Abstract
Context
The current biodiversity crisis has intensified the need to predict species responses to landscape modification and has renewed attention on the fundamental question of what influences the dynamics of species distributions. Landscape composition can affect two main components that dictate distributions: habitat suitability and habitat connectivity. Elucidating the relative importance of these factors and associated landscape features can help prioritize management action for species conservation.
Objectives
Our objective was to use species distribution models and network-based landscape connectivity models to understand which landscape factors were most predictive of the distribution of an anuran, Blanchard’s cricket frog (Acris blanchardi), in an agriculturally-dominated landscape.
Methods
We conducted our study in Ohio, USA, near the edge of the cricket frog’s contracting range. To obtain a current assessment of cricket frog distribution, we surveyed 367 pond and stream locations across three North–South transects. We then tested seven regression models, combining habitat suitability and landscape connectivity metrics, to determine which factors best predicted cricket frog presence.
Results
We detected cricket frogs in 24% of surveyed locations and they were more likely to occupy pond sites than stream sites. Cricket frog presence was best predicted by models with habitat suitability and the number of interconnected habitat patches. We found that, while there was high variation in habitat suitability across the study area, landscape connectivity was relatively uniform where we surveyed.
Conclusions
Agricultural landscapes around the world are often mosaics of land cover types, which may functionally provide connectivity for some species. In such areas, conservation management should focus on preserving and restoring regions of highly suitable habitat. This focus may be particularly relevant for species that do not appear to be dispersal limited and, therefore, able to maintain metapopulation dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current biodiversity crisis has intensified the need to predict species responses to landscape modification and climate change, and in turn has renewed attention on the fundamental question of what influences the dynamics of species distributions and range boundaries (Channell and Lomolino 2000; Elith and Leathwick 2009). At the most fundamental level, species distributions are constrained by physiological, biotic, and abiotic filters such that species persist only where there are appropriate conditions for survival and reproduction (Poff 1997). Often, these factors act at different spatial scales. For example, climatic factors (e.g., temperature and precipitation) often dictate large-scale species distributions, land cover may affect regional distributions, and biotic filters (e.g., predators, prey, competitors) often act on a smaller spatial scale (Guisan and Thuiller 2005; Cord 2011; Gonzalez-Salazar et al. 2013; Pearson and Dawson 2015). For species showing signs of range contraction or declines for reasons that are not clear, landscape-level assessments of occupancy can offer insights into potential drivers of changes in distribution.
One mechanism known to play a large role in dictating species distributions and range limits is dispersal (Brown et al. 1996; Pulliam 2000; Sexton et al. 2009). For instance, dispersal limitation in the context of metapopulation dynamics (Levins 1969) can explain the phenomenon that the availability of quality habitat is not always predictive of species presence; species can be absent from high-quality habitat patches or present in low quality patches (Pulliam 2000). Metapopulation dynamics predict that a patch will change from unoccupied to occupied over time when colonization rate equals or exceeds extinction rate (Levins 1969). The likelihood of colonization depends upon the probability of dispersal as well as the availability of resources at the new patch to sustain a population. In the absence of strong environmental or resource gradients, dispersal becomes a limiting factor in species distributions and range edges (Holt et al. 2005; Bahn et al. 2006).
There are two main factors that must be considered to ensure successful dispersal between patches: (1) the intrinsic dispersal capabilities and motivation of individuals and (2) the spatial configuration of habitat patches the landscape matrix [i.e., structural landscape connectivity (Baguette and Dyck 2007)]. By combining species-specific movement behavior with landscape structure, we are able to study biologically relevant, functional landscape connectivity (Pascual-Hortal and Saura 2006; Baguette and Dyck 2007). Despite the importance of the functional landscape in dictating species distributions, only in the past decade have investigators begun to incorporate dispersal dynamics into models predicting range responses to future environments (Hein et al. 2011; Bateman et al. 2013; Ofori et al. 2017). Similarly, while dispersal limitation is often referenced as an explanation for current ranges (e.g., Svenning et al. 2008; Treasure and Chown 2013) and an important component of management plans (Rudnick et al. 2012; Allen et al. 2020), explicit tests on the impact of landscape connectivity on current species distributions are relatively new (Hartel et al. 2010; Ribeiro et al. 2011; Schivo et al. 2020).
The role of dispersal and functional landscape connectivity may be particularly important for determining species distributions within human-dominated landscapes that have experienced extensive fragmentation and land cover changes. The loss of suitable habitat and increased isolation among habitat patches can reduce dispersal and increase the likelihood of population extinction—especially for declining populations that need rescue by immigrants. This is because losing high-quality habitat can reduce the number of emigrants and losing individuals in an inhospitable landscape matrix will reducing the number of immigrants; the overall loss in dispersing individuals can increase demographic stochasticity and inbreeding depression (Brown and Kodric-Brown 1977; Fahrig 2003; Keyghobadi 2007). Reduced connectivity in fragmented landscapes may explain why a species does not occupy most areas of suitable habitat. In highly modified landscapes, understanding how functional landscape connectivity and availability of suitable habitat patches interact is necessary to understand current occupancy trends and for management planning.
In this study, we used species distribution models and network-based landscape connectivity models to understand which landscape factors were most predictive of the distribution of an anuran in an agriculturally-dominated landscape. Species distribution models infer relationships between environmental variables and the presence of a species (Elith et al. 2011) and can be used to assess habitat suitability. Network-based landscape connectivity models integrate landscape features and species movement abilities to determine areas of high and low functional connectivity (Rayfield et al. 2011). We focused on cricket frogs (Acris blanchardi) in Ohio, U.S. as a model for evaluating the role of both habitat suitability and functional landscape connectivity in determining distribution across the landscape. Cricket frogs are a small (3 cm long) frog that is active throughout the summer months (Gray et al. 2005). They are considered a generalist, pond-breeding species, and can be found in variety of habitats (Trumbo et al. 2012; Youngquist et al. 2017). Cricket frogs were once one of the most abundant amphibian species throughout the midwestern U.S., but have recently experienced enigmatic declines at the northern, western, and eastern edges of their range (Gray et al. 2005). Ohio serves as the eastern edge of their current range. Cricket frogs may be especially dependent on dispersal and landscape connectivity to maintain regional populations because this species functions as an annual species (McCallum 2010; Lehtinen and Witter 2014); a single year of reproductive failure may equate to local extirpation in the absence of immigrants. Recent studies suggest that cricket frog dispersal is negatively affected by forest land cover and highways (Youngquist and Boone 2014; Youngquist et al. 2017). Because of cricket frogs’ sensitivity to land cover, combined with their reliance on dispersal to maintain metapopulation structure, we predicted that incorporation of landscape connectivity would improve predictions of cricket frog presence based only on habitat suitability.
Methods
Current cricket frog distribution
We documented cricket frog presence/absence by conducting call surveys across western Ohio. The landscape of this region is heavily agriculture, with a combination of row-crop (mostly corn and soybean), hay, wheat, and pasture. Areas with higher topographic relief and forested areas are in the southern and southeastern portions of the region. The climate is humid temperate with a 30-year average precipitation of 91 mm, minimum winter temperature of − 5 °C, and maximum summer temperature of 28 °C (NOAA 2021).
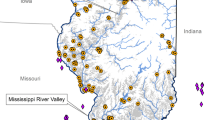
We established three transects within the putative current range of cricket frogs in western Ohio (Lehtinen and Skinner 2006; Lehtinen and Witter 2014). The transects were oriented north–south and placed approximately 45 km apart (hereafter W for “West,” C for “Central,” and E for “East” transect; Fig. 1). Each transect was divided into 31, ~ 90 km2 quadrats (5’ latitude and 7’ longitude), following Lehtinen and Skinner (2006). Within each quadrat we randomly selected two pond and two stream locations to conduct call surveys, for a total of 372 sites. All sites were selected from the National Hydrography Dataset (USGS NHD 2014). Ponds were less than 4 ha in area and less than 400 m from road; streams sites were also within 400 m of the road and were frequently surveyed at bridges.
Map of sampling location in Ohio, USA. North–South transects are W (western), C (central), and E (eastern) from left to right. Points indicate sampled sites of ponds (black circle) and streams (gray triangle). Dotted line indicates the estimated current range of cricket frogs in Ohio based on presence locations 2004–2011. Larger insert shows an enlargement of three sampled quadrats from the E transect, with pond and stream locations within. Small inset show location of Ohio (dark grey) within the United States
We surveyed the W and C transects from 26 May to 30 June 2014 and the E transect 28 May to 25 June 2015. This period corresponds to peak calling for cricket frogs in this region. Our methods are based on the protocol from the North American Amphibian Monitoring Program (NAAMP; Weir and Mossman 2005). At each site we conducted a road-side, 5-min call survey. Our surveys began after 18:00 and finished by 2:00 based on Lehtinen and Skinner (2006). Call surveys were conducted when air temperatures were above 15 °C, winds were relatively calm, and there was no rain; air temperature and wind speed were recorded for occupancy analyses. We recorded call intensity using the NAAMP standard 0–3 qualitative ranking, whereby 0 indicated no individuals heard calling; 1 indicates calls are individually distinct with intervals between calls; 2 indicates overlapping calls but individuals still distinguished; and 3 indicates a full chorus (Weir and Mossman 2005). However, because call intensity varies temporally, we summarized these data into presence or absence of calling males. Furthermore, because of the resolution for our landscape analyses (see below), we recorded all calls within 400 m of our roadside stopping point. If frogs were heard from ponds adjacent to a target stream, or vice versa, we recategorized the site. For instance, if we went to a pond that was dry, but frogs were calling from a stream we recategorized the site to a stream; this resulted in unequal sample sizes between ponds and streams. In a few instances, our target location was dry and we were unable to find an alternative; thus, these sites were removed from the study. Overall, we collected data from 367 locations (pond = 188, stream = 179; Table 1). To assess how survey methods affected our ability to detect cricket frogs, we surveyed 20% of the sites three times (n = 76).
Potential cricket frog habitat in Ohio
We created an independent habitat suitability map for cricket frogs across the entire state of Ohio; this map allowed us to assess the relationship between suitability and presence/absence (described above) as well as assess the potential availability of habitat across the historic range of cricket frogs and the entire state. We used MaxEnt 3.3.3k (Phillips et al. 2006) to develop a model of habitat suitability that was independent of the surveys conducted in 2014 and 2015. MaxEnt applies machine learning to presence-only data, randomly selected “background” points, and a set of environmental layers (e.g., climate, land cover) to obtain an output of predicted environmental suitability for a species. We obtained 204 presence locations from road-side call surveys conducted between 2004 and 2011 from Lehtinen and Skinner (2006), Lehtinen and Witter (2014), and the Ohio Frog and Toad Calling Survey (Pfingsten et al. 2013, J Davis personal communication). To correct for sample bias and spatial autocorrelation in presence locations, we selected points that were 10 km apart; this resulted in 62 presence points. We also created a background bias file (polygon) that approximated the historic putative range in Ohio, which encompasses the western 2/3 of the state (Lehtinen and Skinner 2006).
We tested four categories of environmental variables in our habitat suitability model: climate, land use, hydrologic features, and elevation. Initially, this included 19 bioclimatic variables reflecting average climate from 1970 to 2000 (WorldClim; 30 s resolution); 6 land cover variables—agriculture (row crop plus pasture), urban, forest, canopy cover, impervious surface (2011 National Landcover Dataset [NLCD], Homer et al. 2015) and road density (USGS NTD 2014); three hydrological variables—pond density, stream density, and distance to nearest stream (USGS NHD 2014); and two elevation variables—elevation and slope (OGRIP). We grouped pasture with row crop because behavior and occurrence studies showed cricket frogs respond similarly to these land cover types; further, pasture is a minor component of the landscape and little information is lost by combining these categories. After testing for correlations among variables (r < 0.7) and preliminary optimization of the MaxEnt model (testing variable importance using jackknife tests and overall model performance), we included nine environmental variables in our final model: annual mean temperature, annual temperature range, annual precipitation, mean daily temperature range, percent agriculture (row crop plus pasture), percent forest, percent urban, road density, and slope. Hydrological variables did not contribute to model fit and, in some cases, reduced model performance. Percent land covers were calculated using the National Land Cover Database (Homer et al. 2015; 30 × 30 m cell size) within a 500 × 500 m area; road density was calculated within a 10 km search distance for each raster cell; and slope was initially calculated from a 1 arc-second digital elevation map at 100 m resolution. All layers were resampled to a 500 m cell size and projected to UTM 16 N with the North American Datum of 1983. We chose 500 m as our spatial resolution because this matched the auditory range of our surveys and allowed us to balance resolution with computational processing time.
We ran MaxEnt using most of the default settings with the following exceptions: we set prevalence to 0.3 based on Lehtinen and Witter (2014); we checked the generality of the model using 15-fold cross-validation, whereby occurrence points are split into unique training (57 points) and test datasets (5 points) for each run; and we used jackknife to assess the importance of each environmental variable. We evaluated model fit using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. A value of 0.5 indicates random accuracy and value of 1 indicates perfect discrimination (Hosmer and Lemeshow 2000). We projected the final model to the entire state of Ohio plus a 25 km buffer around the W, C, and E transects (see below). We manipulated data and environmental layers using ArcGIS 10.3 and SDMtoolbox 2.0 (Brown 2014).
Landscape connectivity modeling
To quantify landscape connectivity, we used a landscape graph-based approach (Urban and Keitt 2001). In this approach, habitat patches (nodes, which included pond and lakes plus 100 m buffer) and a landscape resistance layer are input to create a graph—a set of nodes that are connected via links or edges. By using a resistance layer to calculate least cost paths between nodes, the graph edges reflect a biologically relevant landscape for the species. The characteristics of edges, nodes, and their connections are then used to calculate landscape connectivity indices (Pascual-Hortal and Saura 2006). All steps of the landscape analyses were computed with the program Graphab v2.0 (Foltête et al. 2012a). Because of the high computational power needed, we restricted our landscape connectivity modeling to a 25 km buffer around the surveyed transects. This distance prevented any artificial boundary constraints that could affect calculating connectivity metrics at our focal locations.
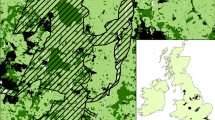
Constructing our landscape graph required three steps (Fig. 2; Foltête et al. 2012a). The first step was to define our nodes (hereafter “habitat patch”). Because cricket frogs preferentially occupy permanent and semi-permanent ponds and lakes, we defined habitat patches as all permanent wetlands (ponds and lakes; USGS NHD 2014) plus a 100 m buffer. The second step was to define a set of edges. We used three land cover layers—2011 NLCD (Homer et al. 2015), streams and rivers (USGS NHD 2014), and roads (USGS NTD 2014)—to create a resistance surface, from which least-cost distance edges were calculated (using cumulative cost along least cost paths; Clauzel et al 2016). We used previous studies of cricket frog movement behavior (Youngquist and Boone 2014) and population structure (Youngquist et al. 2017) to parameterize our resistance surface; these studies demonstrated that cricket frogs avoid traveling through forested land cover and that highways (State, US, and Interstate) limit gene flow. We gave habitat patches and rivers/streams the lowest resistance (value = 1); forest was given an intermediate resistance value (value = 20); State, US, and Interstate highways were given the highest resistance values according to their road class (local = 50; secondary = 75; primary = 100); all other terrestrial land cover types were given a low resistance (value = 5). We used a range of resistances 1–100 because a previous study indicated that analyses are robust to the initial resistance landscape parameterization (Youngquist et al. 2017). The third step was to build our landscape graph based on the previously defined habitat patches and edges. We built a planar threshold graph. ‘Planar’ means no links cross and threshold means maximum link distance is limited by cricket frog dispersal ability. Maximum cricket frog dispersal is estimated at 1.3 km (Gray et al. 2005), which equated to 48.8 cumulative cost distance in the resistance map; this value was obtained using the equation: Cumulative Cost Distance = e^(intercept + β*log(Euclidean Distance)) (Clauzel et al. 2016). Our resistance layer and associated landscape graph had a 100 m cell size.
Landscape connectivity modeling close up views. A The resistance-landscape used to calculate connectivity metrics. Road classes apply to state, US, and interstate highways and indicate road width and traffic speed; local road-class is not shown in the figure. B Graph components showing interconnected habitats used to calculated component order. Blue pond-habitats are connected with least-cost paths (graph-edges), constrained by cricket frog dispersal distance of 48.8 cost units (~ 1.3 km)
We calculated three landscape connectivity indices that represent different ecological meanings at different spatial scales (Pascual-Hortal and Saura 2006; Rayfield et al. 2011; Foltête et al. 2012b). We calculated one landscape-scale metric, component order (CO; Rayfield et al. 2011; Fig. 2), which is the number of interconnected habitat patches within each component (a set of connected patches). Higher component orders indicate a greater number of connected habitats and more potential movement within the landscape. We also calculated two patch level metrics, betweenness centrality and dispersal flux. Betweenness centrality (BC) is a measure of route-specific flux; it examines the role of any given habitat patch to serve as a stepping stone between two other patches (Rayfield et al. 2011). We configured our calculation of BC to approximate long-distance rescue effect (Foltête et al. 2012a, b). Dispersal flux (F) quantifies the potential to move to or from a patch and indicates the amount of movement between any given pair of habitat patches (Rayfield et al. 2011). For BC and F, probability of movement was calculated based on a maximum movement distance of 48.8 cost units (approximately 1.3 km) at a probability p = 0.05. These indices are patch-weighted; weighted indices incorporated habitat patch properties [‘patch capacity’ sensu Foltête et al. (2012a)], such as patch quality or resource potential, that indicate potential immigration and emigration. We used habitat suitability scores from the MaxEnt model as our weight (patch capacity = suitability*100). Patch-level metrics were interpolated across the landscape so we could estimate connectivity at the pond and stream sites surveyed in 2014 and 2015; we assumed that streams locations near highly connected ponds would also be well connected to those ponds. Connectivity indices were interpolated at a 500 m cell size for statistical analyses.
Statistical analyses
To test whether detection probability was affected by our survey methods, we used a single season site occupancy model (MacKenzie et al. 2002). Sites used for these analyses were sampled three times in a single year; no sites were sampled in multiple years. The observation covariates we tested were time of day (minutes after 18:00), sampling day (May 26 = day 1), air temperature, and wind speed; all variables were scaled around the mean. We initially parameterized the models by determining whether linear or quadratic formulations best fit the data using Akaike Information Criteria (AIC). For the final models we used linear formulation of time, day, and wind and quadratic formulation for temperature. We compared eight models: null, each covariate separately, a temporal model, an environmental model, and a full model (Table 2). We used Akaike Information Criteria corrected for small sample size (AICc) to compare and select the best models. We then calculated detection probability for the top model. Using the full dataset (n = 367), we tested whether there were differences in the number of sites with cricket frogs between habitat types (pond vs. stream) and among transects using Chi-square goodness of fit tests.
We modeled cricket frog presence using generalized linear models with a binomial distribution. Cricket frog presence/absence was our response variable. For the 76 sites that were sampled three times, we combined samples such that cricket frogs were recorded as “present” if they were heard calling on at least one date. We built seven different models using our four predictor variables—habitat suitability score, CO, BC, and F (Table 4). We also included transect and habitat type (pond or stream) as covariates. Our seven models included a null model (transect and habitat type), each predictor separately (four univariate models), additive habitat suitability + CO, and a full connectivity model (F + BC + CO). We did not include suitability score in the connectivity models that included BC or F because suitability was incorporated in the calculation of these indices as ‘patch capacity’. To more easily evaluate the relative effect of each variable, we scaled each predictor by centering around the mean and dividing by standard deviation. We compared models using AICc. We also ran the above analyses on pond-only and stream-only locations. We used conditional model averaging to estimate parameters and calculate Wald’s z-statistics. All statistics were conducted in the R statistical environment (R Core Team 2017) using the packages unmarked (Fiske and Chandler 2011) and AICcmodavg (Mazerolle 2019).
Results
Cricket frog surveys
Overall, cricket frogs were detected at 87 locations (24%) and were heard calling more often from pond sites than stream sites (Chi-square = 8.3, df = 1, p = 0.004; Table 1). The number of ponds occupied did not differ among transects (Chi-square = 3.03, df = 2, p = 0.220; Table 1). The probability of detection was affected by the time of day when surveys were conducted; survey day also had a weak effect on detection (Table 2). Average probability of detection was 64 ± 6% (SE); detection probability increased later in the night and decreased over the duration of the survey period (Supplemental Fig. 1).
Habitat suitability and landscape connectivity mapping
The average test AUC for the replicate runs was 0.773, indicating an adequate model of habitat suitability. Areas of suitable habitat were clumped and concentrated within the current cricket frog range. Areas with the highest habitat suitability were southern and western Ohio. Based on the jackknife test of variable importance, the most important variables were annual mean temperature, percent agriculture (crop and pasture), percent forest, and slope. In general, suitable habitats were warmer, had lower percent coverage of agriculture, and intermediate percent cover of forest (heterogeneous landscape), and were relatively flat (Supplemental Figs. 2, 3).
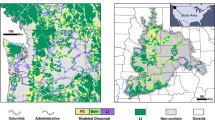
Landscape connectivity metrics had a large range across the landscape and among our study sites (Table 3). However, the overall study area had relatively uniform connectivity—especially where the transects were located and where the sites were sampled (Fig. 3). Histograms of CO, BC, and F were strongly right skewed (Supplemental Fig. 4).
Map of landscape factors within the focal landscape that predict cricket frog presence (black) and absence (white). A Connectivity landscape depicting the land cover categories used for connectivity modeling. B Habitat suitability and C component order were included in top models for the full data set and ponds-only; D betweenness centrality was a competing model for streams-only. Black circles indicate occupied ponds and black triangles indicate occupied stream sites; open circles or triangles indicate unoccupied sites
Cricket frog distribution
For the full dataset (pond and stream sites combined), the model that best explained cricket frog presence was the model that included habitat suitability and CO (Table 4). Cricket frog presence was positively influenced by habitat suitability (Wald’s z = 2.72, p = 0.006); CO was not significantly predictive of presence (Wald’s z = 1.88, p = 0.060; Fig. 3), which suggests the influence of CO on cricket frog presence was small relative to other factors. When evaluating only pond sites, we found a similar outcome: the combined model of habitat suitability and CO was the best model and the habitat suitability-only model was a competing model (Table 4). As with the full dataset, the estimated coefficient for suitability was significantly different from zero (Wald’s z = 3.5, p = 0.0004), but CO (Wald’s z = 1.5, p = 0.12) was not. In contrast, when we examined only streams, there were four competing models: CO, Null, BC, and habitat suitability. Habitat suitability, BC, and CO were all negatively associated with cricket frog presence at stream locations, but their estimate was not significantly different from zero (Table 4; Wald’s z < 0.57, p > 0.57).
Discussion
Understanding how species distributions can be predicted from landscape features is an important step in developing effective management plans and it is particularly valuable in light of continued land-use and climate changes. Two basic landscape components affecting where species might be found are the availability of suitable habitat and the ability to disperse between these areas. Elucidating the relative importance and contribution of habitat availability and landscape connectivity to species occurrences may be key to understanding occupancy in human dominated landscapes. In these predictive models, researchers must consider the landscape at ecologically relevant scales and in an ecologically meaningful context. To this end, we used species-specific parameters to understand the landscape features affecting the distribution of an at-risk amphibian, Blanchard’s cricket frog. We found that areas with higher habitat suitability were more likely to have cricket frogs present, but surprisingly landscape connectivity was not as important as expected.
Isolated populations of species that have a relatively high turnover rate like the cricket frog, which averages ~ 7% extinction rate across habitat types (Lehtinen and Witter 2014), are at high risk for local extirpation. Connectivity between populations is vital to maintain and rescue these populations on the landscape. We predicted that a model including landscape connectivity would be more important than a model with only habitat suitability—a result that was partially supported; our top model included habitat suitability and component order (CO), which is the number of interconnected habitat patches. However, unlike other studies that found a positive relationship between connectivity and occupancy (e.g., Foltête et al. 2012b; Jeliazkov et al. 2019), our results revealed a non-significant but slightly negative relationship between the number of interconnected pond-habitat patches (component order) and cricket frog presence. Betweenness centrality and dispersal flux were not included in top models, despite incorporating habitat suitability in the calculation of these metrics. This lends further support to habitat suitability alone being the best predictor of cricket frog presence.
One possibility for the absence of strong relationship between functional landscape connectivity metrics and cricket frog presence may be a result of relatively uniform connectivity in our sampling areas throughout this agricultural landscape (Fig. 3). In landscapes with more pronounced gradients in landscape connectivity (relative to other predictors), these metrics may better predict presence (e.g., Foltête et al. 2012b). It is also possible that we underestimated long-distance cricket frog dispersal ability (and thus underestimated landscape connectivity), which may frequently be the case with amphibians (Smith and Green 2005). However, Foltête et al. (2012b) showed model stability while changing dispersal distances, indicating that analyses with connectivity metrics are not sensitive to dispersal distance. Furthermore, our preliminary analyses using longer dispersal distances did not change the results; this is likely because increasing the number of interconnected ponds will only make the landscape have more uniform connectivity. Our data suggest the region may be sufficiently connected and allow migration to rescue extinct populations, and other studies have demonstrated that connectivity can be less important than habitat suitability for some species (Gould et al. 2012; Poniatowski et al. 2018). Certainly, our results indicate that cricket frogs are not dispersal limited in this landscape; our models of landscape connectivity that explicitly include dispersal limitation were rejected as predictors of presence. Finally, it is also possible that a negative relationship between connectivity and occupancy could stem from other biotic factors. For example, greater connectivity could increase the probability of exposure to the amphibian chytrid fungus pathogen (Batrachochytrium dendrobatidis; Scheele et al. 2015), which has recently been shown to increase overwinter mortality in cricket frogs (Wetsch et al. unpublished data); in this scenario, more isolated populations would be protected from immigrants carrying and transferring the pathogen.
Overall, habitat suitability was the best predictor of cricket frog presence and was included in top models. For pond-breeding amphibians, habitat suitability can be defined using within-pond characteristics and larger-scale landscape features (e.g., Băncilă et al. 2017; Holtmann et al. 2017). The spatial scale in question can influence the relative importance of suitability and connectivity in predicting species presence. For instance, Gould et al. (2012) found habitat suitability was the best predictor for amphibian presence at larger spatial scales (catchment); while at the wetland scale, landscape connectivity in addition to habitat characteristics were predictive of site occupancy for two of three amphibian species. Interestingly, site occupancy by boreal chorus frogs (Pseudacris maculata), who have life history traits akin to cricket frogs, was only predicted by site characteristics and not landscape connectivity (Gould et al. 2012). In this study, we took a larger-spatial scale approach to model habitat suitability and predict cricket frog presence at a given site. This approach provides a broad brush to identify areas where cricket frogs are most likely to be found and should have priority for conservation. We show that areas of high suitability are clumped into a few general areas in Ohio, with the largest area in the southwest part of the state; southwest Ohio has high habitat heterogeneity and a warmer mean temperature. Highly suitable habitats tend to be around riparian areas, indicating the importance of a riparian habitat for pond-breeding amphibians during the non-breeding season in agricultural landscapes.
Another interesting finding from our habitat suitability model was a relatively high frequency of cricket frogs in sites with lower habitat suitability. One explanation could stem from the model itself, which was an “adequate” model (AUC = 0.773) rather than an “excellent” model (AUC > 0.8). As with all models, our output is only as good as our input. Because presence points were collected from call surveys, there may be a road bias that was not accounted for in our background data points (pseudo absences). However, the high density of roads throughout the study area means this bias likely had a limited effect on the model output. Additionally, there may be other unknown landscape factors that were not included in the model. However, we infer that our habitat model accurately reflected cricket frogs themselves. Previous studies have found no or only very weak relationships between cricket frog presence and land cover (Lehtinen and Skinner 2006; Trumbo et al. 2012; Youngquist et al. 2017). It is, therefore, unsurprising that a land cover-based model might not have as good a fit for a wide-ranging generalist species, like cricket frogs, than with species that have a narrower range of habitat requirements (e.g., Hernandez et al 2006; Evangelista et al. 2008). Furthermore, as a species with relatively high turnover (extinction rates as high as 0.14 and colonization rate of 0.7; Lehtinen and Witter 2014), finding cricket frogs in unsuitable habitat is perhaps expected. Ultimately, our model of habitat suitability has good predictive power to indicate the general regions where cricket frogs should be encountered with higher frequency.
In addition to serving as breeding habitat when water is slow-moving, or in the backwaters, streams may serve as critical habitat corridors to facilitate dispersal and connect populations (Bull 2009; Scherer et al. 2012; Lehtinen and Witter 2014). In our current study, cricket frog presence was highly dependent on the waterbody type surveyed and were more likely to be heard calling from ponds than streams, likely reflecting a preference to breed in and call from lentic habitats (Gray et al. 2005; Lehtinen and Witter 2014); most stream sites in this survey were not still backwaters. However, not detecting calling males does not mean cricket frogs avoid stream habitat. Cricket frogs are frequently found along small rivers and streams (Gray et al. 2005), especially outside the breeding season (MBY personal observations). When looking at model comparisons to predict presence in ponds versus streams, we were surprised that there was no strong support for any metric to predict cricket frog presence in streams. If cricket frogs primarily use streams to travel through the landscape, then their presence on any given night may not be related to large-scale landscape metrics used in this study. However, the low number of stream-occupied sites (low sample size) could obscure our ability to discern associations. Additionally, we note that streams sites were not considered habitat nodes in the landscape connectivity models, but were rather coded as dispersal corridors; we made this decision to simplify the landscape model. We interpolated betweenness centrality and flux across the landscape and made the assumption that stream points near highly connected ponds were also highly connected; streams were not counted as habitat nodes when computing component order and a similar assumption was made. It is unclear how this modeling approach—defining nodes based only on ponds and lakes—may have impacted site-specific connectivity values for stream locations. Our approach could have made our stream sites appear less connected than they really are. If this is the case, then by treating all streams as habitat we would expect to see an even stronger negative relationship between landscape connectivity and occupancy, owing to the overwhelming absence of cricket frogs from “highly connected” stream sites; overall, our conclusions would be unchanged. Alternatively, treating all streams as habitat could create a relatively uniform landscape of high connectivity; under this scenario, there would still be little variation in connectively between occupied and un-occupies sites and our conclusions would remain much the same with habitat suitability as the main predictor.
Anthropogenic land-use change results in increased edge and open-canopy habitats (Haddad et al. 2015) to which some species will benefit; yet, the extent to which species profit will depend on their ability to disperse to those habitats. Species like the Blanchard’s cricket frog should, at least theoretically, gain habitat from conversion of forest into open-canopy agriculture and pastoral lands dotted with human-made ponds. Certainly, cricket frogs are found in many human-made ponds throughout the area in agricultural lands (Youngquist and Boone 2014; Youngquist et al. 2017), golf courses (Puglis and Boone 2012), and exurban areas (Boone et al. unpublished data). Our results do not negate the importance of landscape connectivity in species conservation and habitat management (Albert et al. 2017; Joly 2019), especially when considering metapopulation dynamics and changing landscapes (Zamberletti et al. 2018; Matos et al. 2019; Allen et al. 2020). Multi-year studies show that site occupancy and colonization can be related to pond isolation (Brooks et al. 2019; Wright et al. 2020). Instead, we show that landscape connectivity does not predict current species distributions better than habitat suitability. In landscapes where pond connectivity is relatively uniform and dispersal does not limit species distributions, management priority should focus on land cover types to define and preserve areas of high suitability. Out of an abundance of caution, focus should also be on regions where clusters of ponds are occupied until we have a better understanding of cricket frog dispersal, biotic and abiotic interactions, and the role of the landscape matrix. Our habitat suitability analysis also shows areas of high suitability outside the current range, indicating a potential for recolonization in the future; a potential that may have already begun to witness (Lehtinen and Witter 2014).
Availability of data and materials
Upon publication, requests can be made to the corresponding Author for data.
Code availability
Upon publication, requests can be made to the corresponding author for code.
References
Albert CH, Rayfield B, Dumitru M, Gonzalez A (2017) Applying network theory to prioritize multispecies habitat networks that are robust to climate and land-use change. Conserv Biol 31:1383–1396
Allen C, Gonzales R, Parrott L (2020) Modelling the contribution of ephemeral wetlands to landscape connectivity. Ecol Model 419:108944
Baguette M, Dyck H (2007) Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landsc Ecol 22:1117–1129
Bahn V, O’Connor RJ, Krohn WB (2006) Effect of dispersal at range edges on the structure of species ranges. Oikos 115:89–96
Băncilă RI, Cogălniceanu D, Ozgul A, Schmidt BR (2017) The effect of aquatic and terrestrial habitat characteristics on occurrence and breeding probability in a montane amphibian: insights from a spatially explicit multistate occupancy model. Popul Ecol 59:71–78
Bateman BL, Murphy HT, Reside AE, Mokany K, VanDerWal J (2013) Appropriateness of full-, partial- and no-dispersal scenarios in climate change impact modelling. Divers Distrib 19:1224–1234
Boone MD, Davis AY, Dumyahn S, Freund A, Mendoza R, Muniz-Torres A (Unpublished data) Use of exurban lands by pond-breeding amphibians in agricultural areas: factors that predict patch occupation in human-dominated habitats
Brooks GC, Smith JA, Frimpong EA, Gorman TA, Chandler HC, Haas CA (2019) Indirect connectivity estimates of amphibian breeding wetlands from spatially explicit occupancy models. Aquat Conserv Mar Freshw Ecosyst 29:1815–1825
Brown JL (2014) SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic, and species distribution model analyses. Methods Ecol Evol 5:694–700
Brown JH, Kodric-Brown A (1977) Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58:445–449
Brown JH, Stevens GC, Kaufman DM (1996) The geographic range: size, shape, boundaries, and internal structure. Annu Rev Ecol Syst 27:597–623
Bull EL (2009) Dispersal of newly metamorphosed and juvenile western toads (Anaxyrus boreas) in northeastern Oregon, USA. Herpetol Conserv Bio 4:236–247
Channell R, Lomolino MV (2000) Dynamic biogeography and conservation of endangered species. Nature 403:84–86
Clauzel C, Fotete J, Girardet X, Vuidel G (2016) Graphab 2.0 User Manual. 2016-05-12
Cord A (2011) Inclusion of habitat availability in species distribution models through multi-temporal remote sensing data? Ecol Appl 21:3285–3298
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Elith J, Phillips SJ, Hastie T, Dudik M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57
Evangelista PH, Kumar S, Stohlgren TJ, Jarnevich CS, Crall AW, Norman JB, Barnett DT (2008) Modelling invasion for a habitat generalist and specialist plant species. Divers Distrib 14:808–817
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fiske I, Chandler R (2011) unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23
Foltête JC, Clauzel C, Vuidel G (2012a) A software tool dedicated to the modelling of landscape networks. Environ Model Softw 38:316–327
Foltête JC, Clauzel C, Vuidel G, Tournant P (2012b) Integrating graph-based connectivity metrics into species distribution models. Landsc Ecol 27:557–569
Gonzalez-Salazar C, Stephens CR, Marquet PA (2013) Comparing the relative contributions of biotic and abiotic factors as mediators of species’ distributions. Ecol Model 248:57–70
Gould WR, Patla DA, Daley R, Corn PS, Hossack BR, Bennetts RE, Peterson CR (2012) Estimating occupancy in large landscapes: evaluation of amphibian monitoring in the greater Yellowstone ecosystem. Wetlands 32:379–389
Gray RH, Brown LE, Blackburn L (2005) Decline of northern cricket frogs (Acris crepitans). In: Lannoo MJ (ed) Amphibian declines: the status of United States species. University of California Press, Berkeley, pp 441–443
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:1–10
Hartel T, Nemes S, Öllerer K, Cogalniceanu D, Moga C, Arntzen JW (2010) Using connectivity metrics and niche modelling to explore the occurrence of the northern crested newt Triturus cristatus (Amphibia, Caudata) in a traditionally managed landscape. Environ Conserv 37:195–200
Hein CL, Öhlund G, Englund G (2011) Dispersal through stream networks: modelling climate-driven range expansions of fishes. Divers Distrib 17:641–651
Hernandez PA, Graham CH, Master LL, Albert DL (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29:773–785
Holt RD, Keitt TH, Lewis MA, Maurer BA, Taper ML (2005) Theoretical models of species’ borders: single species approaches. Oikos 108:18–27
Holtmann L, Philipp K, Becke C, Fartmann T (2017) Effects of habitat and landscape quality on amphibian assemblages of urban stormwater ponds. Urban Ecosyst 20:1249–1259
Homer CG, Dewitz JA, Yang L, Jin S, Danielson P, Xian GZ, Coulston J, Herold N, Wickham J, Megown K (2015) Completion of the 2011 National Land Cover Database for the conterminous United States—representing a decade of land cover change information. Photogramm Eng Remote Sens 81(5):345–354
Hosmer DW, Lemeshow S (2000) Chapter 5—applied logistic regression, 2nd edn. Wiley, New York, NY, pp 160–164
Jeliazkov A, Lorrillière R, Besnard A, Garnier J, Silvestre M, Chiron F (2019) Cross-scale effects of structural and functional connectivity in pond networks on amphibian distribution in agricultural landscapes. Freshw Biol 64:997–1014
Joly P (2019) Behavior in a changing landscape: using movement ecology to inform the conservation of pond-breeding amphibians. Front Ecol Evol 7:1–17
Keyghobadi N (2007) The genetic implications of habitat fragmentation for animals. Can J Zool 85:1049–1064
Lehtinen RM, Skinner AA (2006) The enigmatic decline of Blachard’s cricket frog (Acris crepitans blanchardi): a test of the habitat acidification hypothesis. Copeia 2006:159–167
Lehtinen RM, Witter JA (2014) Detecting frogs and detecting declines: an examination of occupancy and turnover patterns at the range edge of Blanchard’s Cricket Frog (Acris blanchardi). Herpetol Conserv Bio 9:502–515
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15:237–240
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
Matos C, Petrovan SO, Wheeler PM, Ward AI (2019) Landscape connectivity and spatial prioritization in an urbanizing world: a network analysis approach for a threatened amphibian. Biol Conserv 237:238–247
Mazerolle MJ (2019) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.2-1
McCallum ML (2010) Future climate change spells catastrophe for Blanchard’s cricket frog, Acris blanchardi (Amphibia:Anura:Hylidae). Acta Herpetol 5:119–130
NOAA (2021) 1991–2020 US Climate Normals for Lima OH. National Centers for Environmental Information. National Oceanic and Atmospheric Administration. https://www.ncei.noaa.gov/access/us-climate-normals/
Ofori BY, Stow AJ, Baumgartner JB, Beaumont LJ (2017) Combining dispersal, landscape connectivity and habitat suitability to assess climate-induced changes in the distribution of Cunningham’s skink, Egernia cunninghami. PLoS ONE 12:e0184193
OGRIP. Ohio geographically referenced information program: digital elevation model. Data downloaded from https://ogrip.oit.ohio.gov/ServicesData/GEOhioSpatialInformationPortal/USGSGeodataDistribution(Historical)/DEM.aspx. Accessed 26 Feb 2021
Ohio Frog and Toad Calling Survey. Data provided by Jeff Davis. http://Ohioamphibians.com. Accessed 26 Feb 2021
Pascual-Hortal L, Saura S (2006) Comparison and development of new graph-based landscape connectivity indices: towards the prioritization of habitat patches and corridors for conservation. Landsc Ecol 21:959–967
Pearson RG, Dawson TP (2015) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr 12:361–371
Pfingsten RA, Davis JG, Matson TO, Lipps G, Wynn D, Armitage BJ (2013) Amphibians of Ohio. Ohio Biological Survey. 900 pp
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Poff NL (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J N Am Benthol Soc 16:391–409
Poniatowski D, Stuhldreher G, Loffler F, Fartmann T (2018) Patch occupancy of grassland specialists: habitat quality matters more than habitat connectivity. Biol Conserv 225:237–244
Puglis HJ, Boone MD (2012) Effects of terrestrial buffer zones on amphibians on golf courses. PLoS ONE 7:e39590
Pulliam HR (2000) On the relationship between niche and distribution. Ecol Lett 3:349–361
R Core (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rayfield B, Fortin M-J, Fall A (2011) Connectivity for conservation: a framework to classify network measures. Ecology 92:847–858
Ribeiro R, Carretero MA, Sillero N, Alarcos G, Ortiz-Santaliestra M, Lizana M, Llorente GA (2011) The pond network: can structural connectivity reflect on (amphibian) biodiversity patterns? Landsc Ecol 26:673–682
Rudnick DS, Ryan SJ, Beier P, Cushman SA, Dieffenbach F, Epps CW, Gerber LR, Hartter J, Jenness JS, Kintsch J, Merenlender AM, Perkl RM, Reziosi DV, Trombulak SC (2012) The roll of landscape connectivity in planning and implementing conservation and restoration priorities. Issues Ecol 16:1–20
Scheele BC, Driscoll DA, Fischer J, Fletcher AW, Hanspach J, Voros J, Hartel T (2015) Landscape context influences chytrid fungus distribution in an endangered European amphibian. Anim Conserv 18:480–488
Scherer RD, Muths E, Noon BR (2012) The importance of local and landscape-scale processes to the occupancy of wetlands by pond-breeding amphibians. Popul Ecol 54:487–498
Schivo F, Mateo-Sanchez MC, Bauni V, Quintana RD (2020) Influence of land-use/land-cover change on landscape connectivity for an endemic threatened amphibian (Argenteohyla siemersi pederseni, Anura: Hylidae). Landsc Ecol 35:1481–1494
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:110–128
Svenning J-C, Normand S, Skov F (2008) Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography 31:316–326
Treasure AM, Chown SL (2013) Contingent absences account for range limits but not the local abundance structure of an invasive springtail. Ecography 36:146–156
Trumbo DR, Burgett AA, Hopkins RL, Biro EG, Chase JM, Knouft JH (2012) Integrating local breeding pond, landcover, and climate factors in predicting amphibian distributions. Landsc Ecol 27:1183–1196
Urban D, Keitt T (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82:1205–1218
USGS NHD 2014: United States Geological Survey: National hydrography dataset—Best resolution. Downloaded from The National Map. https://apps.nationalmap.gov/viewer. Accessed 26 Feb 2021
USGS NTD 2014: United States Geological Survey: National Transportation Dataset. Downloaded from The National Map. https://apps.nationalmap.gov/viewer/. Accessed 26 Feb 2021
Weir LA, Mossman MJ (2005) North American amphibian monitoring program (NAAMP). In: Lannoo M (ed) Amphibian declines: conservation status of United States amphibians. University of California Press, Berkeley, CA, pp 307–313
Wetsch O, Strasburg M, McQuigg J, Boone MD (Unplublished data) Is overwintering mortality driving enigmatic declines? Evaluating the impacts of multiple parasites on an anuran across life stages
WorldClim. Bioclimatic variables. https://worldclim.org/. Data downloaded 4/2014. Accessed 25 Feb 2021
Wright AD, Grant EHC, Zipkin EF (2020) A hierarchical analysis of habitat area, connectivity, and quality on amphibian diversity across spatial scales. Landsc Ecol 35:529–544
Youngquist MB, Boone MD (2014) Movement of amphibians through agricultural landscapes: the role of habitat on edge permeability. Biol Conserv 175:148–155
Youngquist MB, Inoue K, Berg DJ, Boone MD (2017) Effects of land use on population presence and genetic structure of an amphibian in an agricultural landscape. Landsc Ecol 32:147–162
Zamberletti P, Zaffaroni M, Accatino F, Creed IF, Michele CD (2018) Connectivity among wetlands matters for vulnerable amphibian populations in wetlandscapes. Ecol Model 384:119–127
Acknowledgements
This project was funded by National Science Foundation Doctoral Dissertation Improvement Grant (DDIG) to MBY. We thank Dr. R. Lehtinen and J. Davis for providing data of cricket frog presence across Ohio; T. Drought and K. Inoue for assisting with surveys; M. Strasburg, J. McQuigg, M. Murphy, C. Dvorsky, and O. Wetsch for reading earlier versions of this paper; and S. Rumschlag and T. Hoskins for general support.
Funding
Funding for this project was provided by the National Science Foundation Doctoral Dissertation Improvement Grant (DDIG) to MBY. Award Number: 1406814.
Author information
Authors and Affiliations
Contributions
MBY designed the study, conducted the investigation, analyzed the data, and wrote the manuscript, and was awarded funding as co-PI. MDB assisted in study design and advised MBY on all aspects of the study, provided editorial advice, and was awarded funding as PI.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Methods were approved by Miami University Institutional Animal Care and Use Committee. IACUC Project Number: 914.
Consent for publication
All authors consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Youngquist, M.B., Boone, M.D. Making the connection: combining habitat suitability and landscape connectivity to understand species distribution in an agricultural landscape. Landscape Ecol 36, 2795–2809 (2021). https://doi.org/10.1007/s10980-021-01295-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-021-01295-7