Construction of the pET28a/SUMO-Gigantoxin-4-4D5 scFv
The gene encoding human 4D5 scFv (GenBank No. KM016462), Gigantoxin-4 (GenBank No. JQ353486), and SUMO (GenBank No. NM_001180818) were synthesized by Shanghai Sangon Biological Engineering Technology and Services Co. Ltd. (Shanghai, China). Illustrations of the target sequence construction were shown in Fig. 1. The flexible linker (G4S)3 was used to conjugate the C-terminus of Gigantoxin-4 to the N-terminus of 4D5 scFv. The gene encoding SUMO was fused to the N-terminus of Gigantoxin-4-4D5 scFv. The primers designed were shown in Table 1. Overlapping PCR was applied to amplify the gene encoding SUMO-Gigantoxin-4-4D5 scFv by the forward and reverse primers (P1, P6) containing Nco I and Xho I endonuclease sites, respectively. The PCR product digested with endonuclease was cloned into pET28a.
The control recombination vector pET28a/SUMO-Gigantoxin-4 was also obtained by similar method. The two plasmids were confirmed by gene sequencing.
Expression and purification of Gigantoxin-4-4D5 scFv
The recombinants were cultured in fresh Luria-Bertani (LB) medium containing 50 μg/mL Kanamycin at 37 °C on an orbital shaker (200 rpm). When the OD reached 0.8, 0.1 mM IPTG was added to induce the expression of Gigantoxin-4-4D5 scFv at 18 °C for 16 h. The cells were harvested by centrifugation and washed with PBS, then resuspended in the lysis buffer (PBS containing 10 mM imidazole, pH 7.4) and lysed by sonication. The samples were identified by SDS-PAGE using 12 % polyacrylamide gels. Then, the supernatant was applied to Ni-NTA sepharose column. The fusion protein with His6 tag was eluted with elution buffer (PBS containing 200 mM imidazole, pH 7.4). The fractions containing fusion protein were dialyzed overnight at 4 °C in PBS (pH 7.4). Then, fusion protein was digested with SUMO protease to release Gigantoxin-4-4D5 scFv and loaded onto Ni-NTA sepharose column again. Gigantoxin-4-4D5 scFv without His6 tag was shed out; SUMO, SUMO protease, and SUMO-Gigantoxin-4-4D5 scFv with His6 tag were combined to the column. Then, Gigantoxin-4-4D5 scFv was dialyzed in the PB buffer (pH 6.0) at 4 °C for 8 h and applied to CM Sepharose column. The target protein was eluted when the concentration of NaCl was 50 mM in the eluent. Eventually, the concentration and purity of target protein were determined by Bradford protein assay and SDS-PAGE. The protein Gigantoxin-4 was prepared using the same method.
Hemolytic assay
The hemolytic activity on fresh healthy human red blood cells was measured as the method described previously (de Oliveira et al. 2006), with slight modifications. Different concentrations of Gigantoxin-4-4D5 scFv were added into 2 % red cell suspension. Background or total cell lysis was performed by incubation of 2 % red cell suspension with PBS or 0.5 % Triton X-100 in PBS, respectively. The reaction system was incubated at 37 °C for 1 h and then centrifuged at 3000×g for 5 min. The supernatant was added to 96-well plates to read the absorption at 420 nm using microplate reader (Bio-Rad 680, Bio-Rad, Hercules, CA).
Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was applied to measure the viability of the cells that incubated with Gigantoxin-4-4D5 scFv. Eight thousand cells per well were added into 96-well plates in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % fetal bovine serum and incubated overnight at 37 °C in 5 % CO2. Gigantoxin-4-4D5 scFv in different concentrations were added. After incubation for 24 h, 10 μL MTT (5 mg/mL) was added to each well and incubated for 4 h. Then, the total supernatant was drained carefully and 150 μL DMSO was added into each well to incubate at 37 °C for 10 min to dissolve the formazan. The absorbance of each well was measured at 490 nm with microplate reader. The experiments were carried out in triplicate.
Immunofluorescence analysis
Immunofluorescence was applied to analyze the selectivity of Gigantoxin-4-4D5 scFv on SK-OV-3 and HEK-293 cells. Gigantoxin-4-4D5 scFv was dialyzed in sodium carbonate buffer (pH 9.0) for three times and then incubated with FITC in the ratio of 1 mg protein with 15 μg FITC in the dark for 8 h. Ammonium chloride was added to the final concentration of 50 mM and incubated for 2 h to stop the reaction. The mixture was dialyzed in PBS for four times. Cells were seeded at 1 × 105 cells/well density on confocal dish (25 mm). Then, 10 nM FITC-labeled Gigantoxin-4-4D5 scFv was added and incubated at 4 °C for 1 h. The nuclei had already been stained with Hoechst 33342. Visualization of immunofluorescence was detected using a confocal laser scanning microscopy (LSM700, Carl Zeiss Inc., Germany). The same concentration of Gigantoxin-4 that was labeled with FITC was also performed.
Competition of Gigantoxin-4-4D5 scFv cytotoxicity
SK-OV-3 cells were incubated with 0.432 nM Gigantoxin-4-4D5 scFv alone or in the presence of different concentrations of 4D5 scFv which was prepared as Gigantoxin-4-4D5 scFv. After incubation at 37 °C for 24 h, cell viability was determined by MTT assay. At the same time, the viability of SK-OV-3 cells that were incubated with 147.2 nM Gigantoxin-4 and different concentrations of 4D5 scFv was also detected.
Scanning election microscope detection
SK-OV-3 cells were seeded on the slide with 10,000 cells per well and incubated overnight at 37 °C in 5 % CO2. 0.432 nM Gigantoxin-4-4D5 scFv, Gigantoxin-4, or 4D5 scFv was added respectively and incubated for 24 h. The cells were washed carefully with PBS and fixed in 2.5 % glutaraldehyde overnight at 4 °C, then washed with 0.1 M phosphate buffer for three times. Next, cells were dehydrated by gradient concentration of ethanol and put into the vacuum freeze drier overnight. Lastly, cells were coated by gold and observed with the scanning electron microscope (SEM; S-3400, Hitachi, Japan).
Detection of released lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity was measured by Pierce™ LDH cytotoxicity assay kit (Thermo Scientific, Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s recommendations. This test is a colorimetric assay for the quantification of cell death and cell lysis based on the measurement of LDH activity released from the cytosol of damaged cells into the supernatant. The released LDH activity detected in the culture supernatant correlates with the proportion of lysed cells (Abdel-Latif et al. 2006). The released LDH activity of Gigantoxin-4-4D5 scFv, Gigantoxin-4 and 4D5 scFv toward SK-OV-3 and SK-BR-3 cells were measured.
Flow cytometric analysis
Flow cytometric was used to analyze apoptosis or necrosis of the cells that were incubated with Gigantoxin-4-4D5 scFv, Gigantoxin-4, and 4D5 scFv. 8 × 105 cells per well were added into six-well plates, and different concentrations of proteins were added to incubate for 24 h, respectively. Then, cells were digested by tyrisin and washed with PBS for three times. The binding buffer, Annexin V-FITC, and PI were added to each plate and incubated at room temperature for 10 min. The samples were then detected by flow cytometer (BD Calibur, BD Bioscience, NJ, USA). The results were analyzed with Flow Jo 7.6 software.
Statistical analysis
All results were expressed as means ± standard error (mean ± SE). The statistical differences between groups of data were analyzed by Students’ t test. A level of P < 0.05 was considered to be significant (*), and P < 0.01 was considered to be very significant (**).
Results
Construction and expression of Gigantoxin-4-4D5 scFv
A SUMO tag was fused to the N-terminus of Gigantoxin-4-4D5 scFv for the purpose of soluble expression. Six primers, named P1 to P6, were used to amplify the target gene encoding SUMO-Gigantoxin-4-4D5 scFv (Fig. 1). The PCR product was cloned into pET28a. As control, the plasmid pET28a/SUMO-Gigantoxin-4 was also constructed with the similar method. The recombinant plasmids were sequenced to confirm the accuracy of the target genes.
0.1 mM IPTG was added to express the fusion protein, and SDS-PAGE was applied to confirm the level of protein expression. The results showed that SUMO-Gigantoxin-4-4D5 scFv and SUMO-Gigantoxin-4 were expressed with different degrees of solubility. After analysis by Gel Pro Analyzer 4.0, the soluble expression level of SUMO-Gigantoxin-4-4D5 scFv and SUMO-Gigantoxin-4 reached 9.8 and 45.1 % of the supernatant proteins, respectively (Fig. 2).
SDS-PAGE analysis of the expression of SUMO-Gigantoxin-4-4D5 scFv and SUMO-Gigantoxin-4. a Lane 1 soluble lysate fraction from E. coli containing pET28/SUMO-Gigantoxin-4-4D5 scFv induced for 16 h; lane 2 insoluble lysate fraction from E. coli containing pET28/SUMO-Gigantoxin-4-4D5 scFv; M protein molecular weight marker (from top to bottom: 116, 66.2, 45, 35, 25, 18.4, 14.4 kDa, respectively); lane 3 pre-induced soluble lysate fraction from E. coli containing pET28/SUMO-Gigantoxin-4-4D5 scFv. b M protein molecular weight marker; lane 1 pre-induced soluble lysate fraction from E. coli containing pET28/SUMO-Gigantoxin-4; lane 2 soluble lysate fraction from E. coli containing pET28/SUMO-Gigantoxin-4 induced for 16 h
Purification of Gigantoxin-4-4D5 scFv
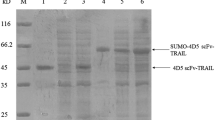
The samples were loaded onto the Ni-NTA Sepharose column and fusion protein was eluted with 200 mM imidazole. After removing the imidazole by dialysis, the protein was cleaved with SUMO protease and loaded onto the Ni-NTA Sepharose again to collect the target protein. The purity of Gigantoxin-4-4D5 scFv and Gigantoxin-4 was 56.2 and 98 %, respectively (Fig. 3a, b). Then, the sample containing Gigantoxin-4-4D5 scFv was passed through CM Sepharose column with a pH 6.0 and the target protein was eluted when the concentration of NaCl reached 50 mM (Fig. 3c). The purity of Gigantoxin-4-4D5 scFv reached as high as 96 %. In addition, the yield of Gigantoxin-4-4D5 scFv and Gigantoxin-4 was 14.3 and 32.5 mg/L, respectively.
SDS-PAGE analysis of SUMO-Gigantoxin-4 and SUMO-Gigantoxin-4-4D5 scFv digested by SUMO protease and purified target proteins. a lane 1 the purified SUMO-Gigantoxin-4; lane 2 SUMO-Gigantoxin-4 digested by SUMO protease; lane 3 purified Gigantoxin-4; lane 4 eluted SUMO; M protein molecular weight marker. b lane 1 the purified SUMO-Gigantoxin-4-4D5 scFv; M protein molecular weight marker; lane 2 SUMO-Gigantoxin-4-4D5 scFv digested by SUMO protease; lane 3 purified proteins containing Gigantoxin-4-4D5 scFv; lane 4 eluted proteins containing SUMO. c SDS-PAGE analysis of Gigantoxin-4-4D5 scFv purified by CM Sepharose column. M protein molecular weight marker; Lane 1 unbounded proteins; lane 2 purified Gigantoxin-4-4D5 scFv eluted by 50 mM NaCl; lane 3 proteins eluted by 100 mM NaCl; lane 4 proteins eluted by 150 mM NaCl
The hemolytic activity of Gigantoxin-4-4D5 scFv
The consequence of the hemolytic activity of Gigantoxin-4-4D5 scFv and Gigantoxin-4 was shown in Fig. 4a. Gigantoxin-4-4D5 scFv and Gigantoxin-4 lysed erythrocytes in a dose-dependent manner. The HC50 of Gigantoxin-4-4D5 scFv and Gigantoxin-4 was 18.9 and 5.3 nM, respectively. 5 μM 4D5 scFv did not induce lysis of red blood cells. Compared with Gigantoxin-4, the hemolytic activity of Gigantoxin-4-4D5 scFv exhibited approximately a threefold decrease. This might be attributed to the fact that the molecular weight of Gigantoxin-4-4D5 scFv was higher and Gigantoxin-4-4D5 scFv had a greater difficulty creating holes in the membrane.
Cytotoxicity of Gigantoxin-4 and Gigantoxin-4-4D5 scFv against normal cells and ovarian carcinoma SK-OV-3 cells. a Hemolytic activity of Gigantoxin-4 and Gigantoxin-4-4D5 scFv on human erythrocytes. Data represented mean ± S.E.M of three repeats. *p < 0.05 and ** p < 0.01, in comparison to the group treated with Gigantoxin-4. b Cytotoxicity of Gigantoxin-4 on SK-OV-3 and HEK-293 cells. Cell viability was determined after incubation with different concentrations of Gigantoxin-4 for 24 h by MTT assay. Gigantoxin-4 inhibited the proliferation of SK-OV-3 and HEK-293 cells in a dose-dependent manner. c Cytotoxicity of Gigantoxin-4-4D5 scFv on SK-OV-3 and HEK-293 cells evaluated by MTT assay. Gigantoxin-4-4D5 scFv showed highly cytotoxicity on SK-OV-3 cells, whereas it had little effect on the growth of HEK-293 cells. Data represented mean ± S.E.M of three repeats
The cytotoxicity of Gigantoxin-4-4D5 scFv toward SK-OV-3 cells improved markedly
An MTT assay was applied to analyze the viability of the cells that were incubated with different concentrations of Gigantoxin-4-4D5 scFv and Gigantoxin-4 for 24 h. As shown in Fig. 4b, Gigantoxin-4 suppressed the proliferation of SK-OV-3 and HEK-293 cells in a dose-dependent manner. The IC50 of Gigantoxin-4 on SK-OV-3 and HEK-293 cells was 74.1 and 112.2 nM, respectively. In addition, Gigantoxin-4-4D5 scFv could inhibit the growth of SK-OV-3 cells at relatively low concentrations and the IC50 was 0.171 nM. For another HER2/neu positive cancer cell SK-BR-3, Gigantoxin-4-4D5 scFv could also exert high cytotoxicity and the IC50 was 0.158 nM (Fig. S1a). However, the same concentration of Gigantoxin-4-4D5 scFv could not cause cytotoxicity against HER2/neu negative ovarian cancer OVCAR-3 cells (Fig. S1b). Moreover, 0.432 nM Gigantoxin-4-4D5 scFv had no effect on HEK-293 cells but demonstrated a high degree of cytotoxicity against HER2/neu positive cells (Fig. 4c). In addition, the IC50 of Gigantoxin-4-4D5 scFv on HEK-293 cells was 719.5 nM and 5 μM 4D5 scFv had no effect on either SK-OV-3 or HEK-293 cells (data not shown).
Gigantoxin-4-4D5 scFv selectively bound to SK-OV-3 cells
Ten nanomole Gigantoxin-4-4D5 scFv labeled with FITC was incubated with SK-OV-3 and HEK-293 cells at 4 °C for 1 h. Using confocal microscopy, we observed that Gigantoxin-4-4D5 scFv was specifically located on the membrane of SK-OV-3 cells, and no fluorescence signal was observed in HEK-293 cells (Fig. 5a). The same concentration of Gigantoxin-4 did not accumulate on either the SK-OV-3 or the HEK-293 cells. Thus, Gigantoxin-4-4D5 scFv can specifically recognize the cells that overexpress HER2/neu.
Confocal laser scanning microscopy images and competitive experiments analysis of the selectivity of Gigantoxin-4-4D5 scFv. a Confocal fluorescence images of SK-OV-3 and HEK-293 cells that were stained with Hoechst 33342 and then incubated with 10 nM FITC-labeled Gigantoxin-4 or Gigantoxin-4-4D5 scFv at 4 °C for 1 h. Images were captured at ×600 amplification. b The cytotoxic activity of 0.432 nM Gigantoxin-4-4D5 scFv and 147.2 nM Gigantoxin-4 in the absence or presence of different concentrations of 4D5 scFv on SK-OV-3 cells. Cell viability was measured by MTT assay. Data were mean ± S.E.M of three independent experiments. *p < 0.05 and **p < 0.01, in comparison to the group without 4D5 scFv
4D5 scFv inhibited the activity of Gigantoxin-4-4D5 scFv on SK-OV-3 cells
It has been previously reported that 4D5 scFv can interact with HER2/neu antigen (Willuda et al. 1999). If 4D5 scFv and Gigantoxin-4-4D5 scFv are both incubated with SK-OV-3 cells, 4D5 scFv can compete to interact with HER2/neu so that the inhibition of SK-OV-3 cells caused by Gigantoxin-4-4D5 scFv declines. The results showed that as the concentration of 4D5 scFv increased, the viability of SK-OV-3 cells was higher even though the cells were also incubated with 0.432 nM Gigantoxin-4-4D5 scFv. When the concentration of 4D5 scFv reached 54 nM, the cytotoxicity of Gigantoxin-4-4D5 scFv on SK-OV-3 cells was almost completely inhibited (Fig. 5b). However, the effect of Gigantoxin-4 on SK-OV-3 cells was not hampered by 4D5 scFv.
Gigantoxin-4-4D5 scFv destroyed the SK-OV-3 cell membrane
Scan electron microscopy was applied to analyze the surface morphology of SK-OV-3 cells that were incubated with 0.432 nM fusion proteins for 24 h. Compared with controls, the morphology of SK-OV-3 cells incubated with Gigantoxin-4-4D5 scFv exhibited significant changes. The membranes were markedly rough, shrinked, and showed some disintegration that lead to irreversible cytolysis and death of the target cells. However, the cells treated with the same concentration of Gigantoxin-4 and 4D5 scFv were similar in appearance to the controls (Fig. 6a).
Scanning electron microscopy and LDH activity analysis of morphological changes of SK-OV-3 cells and necrosis. a The morphological observation of SK-OV-3 cells that were treated with 0.432 nM Gigantoxin-4-4D5 scFv, Gigantoxin-4 or 4D5 scFv for 24 h using scanning electron microscopy. b Analysis of extracellular LDH activity. After SK-OV-3 cells incubated with Gigantoxin-4-4D5 scFv, Gigantoxin-4 or 4D5 scFv for 24 h, extracellular LDH activity was determined. The control group was incubated with PBS. Data were mean ± S.E.M of three independent experiments. *p < 0.05 and **p < 0.01, in comparison to the control group
Gigantoxin-4-4D5 scFv caused LDH leakage in SK-OV-3 cells
LDH is a stable cytoplasmic enzyme that exits in all the cells. It is rapidly released following damage to the plasma membrane and is an indicator of necrotic cell death (Blanco et al. 2003). In contrast, extracellular LDH cannot be detected when the membrane is intact during apoptosis (Deyev and Lebedenko 2008). Our results showed that with an increase in the concentration of Gigantoxin-4-4D5 scFv, the activity of extracellular LDH also increased proportionally; 0.216 nM Gigantoxin-4-4D5 scFv caused approximately 50 % LDH release. However, when the concentration of Gigantoxin-4 and 4D5 scFv reached 0.432 nM, extracellular LDH was still not detected (Fig. 6b). Therefore, the LDH assay indicates that Gigantoxin-4-4D5 scFv caused SK-OV-3 cell death primarily via necrosis. At the same time, the similar results could also been observed on the SK-BR-3 cells (Fig. S1c).
Flow cytometry analysis of SK-OV-3 and HEK-293 cells treated with Gigantoxin-4-4D5 scFv
Based on the results of the morphological observations and the extracellular LDH analysis, we speculated that Gigantoxin-4-4D5 scFv inhibited SK-OV-3 cells primarily through the destruction of the cell membrane and by inducing necrosis. We employed the use of flow cytometry to determine the extent of cell death via apoptosis. As shown in Fig. 7, the ratio of necrosis and late apoptosis of SK-OV-3 cells caused by 0.864 nM Gigantoxin-4-4D5 scFv was 47.2 %. Early apoptosis was also detected and the ratio was 18.7 %. SK-OV-3 cells treated with Gigantoxin-4-4D5 scFv exhibited necrosis as well as apoptosis. 0.864 nM Gigantoxin-4 and 4D5 scFv did not induce obvious necrosis or apoptosis of SK-OV-3 cells. Furthermore, neither necrosis nor apoptosis of HEK-293 cells incubated with the same concentration of proteins was observed (Fig. 8). In addition, the apoptosis and necrosis were also detected in HER2/neu overexpressing SK-BR-3 cells but not in HER2/neu negative OVCAR-3 cells (Fig. S2, S3).
Flow cytometry analysis of SK-OV-3 cells treated with Gigantoxin-4-4D5 scFv, Gigantoxin-4 or 4D5 scFv for 24 h. The experiment was repeated thrice at least. The cells that were not dyed by Annexin V-FITC or PI represented living cells. The cells only stained by Annexin V-FITC represented early apoptotic cells. The cells stained by both Annexin V-FITC and PI represented late apoptotic and necrotic cells. The cells only stained by PI represented mechanically damaged cells. Gigantoxin-4-4D5 scFv could induce apoptosis and necrosis to SK-OV-3 cells. Neither apoptosis nor necrosis were observed when SK-OV-3 cells were treated with Gigantoxin-4 and 4D5 scFv
Flow cytometry analysis of HEK-293 cells treated with Gigantoxin-4-4D5 scFv, Gigantoxin-4 or 4D5 scFv for 24 h. The experiments were repeated thrice at least. The cells that were not dyed by Annexin V-FITC or PI represented living cells. The cells only stained by Annexin V-FITC represented early apoptotic cells. The cells stained by both Annexin V-FITC and PI represented late apoptotic and necrotic cells. The cells only stained by PI represented mechanically damaged cells. All the proteins could not induce apoptosis and necrosis to HEK-293 cells
Discussion
Immunotoxins have attracted growing attention since they were successfully made in the 1980s (Blythman et al. 1981). In general, the toxins used to conjugate antibodies are from plants and bacteria. Unfortunately, there are several disadvantages such as the need to be internalized into the cytoplasm, digestion by lysosomes, and resulting in drug resistance (Abdel-Latif et al. 2006; Thrush et al. 1996). Moreover, immunotoxins formed by chemical cross-linking are often unstable, inhomogeneous, and difficult to repeat (Pastan et al. 2007).
In this study, we firstly combined sea anemone cytolysin Gigantoxin-4 with 4D5 scFv using a flexible hinge (G4S)3 by genetic engineering. The fusion immunotoxin Gigantoxin-4-4D5 scFv is advantageous as it does not have the same shortcomings as other immunotoxins as described above. Gigantoxin-4-4D5 scFv can damage the target cell membrane and does not require internalization.
To achieve soluble expression of Gigantoxin-4-4D5 scFv, the SUMO tag was fused to its N-terminus. Lastly, the expression of SUMO-Gigantoxin-4-4D5 scFv accounted for 9.8 % of the proteins in supernatant. After the cleavage of SUMO and subsequent purification, the purity of Gigantoxin-4-4D5 scFv reached 96 % and the yield was 14.3 mg/L in shaking fermentation.
The mechanism of sea anemone cytolysin damage to the cell membrane is a complicated multistep process. It involves the binding, insertion, and oligomerization of three to four monomers into a functional channel with an inner effective hydrodynamic radius of approximately 1 nm (Anderluh and Menestrina 2001; Tejuca et al. 1996, 2001; Varanda and Finkelstein 1980). It was reasonable that the hemolytic activity of Gigantoxin-4-4D5 scFv was more than threefold lower compared with Gigantoxin-4. This could be due to the fact that 4D5 scFv fused to Gigantoxin-4 has a larger molecular weight that may affect the efficiency of the membrane disruption. However, 4D5 scFv could make Gigantoxin-4-4D5 scFv bind to HER2/neu overexpressed on cancer cells to improve overall cytotoxicity. Observed by confocal microscopy, Gigantoxin-4-4D5 scFv accumulated on the membrane of SK-OV-3 cells but not obviously on HEK-293 cells. In an MTT assay, the IC50 of Gigantoxin-4-4D5 scFv on SK-OV-3 cells was 0.171 nM, much lower than the IC50 of 74.1 nM of Gigantoxin-4 on SK-OV-3 cells. The IC50 of Gigantoxin-4-4D5 scFv on SK-BR-3 cells was 0.158 nM. In addition, the IC50 of Gigantoxin-4-4D5 scFv on HEK-293 cells was improved to 719.5 nM. Gigantoxin-4-4D5 scFv exhibited nearly undetectable effect on OVCAR-3 cells. The results indicated that Gigantoxin-4-4D5 scFv was highly cytotoxic toward HER2-overexpressing SK-OV-3 and SK-BR-3 cells but not displaying marked cytotoxicity against HER2 negative OVCAR-3 cells and HEK-293 cells. It is worth noting that HER2 is weakly expressed in HEK-293 cells (Lanteri et al. 2005). These allowed for Gigantoxin-4-4D5 scFv to successfully damage the HER2/neu-positive SK-OV-3 cells without harming normal cells.
Lactate dehydrogenase (LDH) is one of the intrinsic enzymes in the cytoplasm. Normally, LDH is not released unless the cell membrane is damaged, and thus extracellular LDH is an indicator of necrosis (Blanco et al. 2003). Our results showed that Gigantoxin-4-4D5 scFv induced cellular LDH release in a concentration-dependent manner. Necrosis was the primary mechanism of Gigantoxin-4-4D5 scFv killing of SK-OV-3 cells. Additionally, SEM demonstrated that the membrane was destroyed after incubation with Gigantoxin-4-4D5 scFv. Gigantoxin-4-4D5 scFv was able to lyse the cell membrane and caused SK-OV-3 cell necrosis. Annexin V staining indicated phosphatidylserine externalization and represented the early stages of apoptosis. Both Annexin V and PI staining represented not only late stages of apoptosis but also necrosis. Since the necrotic cell membrane was damaged, Annexin V and PI could penetrate into the cells (Sabine et al. 2015; Van and Van den 2002). Based on the results of our general flow cytometry analysis, Gigantoxin-4-4D5 scFv could induce both apoptosis and necrosis on SK-OV-3 cells. However, the concrete mechanism by which this occurs requires further research.
Compared with the original Gigantoxin-4, Gigantoxin-4-4D5 scFv can specifically bind to HER2/neu overexpressed on SK-OV-3 cells and induce cell death by damaging cell membrane and inducing both necrosis and apoptosis. We identified that the cytotoxic activity of Gigantoxin-4-4D5 scFv on SK-OV-3 cells was approximately 430 times higher than Gigantoxin-4 in vitro. Future studies should be conducted to verify the effectiveness of Gigantoxin-4-4D5 scFv in vivo.
In conclusion, the novel recombinant immunotoxin Gigantoxin-4-4D5 scFv demonstrates high cytotoxicity and selectivity on the HER2/neu-positive SK-OV-3 cells. Therefore, this immunotoxin is a novel potential candidate for treating HER2/neu-positive ovarian carcinoma.
References
Abdel-Latif L, Murray BK, Renberg RL, O’Neill KL, Porter H, Jensen JB, Johnson FB (2006) Cell death in bovine parvovirus infected embryonic bovine tracheal cells is mediated by necrosis rather than apoptosis. J Gen Virol 87:2539–2548. doi:10.1099/vir.0.81915-0
Anderluh G, Menestrina G (2001) Pore-forming proteins from sea anemones and the construction of immunotoxins for selective killing of harmful cells. In: Fingerman M, Nagabhushanam R (eds) Bio-organic compounds: chemistry and medical applications. Science, USA, pp. 131–148
Antignani A, Fitzgerald D (2013) Immunotoxins: the role of the toxin. Toxins (Basel) 5:1486–1502. doi:10.3390/toxins5081486
Avila AD, de Acosta MC, Lage A (1988) A new immunotoxin built by linking a hemolytic toxin to a monoclonal antibody specific for immature T lymphocytes. Int J Cancer 42:568–571. doi:10.1002/ijc.2910420417
Avila AD, de Acosta MC, Lage A (1989) A carcinoembryonic antigen-directed immunotoxin built by linking a monoclonal antibody to a hemolytic toxin. Int J Cancer 43:926–929. doi:10.1002/ijc.2910430533
Bárbara F, Vasconcelos V, Antunes A (2012) Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview. Mar Drugs 10:1812–1851. doi:10.3390/md10081812
Blanco R, Carrasco L, Ventoso I (2003) Cell killing by HIV-1 protease. J Biol Chem 278:1086–1093. doi:10.1074/jbc.M205636200
Blythman HE, Casellas P, Gros O, Gros P, Jansen FK, Paolucci F, Pau B, Vidal H (1981) Immunotoxins: hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature 290:145–146. doi:10.1038/290145a0
de Oliveira JS, Zaharenko AJ, de Freitas JC, Konno K, de Andrade SA, Portaro FCV, Richardson M, Sant’Anna OA, Tambourgi DV (2006) Caissarolysin I (Bcs I), a new hemolytic toxin from the Brazilian sea anemone Bunodosoma caissarum: purification and biological characterization. Biochim Biophys Acta 1760:453–461. doi:10.1016/j.bbagen.2005.12.018
Deyev SM, Lebedenko EN (2008) Multivalency: the hallmark of antibodies used for optimization of tumor targeting by design. BioEssays 30:904–918. doi:10.1002/bies.20805
Farah RA, Clinchy B, Herrera L, Vitetta ES (1998) The development of monoclonal antibodies for the therapy of cancer. Crit Rev Eukaryot Gene Expr 8:321–356. doi:10.1615/CritRevEukarGeneExpr.v8.i3-4.50
Hu B, Guo W, Wang LH, Wang JG, Liu XY, Jiao BH (2011) Purification and characterization of gigantoxin-4, a new actinoporin from the sea anemone Stichodactyla gigantea. Int J Biol Sci 7:729–739. doi:10.7150/ijbs.7.729
Kreitman RJ (1999) Immunotoxins in cancer therapy. Biosci Rep Opin Immunol 11:570–578. doi:10.1016/S0952-7915(99)00005-9
Kreitman RJ (2009) Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs 23:1–13. doi:10.2165/00063030-200923010-00001
Kubetzko S, Balic E, Waibel R, Zangemeister-Wittke U, Pluckthun A (2006) PEGylation and multimerization of the antip185HER-2 single chain Fv fragment 4D5: effects on tumor targeting. J Biol Chem 281:35186–35201. doi:10.1074/jbc.M604127200
Kwok KHH, Law KB, Wong RNS, Yung KKL (1999) Immunolesioning of nerve growth factor p75 receptor-containing neurons in the rat brain by a novel immunotoxin: anti-p75-anti-mouse IgG-trichosanthin conjugates. Brain Res 846:154–163. doi:10.1016/S0006-8993(99)01999-X
Lanteri M, Ollier L, Giordanengo V, Lefebvre JC (2005) Designing a HER2/neu promoter to drive α1,3galactosyltransferase expression for targeted anti-α Gal antibody-mediated tumor cell killing. Breast Cancer Res 7:487–494. doi:10.1186/bcr1034
Mayra T, Anderluh G, Serra MD (2009) Sea anemone cytolysins as toxic components of immunotoxins. Toxicon 54:1206–1214. doi:10.1016/j.toxicon.2009.02.025
Miguel A, Pardo-Cea, Castrillo I’s, Alegre-Cebollada J, Martı’nez-del-Pozo A’l, Gavilanes JG, Bruix M (2011) Intrinsic local disorder and a network of charge-charge interactions are key to actinoporin membrane disruption and cytotoxicity. Febs J 278:2080–2089. doi:10.1111/j.1742-4658.2011.08123.x
Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J (2001) Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol 19:376–388. doi:10.1016/s0923-1811(98)84232-1
Pastan I, Raffit H, FitzGerald David J, Kreitman Robert J (2007) Immunotoxin treatment of cancer. Annu Rev Med 58:221–237. doi:10.1146/annurev.med.58.070605.115320
Potrich C, Viero G, Tejuca M, Anderluh G, Macek P, Menestrina G (2000) Construction of new immunotoxins by linking equinatoxin II to monoclonal antibodies via the biotin-avidin interaction. cytotoxic effects on human tumor cells. Acta Biol Slov 43:47–51
Sabine P, Schmidt JH, Lavrik IN (2015) Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J Immunol Methods 423:99–103. doi:10.1016/j.jim.2015.04.025
Smaldone MC, Davies BJ (2010) BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther 12:607–616
Tejuca M, Dalla Serra M, Potrich C, Alvarez C, Menestrina G (2001) Sizing the radius of the pore formed in erythrocytes and lipid vesicles by the toxin sticholysin I from the sea anemone Stichodactyla helianthus. J Membr Biol 183:125–135. doi:10.1007/s00232-001-0060-y
Tejucaa M, Dı’az I, Figueredo R, Roque L, Pazos F, Martínez D, Iznaga-Escobar N, Pe’rez R, Alvarez C, Lanio ME (2004) Construction of an immunotoxin with the pore forming protein St1 and ior C5, a monoclonal antibody against a colon cancer cell line. Int Immunopharmacol 4:731–744. doi:10.1016/j.intimp.2004.02.010
Tejuca M, Serra MD, Ferreras M, Lanio ME, Menestrina G (1996) The mechanism of membrane permeabilisation by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 35:14947–14957
Thrush GR, Lark LR, Clinchy BC, Vitetta ES (1996) Immunotoxins: an update. Annu Rev Immunol 14:49–71. doi:10.1146/annurev.immunol.14.1.49
Van CS, Van den BW (2002) Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 31:214–223. doi:10.1046/j.1439-0264.2002.00398.x
Varanda A, Finkelstein A (1980) Ion and nonelectrolyte permeability properties of channels formed in planar lipid bilayer membranes by the cytolytic toxin from the sea anemone, Stoichactis helianthus. J Membr Biol 55:203–211. doi:10.1007/BF01869461
Veenendaal LM, Jin H, Ran S, Cheung L, Navone N, Marks JW, Waltenberger J, Thorpe P, Rosenblum MG (2002) In vitro and in vivo studies of a VEGF121/rGelonin chimeric fusion toxin targeting the neovasculature of solid tumors. Proc Natl Acad Sci 99:7866–7871. doi:10.1073/pnas.162346299
Weldon JE, Pastan I (2011) A guide to taming a toxin-recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. Febs J 278:4683–4700. doi:10.1111/j.1742-4658.2011.08182.x
Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, Pluckthun A (1999) High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res 59:5758–5767
Zhou XK, Qiu J, Wang Z, Huang NY, Li XL, Li Q, Zhang YB, Zhao CJ, Li C, Zhang NN, Teng X, Chen ZW, Liu X, Yu XL, Wu WL, Wei YQ, Li J (2012) In vitro and in vivo anti-tumor activities of anti-EGFR single-chain variable fragment fused with recombinant gelonin toxin. J Cancer Res Clin Oncol 138:1081–1090. doi:10.1007/s00432-012-1181-7
Acknowledgments
We thank Wei He and Xinwei Xu for excellent technical assistance. This work was supported by the grants from the National major science and technology projects of China (Grant No. 2012ZX09304009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was funded by the National major science and technology projects of China (Grant No. 2012ZX09304009)
Electronic supplementary material
ESM 1
(PDF 4517 kb)
Rights and permissions
About this article
Cite this article
Lv, X., Zhang, J., Xu, R. et al. Gigantoxin-4-4D5 scFv is a novel recombinant immunotoxin with specific toxicity against HER2/neu-positive ovarian carcinoma cells. Appl Microbiol Biotechnol 100, 6403–6413 (2016). https://doi.org/10.1007/s00253-016-7487-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7487-7