Abstract
Context
The effect of landscape complexity on biodiversity is an important topic in landscape ecology, and spatial scale is key to understand true species-landscape relationships.
Objectives
We assessed the effect of landscape complexity on the occurrence of small mammal species and species richness at different spatial scales in an agroecosystem of central Argentina.
Methods
We performed two capture-recapture samplings in 50 sites with different landscape complexity covering a ~ 452 km2 area. We used a multi-species analysis following a Bayesian approach. We modeled species occurrence as a function of landscape complexity (estimated through the Shannon habitat diversity index) at six spatial scales.
Results
We found that the occurrence probability of species that are favored by agriculture intensification increased with the decrease of landscape complexity, whereas that of species dependent on natural habitats decreased. Some species occurred over the whole range of landscape complexity, one species was only present in the simplest landscapes and the others occurred at intermediate and high values of landscape complexity. Species richness increased with landscape complexity. On average, our results suggest that landscape complexity is perceived by small mammals at a spatial scale of 150-200 m.
Conclusions
Landscape heterogeneity is a key factor to maintain biodiversity and species persistence in agroecosystems. An important finding of our study is that a complex landscape at 200 m (16 ha) spatial scale would benefit most small mammal assemblage species. This result would be key to define management strategies for biodiversity conservation in agricultural landscapes of central Argentina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans have transformed natural ecosystems across more than three-quarters of the terrestrial biosphere surface (Sanderson et al. 2002), and consequently have altered global patterns of biodiversity and ecosystem processes (Ellis and Ramankutty 2008). The anthrome framework, which maps global ecological patterns created by sustained direct human interactions with ecosystems, presents an alternative view of the terrestrial biosphere (Ellis and Ramankutty 2008; Martin et al. 2014).
Under this approach, cropland anthromes or agricultural landscapes are the second most extensive, covering about 20% of Earth’s ice-free land, and contain an agricultural matrix and patches and/or linear habitats with natural vegetation. These areas present a challenge for setting biodiversity conservation goals and management outside protected areas (Quinn et al. 2014). Therefore, the contribution of agricultural areas is critical for successful biodiversity conservation in the long term (Ellis and Ramankutty 2008).
Biodiversity conservation in agricultural landscapes requires a proper understanding of the relationship between landscape heterogeneity and biodiversity itself (Tscharntke et al. 2005; Fahrig et al. 2011). Landscape heterogeneity has two components: compositional heterogeneity (the variety of different cover types) and configurational heterogeneity (spatial patterning of cover types) (Fahrig et al. 2011). Some studies show that the increase of these two components benefits biodiversity in agricultural landscapes (Weibull et al. 2003; Lindsay et al. 2013; Mitchell et al. 2014; Jackson and Fahrig 2015; Novotný et al. 2015).
The effects of landscape heterogeneity on ecological processes can be misleading if the scale chosen to measure the environmental variable is wrong (Smith et al. 2011). Two of the most important components of scale are grain and extent. It is well known that ecological processes depend on the spatial extents (hereafter scales) at which organisms perceive landscape heterogeneity (Wiens 2002; Thies et al. 2003). The optimal scale is the one at which the ecological response in the focal area is best predicted by the landscape structure, i.e. the scale at which the relationship is the strongest (scale of effect; Jackson and Fahrig 2015; Miguet et al. 2016). The most common method for estimating the appropriate scale is to model the relationship between landscape complexity and biodiversity at different spatial scales and determine which scale yields the best fit, i.e., using empirical data in a study at different spatial scales to find the steepest slope (Miguet et al. 2016).
The effects of landscape complexity on populations vary with the habitat specialization degree of species (Levins 1968; Devictor et al. 2008). Habitat specialist species rely on local habitat quality and are more affected by habitat disturbance than generalist species. The latter are able to exploit a wider array of habitats, including the matrix and resources available there (Bentley et al. 2000; Zollner 2000; Millan de la Pena et al. 2003; Filippi-Codaccioni et al. 2010; Fischer and Schröder 2014; King et al. 2014). Thus, generalist species would be less affected by habitat homogenization produced by agriculture than specialist species (Coda et al. 2015, 2016).
Agriculture has been highlighted as one of the main global drivers in the reduction of landscape heterogeneity, which affects a variety of ecological processes in several taxa (Benton et al. 2003; Fahrig et al. 2011; Fischer et al. 2011; Stanton et al. 2018; Zingg et al. 2018). Particularly in Argentina, the rapid expansion and intensification of crop production occurred during the last 25 years have resulted in habitat loss and reduced spatial heterogeneity (Gavier-Pizarro et al. 2012; Bedano and Domínguez 2016). These changes have led to drastic modifications in agricultural landscapes of central Argentina, where pastures, grasslands and forests, whether natural or used for cattle grazing, have been converted to crop production (Viglizzo et al. 1997; Paruelo et al. 2005; Baldi and Paruelo 2008). Furthermore, plot size has been enlarged and field borders have been removed to enlarge crop areas (Aizen et al. 2009), leading to a decrease in landscape complexity (Bilenca et al. 2007; Baldi and Paruelo 2008; Gomez et al. 2015).
Studies carried out in agroecosystems of central Argentina showed that the increase in agriculture intensification affected small mammal diversity and abundance (Coda et al. 2014, 2015; Gomez et al. 2015, 2018). Some rodent species in the assemblage can occur in almost all types of habitats within the agricultural landscape (habitat generalist) while others occur only in habitats with high vegetation cover similar to natural habitats (habitat specialist). Thus, assemblage species were ranked from generalists to specialists: Calomys musculinus, C. laucha, Akodon azarae, Oligoryzomys flavescens, C. venustus, A. dolores and Oxymycterus rufus (Martínez et al. 2014). Previous studies show that C. musculinus and C. laucha are favored by agriculture intensification, whereas A. azarae, O. rufus, O. flavescens and the marsupial species Monodelphis dimidiata and Thylamys pallidior are negatively affected (Medan et al. 2011; Fraschina et al. 2012; Coda et al. 2014, 2015; Gomez et al. 2015, 2018). These studies, however, were conducted at constant grain and spatial extent. Therefore, little is known about the responses of these small mammals to landscape complexity at different spatial extent.
The aim of this study was to assess the relationship between landscape complexity and the occurrence and richness of small mammal species at different spatial scales in agroecosystems of central Argentina, through the implementation of hierarchical occupancy models. We predict that occurrence probability of species favored by agricultural intensification will increase with decreasing landscape complexity, whereas the occurrence probability of species dependent on natural or semi natural habitats will decrease. Small mammal species richness will decrease together with landscape complexity. It was also our aim to find the spatial scale at which the relationships are the strongest for each species.
Methods
Study area
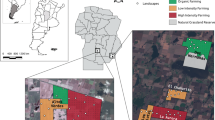
We carried out this study in an agroecosystem of the center-south of Córdoba Province, Argentina (Fig. 1). The area belongs to the Espinal ecoregion (Burkart et al. 1999), but its physiognomy has undergone a marked transformation due to intensive agriculture and livestock practices. Currently, the landscape is composed of a matrix of crop/pasture fields surrounded by border habitats, railways and other types of linear habitats where remnants of original flora are found. Besides, there are few small patches of grasslands and woodlands.
Small mammal’s surveys
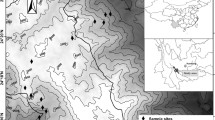
We performed two capture-recapture samplings of two weeks each, in March and May 2016 (Austral late summer and autumn, respectively). We selected 50 sampling sites (25 per week) in a ~ 452 km2 area. Sampling sites covered a gradient from extremely simple to complex landscapes (98% and 23% of arable land, respectively). In each site we set up 1 trapping line of 20 live traps along a linear habitat. Contiguous trapping lines were separated by at least 1.2 km. Traps within lines were separated by a distance of 10 m and they were baited with a mixture of peanut butter and cow fat. Trapped animals were identified, sexed, weighed and ear-tagged. Body and tail length were also recorded.
Landscapes variables
We analyzed the landscape complexity of each sampling site at six spatial extents considering radii of 150 m (9 ha), 200 m (16 ha), 300 m (35 ha), 400 m (62 ha), 500 m (97 ha) and 600 m (140 ha) around the center of each trapping line. Grain size did not change along the study. Following Jackson and Fahrig (2015), we selected these scales because they cover from four to nine times the average dispersal distance of species. The average movement distance of our studied species is 70 m (Sommaro et al. 2010; Gomez et al. 2011).
Maps were drawn at the scale 1:1250 from Google Earth images corresponding to a date close to the field surveys, using the OpenLayer plugin within QGIS 2.7 (QGIS Development Team 2017). A polygon vector layer was created digitizing every type of cover land. We quantified the compositional heterogeneity through percentages of arable land (crop and pastures), grassland and woodland and Shannon habitat diversity index. Shannon index was estimated as \(H = - \sum\nolimits_{i = 1}^m {{P_i}\log {P_i}}\), where m is the number of habitat types in each sampling site and Pi is the proportion of each habitat type from all available habitat types within each sampling site (Fahrig et al. 2011). Habitat types could be arable land, grassland, woodland, railway, border habitats (1.5–2.5 m wide vegetation strip along field wire fences) and man-made structures (farmhouses, sheds, storehouses). Furthermore, we calculated the configurational heterogeneity by perimeter–area ratio, \(P/{A_{cropplot}} = \sum\nolimits_{i = 1}^m {{P_i}/\sum\nolimits_{i = 1}^m {A_i} }\) where P is the perimeter (border habitat), A the crop plot area, i = 1,…,m the crop plot number, and m is the number of plots in the landscape (Fischer and Schröder 2014). All landscape predictors were measured at each spatial scale and standardized to allow comparison of regression coefficients. Correlation analyses were performed to test for multicollinearity among landscape variables for each radius (either Pearson or Spearman tests according to normality/non-normality). We showed these results in Online Resource 1, Table A1. We considered significant correlation between variables when p < 0.05.
We selected the Shannon habitat diversity index to study the relationship between species occurrence and landscape complexity because this metric is a robust parameter for the quantification of landscape complexity (Fahrig et al. 2011). However, we also showed results of the relationship between perimeter/areacrop plot ratio and species occupancy.
Occupancy and species richness analyses
Hierarchical multi-species occupancy models using a Bayesian approach were used to assess the influence of landscape complexity on small mammal species (Dorazio et al. 2005; Royle and Dorazio 2008; Zipkin et al. 2009; Kéry and Royle 2016). These models incorporate detection probabilities to overcome sampling biases related to differences in species detection that can affect the estimation of the relationship between species occurrence and habitat covariates (Royle and Dorazio 2008). The implementation of hierarchical multi-species models allows more robust inferences and increases the accuracy of occupation estimators compared to those models that consider individual species. These models have several key advantages since they permit inference at the level of the whole assemblage and at each individual species. The assemblage model is a “hypermodel” for abundance or distribution of a set of species. The parameters of each species are treated as random effects endowed with prior distributions and the hyperparameters of those priors describe the assemblage (Kéry and Royle 2016). This becomes more relevant for those species that are less detected, and estimates can be made about them (Dorazio et al. 2005; Mackenzie et al. 2006; Royle and Dorazio; 2008; Kéry et al. 2009). In this way, our approach allows inferences to be made about the effect of landscape heterogeneity on each species and on the assemblage, allowing different scales of effect to be selected for each species.
Occupancy estimation accounts for imperfect detection probabilities of each species (p < 1), so that if a species is not observed at a certain point, it can be either truly absent, or present but undetected (Mackenzie et al. 2002, 2006; Tyre et al. 2003). Sites occupancy models can be formulated as a hierarchical state-space model, linking two binary regression models: a process model for occupancy of each species, and an observation model for detection conditional on occupancy (Kéry and Royle 2016). Occurrence (zi,k,t) for each species (k) at each site (i) and season (t) is specified as a Bernoulli random variable, zi,k,t ~ Bern (ψi,k,t), where ψi,k,t is the probability that species k occurs at site i and season t. True occurrence is imperfectly observed, where zi,k,t = 1 when the species is present and zero otherwise. The observation model also follows a Bernoulli distribution as yi,j,k,t ~ Bern (pi,j,k,t * zi,k,t), where pi,j,k,t is the probability that species k at site i is detected at night j and season t. This formulation is conditional on the species being present (i.e., zi,k,t = 1).
Following a hierarchical multi-species occupancy approach, we modeled species occurrence as a function of Shannon habitat diversity index separately for each spatial scale. For example, one of our occupancy models was:
where both the parameter denoting covariate effects (SI, Shannon habitat diversity index) and the intercept β for each species k = 1, 2…N were estimated for the 150 m spatial scale. We obtained the most relevant spatial scale from the model with the highest absolute value of β coefficient for SI (steepest slope) and through inferences based on the 95% credible intervals (95% CRI), assuming a strong effect when CRI did not overlap zero, and an important effect when the interval overlapped zero less than 25% (i.e. 75% of the interval had the same sign of the mean effect) (Gomez et al. 2018). Models were run using R 3.1.2 (R Core Team 2018) and JAGS software, through the package jagsUI (Plummer et al. 2016), which uses Markov Chain Monte Carlo (MCMC) to find the posterior distribution of the parameters of interest. We ran three chains of length 100,000 each and discarded the first 50,000 as a burn in, with a thinning rate of two. We used weak priors for all parameters (Kellner 2017). We assessed convergence using the Gelman and Rubin diagnostic (\(\hat R\)), which includes the variance between the means from the parallel chains and the average of the within-chain variances. Convergence is reached when \(\hat R\) is near 1 (Gelman and Rubin 1992). We used the same procedure to model species occurrence as a function of perimeter/areacrop plot ratio.
Species richness cannot be modeled as a structural parameter in the occupancy model but it is a quantity computed from the matrix of the individual species presence indicators (Kéry and Royle 2016). Thus, we calculated site-specific species richness by summing the estimated number of species, i.e. the latent occurrences, Z matrix values (Online Resource 3). Following Kery and Royle (2016), we explored the relationship between estimated species richness and SI for each scale through the fit of a regression model with quadratic polynomials of SI. We made predictions of species richness for a complete range of SI (Online Resource 3).
Results
Arable land percentages increased with the spatial scale, whereas SI and perimeter/areacrop plot ratio decreased (Fig. 2). Arable land percentages were on average higher than 58% in all spatial scales (Fig. 2c). Other types of habitats (railway, border habitats and man-made structures) were not included in the figure due to their low percentages.
We trapped a total of 774 individuals, including rodent and marsupial species. Calomys musculinus and A. azarae were the most frequently captured species (30.88% and 35.4% respectively), while A. dolores and T. pallidior were the least common (0.25% and 0.39% respectively) (Online Resource 1, Table A2).
We were able to estimate occupancy probabilities for all the species in the assemblage. We observed that the scale of effect of SI over occupancy probabilities varied among species. Scale of effect (the highest absolute value of β coefficient and CRI) was 150 m for A. dolores and C. venustus, 200 m for A. azarae, O. flavescens and C. musculinus, 400 m for C. laucha and O. rufus and, 600 m for M. dimidiata and T. pallidior (Fig. 3, Online Resource 1—Table A3). Based on these results, we analyzed both the detection and occupation probabilities at the most relevant scale for each species.
Shannon habitat index coefficients in the logit scale (\(\hat \beta\), 95% CRI) on logit occupancy (logit \(\hat \varPsi\)) of assemblage and individual small mammal species at 6 spatial scales (150 m, 200 m, 300 m, 400 m, 500 m and 600 m). In solid, lines where at least 75% of the interval had the same sign of the mean effect. In black, the scale of effect for each species
Detection probabilities varied by species but not by night, and they were generally low (p < 0.5). Calomys musculinus and A. azarae showed the highest detection probabilities, and M. dimidiata the lowest (Fig. 4). Mean occupancy probabilities of C. musculinus and C. laucha were negatively affected by SI (Fig. 5b and c). Moreover, at low landscape complexity C. musculinus had higher occupancy probabilities than C. laucha. Indeed, C. laucha was almost extinct in more complex landscapes (Fig. 5c). Shannon habitat diversity index had a positive effect on occupancy of species known to be more dependent on habitat quality. Akodon azarae, C. venustus, O. rufus and M. dimidiata reached the highest occupancy probabilities at the highest landscape complexity values. However, these species responded differently to low landscape complexity. We observed a gradient in occupancy probabilities from more tolerant to more sensitive species according to their habitat requirements, i.e., A. azarae, C. venustus, O. rufus and M. dimidiata (Fig. 5a–d). The other species, A. dolores, O. flavescens and T. pallidior were only observed in sites with SI values higher than 0.2 (Fig. 5a, b, and d). Inferences about A. dolores and T. pallidior should be considered with caution based on their low captures (Online Resource 1—Table A2). The community mean effect was positive and estimated at 0.081 (Fig. 3). The derived number of species by site in relation to SI and spatial scale is shown in Fig. 6. In general, species richness increased with landscape complexity, and it was highest at 150–200 m.
The perimeter/areacrop plot ratio explains occupancy probabilities in a similar way to the SI but with a smaller slope in those species more tolerant to landscape simplification (C. laucha and C. musculinus). However, it would not be a good index of landscape complexity to analyze the occupancy of those species more dependent on natural or semi-natural habitats (Fig. 7).
Discussion
Land use modifies the landscape structure causing habitat alteration and fragmentation (Borges-Matos et al. 2016). Understanding landscape structure can lead to a better comprehension of species persistence. The role of landscape complexity on biodiversity is an important topic in landscape ecology. However, the spatial scale at which landscape structure is measured can affect species-landscape relationships (Jackson and Fahrig 2015). This scale of effect is related to the spatial scale at which species perceive and interact with landscapes (Miguet et al. 2016). Our sampling design allowed us to maximize our ability to detect species-landscape relationships, since we compared the effect of landscape complexity on species occupancy and richness at multiple spatial scales. Although there are several studies about the relationship between small mammals and environmental variables in central Argentina croplands (Andreo et al. 2009; Simone et al. 2010; Polop et al. 2012; Martínez et al. 2014; Coda et al. 2015; Gomez et al. 2015), none of them have followed this type of approach.
We found support to our predictions that occurrence probabilities of species that are favored by agriculture intensification (C. laucha and C. musculinus) increase with the decrease of landscape complexity whereas those of species dependent on natural habitats (A. azarae, C. venustus, O. rufus, O. flavescens, A. dolores, M. dimidiate and T. pallidior) decrease. Thus, our results showed that landscape structure divides species assemblage in two groups, i.e., species negatively affected and species positively affected. The scale of effect varied among species though.
The two species that benefit from agriculture had a negative relationship with landscape complexity. Calomys laucha was only found in simple landscapes. It showed a negative curvilinear relationship between occupancy probability and Shannon habitat index, becoming extinct at intermediate habitat complexity values. Despite C. musculinus occupancy probability showed a negative linear relationship with habitat complexity, this species occurred all along the habitat complexity range. Therefore, C. musculinus might be considered a habitat generalist. Although A. azarae and C. venustus occupancy probabilities increased with landscape complexity, these species are also being able to occupy the whole range of habitats. Oligoryzomys flavescens, A. dolores and T. pallidior appeared to be the most harmed by agriculture. Occupancy probabilities of these species showed a positive curvilinear relationship with habitat complexity, they were only captured at intermediate or high values of Shannon habitat index.
Our findings about the relationship between species occurrence and landscape complexity allow us to revise our characterization of species in habitat generalist and habitat specialist (Martínez et al. 2014; Gomez et al. 2015). The use of a landscape approach allows us to define three groups of species, cropland specialists (mainly occur in croplands), natural or semi-natural habitat specialists (mainly occur in natural/semi-natural habitats) and habitat generalists (occur in almost all habitats within the agroecosystems). Thus, C. laucha would be cropland specialist; O. flavescens, A. dolores and T. pallidior would be natural and semi-natural habitat specialists and C. musculinus, A. azarae, O. rufus, C. venustus and M. dimidiata would be habitat generalists.
Our results also supported the prediction about the effect of landscape complexity on small mammal species richness. Indeed, species richness increased with the availability of natural and semi-natural habitats typical of complex landscapes. Higher landscape complexity would benefit biodiversity by increasing habitat connectivity, providing shelter from predators and more resources for species persistence (Fischer et al. 2011; Gomez et al. 2015; Monck-Whipp et al. 2018).
Besides, Shannon index also seems to be a better index of landscape complexity than the perimeter/areacrop plot ratio since the latter reflects only the amount of linear habitats in agroecosystems, and complex landscapes are not only constituted by these habitats but by other elements that would favor the whole small mammal assemblage.
Our results suggest that on average, the best spatial scales to study the effects of landscape complexity on small mammal assemblage of agroecosystems of central Argentina would be 150 and 200 m. These scales allow us to elucidate the true relationships between species occurrence and landscape. For example, the scale of effect for C. venustus and A. dolores highlights the importance of finding the correct direction of the effect, i.e., landscape complexity positively affected C. venustus and A. dolores at 150 and 200 m, but negatively at greater spatial scales. It is important to note that due to the hierarchical approach used in our study, the method used to select the most important spatial scale could have some limitations. However, we gave priority to the advantages of obtaining a reliable response of each species to landscape complexity in the context of the assemblage to which it belongs.
Conclusion
As it was previously determined in other agroecosystems (Weibull et al. 2003; Mitchell et al. 2014; Jackson and Fahrig 2015), our work shows that landscape heterogeneity is a key factor to maintain biodiversity and species persistence. A higher level of landscape heterogeneity does not only mean a higher proportion of natural habitats but also man made habitats such as crop plots, and clearly some species benefit from them. The conservation of natural and semi-natural habitats, however, is important because they maximize species occurrence and richness. A relevant finding of our study is that landscape complexity at 200 m (16 ha) spatial scale would benefit most of the species in the assemblage. This result is key to define management strategies for biodiversity conservation in agricultural landscapes of central Argentina. Further studies are now needed to understand which are the most important habitats and, whether border habitats are enough to ensure landscape heterogeneity at a 16 ha spatial scale. This will allow to link our results with future management strategies.
References
Aizen MA, Garibaldi LA, Dondo MD (2009) Expansión de la soja y diversidad de la agricultura argentina. Ecol Austral 19:45–54
Andreo V, Lima MA, Provensal C, Priotto JW, Polop JJ et al (2009) Population dynamics of two rodent species in agro-ecosystems of central Argentina: intra-specific competition, land-use, and climate effects. Popul Ecol 51:297–306
Baldi G, Paruelo JM (2008) Land-use and land cover dynamics in South American temperate grasslands. Ecol Soc 13:6
Bedano JC, Domínguez A (2016) Large-scale agricultural management and soil meso- and macrofauna conservation in the Argentine Pampas. Sustain 8:653
Bentley J, Catterall C, Smith G (2000) Effects on fragmentation of Araucarian vine forest on small mammal communities. Conserv Biol 14:1075–1087
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol Evol 4:182–188
Bilenca DN, González-Fischer CM, Teta P, Zamero M (2007) Agricultural intensification and small mammal assemblages in agroecosystems of the Rolling Pampas, central Argentina. Agric Ecosyst Environ 121:371–375
Borges-Matos C, Aragón S, da Silva MNF, Fortin MJ, Magnusson WE (2016) Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204:417–425
Burkart R, Bárbaro N, Sánchez RO, Gómez DA (1999) Eco-regiones de la Argentina. Administración de Parques Nacionales - Programa de Desarrollo Institucional Ambiental, Buenos Aires
Coda JA, Gomez MD, Martínez JJ, Steinmann AR, Priotto JW (2016) The use of fluctuating asymmetry as a measure of farming practice effects in rodents: a species-specific response. Ecol Indic 70:269–275
Coda JA, Gomez MD, Steinmann AR, Priotto JW (2014) The effects of agricultural management on the reproductive activity of female rodents in Argentina. Basic Appl Ecol 15:407–415
Coda JA, Gomez D, Steinmann AR, Priotto JW (2015) Small mammals in farmlands of Argentina: responses to organic and conventional farming. Agric Ecosyst Environ 211:17–23
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514
Dorazio RM, Royle A, Royle JA (2005) Estimating size and composition of biological communities by modeling the occurrence of species. J Am Stat Assoc 100:389–398
Ellis EC, Ramankutty N (2008) Putting people in the map: anthropogenic biomes of the world. Front Ecol Environ 6:439–447
Fahrig L, Baudry J, Brotons LL, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Filippi-Codaccioni O, Devictor V, Bas Y, Clobert J, Julliard R (2010) Specialist response to proportion of arable land and pesticide input in agricultural landscapes. Biol Conserv 143:883–890
Fischer C, Schröder B (2014) Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric Ecosyst Environ 189:145–153
Fischer C, Thies C, Tscharntke T (2011) Small mammals in agricultural landscapes: opposing responses to farming practices and landscape complexity. Biol Conserv 144:1130–1136
Fraschina J, León VA, Busch M (2012) Long-term variations in rodent abundance in a rural landscape of the Pampas, Argentina. Ecol Res 27:191–202
Gavier-Pizarro GI, Calamari NC, Thompson JJ, Canavelli SB, Solari LM, Decarre J, Goijman AP, Suarez RP, Bernardos JN, Zaccagnini ME (2012) Expansion and intensification of row crop agriculture in the Pampas and Espinal of Argentina can reduce ecosystem service provision by changing avian density. Agric Ecosyst Environ 154:44–55
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472
Gomez MD, Coda JA, Simone I, Martínez JJ, Bonatto F, Steinmann AR, Priotto JW (2015) Agricultural land-use intensity and its effects on small mammals in the central region of Argentina. Mammal Res 60:415–423
Gomez MD, Goijman AP, Coda JA, Serafini VN, Priotto JW (2018) Small mammal responses to farming practices in central Argentinian agroecosystems: The use of hierarchical occupancy models. Austral Ecol 43:828–838
Gomez D, Sommaro L, Steinmann AR, Chiappero M, Priotto JW (2011) Movement distances of two species of sympatric rodents in linear habitats of Central Argentine agro-ecosystems. Mamm Biol 76:58–63
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr 24:52–63
Kellner K (2017) jagsUI: a wrapper around ‘rjags’ to streamline ‘JAGS’ analyses. https://CRAN.R-project.org/package=jagsUI
Kéry M, Royle JA (2016) Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and BUGS. Prelude and static models, vol 1. Elsevier Inc., Amsterdam
Kéry M, Royle JA, Plattner M, Dorazio RM (2009) Species richness and occupancy estimation in communities subject to temporary emigration. Ecology 90(5):1279–1290
King KL, Homyack JA, Wigley TB, Miller DA, Kalcounis-Rueppell MC (2014) Response of rodent community structure and population demographics to intercropping switchgrass within loblolly pine plantations in a forest-dominated landscape. Biomass Bioenerg 69:255–264
Levins R (1968) Evolution in changing environment. Princenton University Press, Princenton
Lindsay KE, Kirk DA, Bergin TM, Louis B, Sifneos JC, Smith J, Sifneos JC (2013) Farmland heterogeneity benefits birds in american mid-west watersheds. Am Midl Nat 170:121–143
Mackenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
Mackenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey L, Hines J (2006) Occupancy estimation and modeling. Inferring patterns and dynamics of species occurrence. Elsevier Inc., Amsterdam
Martin LJ, Quinn JE, Ellis EC, Shaw R, Dorning M, Hallett L, Heller N, Hobbs R, Kraft C, Law E, Michel N, Perrig M, Shirey P, Wiederholt R (2014) Conservation opportunities across the world’s anthromes. Divers Distrib 20:745–755
Martínez JJ, Millien V, Simone I, Priotto JW (2014) Ecological preference between generalist and specialist rodents: spatial and environmental correlates of phenotypic variation. Biol J Linn Soc 112:180–203
Medan D, Torretta JP, Hodara K, Fuente EB, Montaldo NH (2011) Effects of agriculture expansion and intensification on the vertebrate and invertebrate diversity in the Pampas of Argentina. Biodivers Conserv 20:3077–3100
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landscape Ecol 31:1–18
Millan de la Pena N, Butet A, Delettre Y, Morant P, Le Du L, Burel FG (2003) Response of the small mammal community to changes in western French agricultural landscapes. Landscape Ecol 18:265–278
Mitchell MGE, Bennett EM, Gonzalez A (2014) Agricultural landscape structure affects arthropod diversity and arthropod-derived ecosystem services. Agric Ecosyst Environ 192:144–151
Monck-Whipp L, Martin AE, Francis CM, Fahrig L (2018) Farmland heterogeneity benefits bats in agricultural landscapes. Agric Ecosyst Environ 253:131–139
Novotný D, Zapletal M, Kepka P, Beneš J, Konvička M (2015) Large moths captures by a pest monitoring system depend on farmland heterogeneity. J Appl Entomol 139:390–400
Paruelo JJM, Guerschman JJP, Verón SRS (2005) Expansión agrícola y cambios en el uso del suelo. Cienc Hoy 15:14–23
Plummer M, Stukalov A, Denwood M (2016) Package “rags”. https://CRAN.R-project.org/package=rjags
Polop F, Provensal MC, Priotto JW, Steinmann A, Polop J (2012) Differential effects of climate, environment, and land use on two sympatric species of Akodon. Stud Neotrop Fauna Environ 47:147–156
QGIS Development Team (2017) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. Accessed 17 Jul 2017
Quinn JE, Johnson RJ, Brandle JR (2014) Identifying opportunities for conservation embedded in cropland anthromes. Landscape Ecol 29:1811–1819
R Core Team (2018) R: the R project for statistical computing
Royle JA, Dorazio RM (2008) Hierarchical modeling and inference in ecology: the analysis of data from populations. Metapopulations and Communities. Elsevier Inc., Amsterdam
Sanderson EW, Malanding J, Levy MA, Redford KH, Wannebo AV, Woolmer G (2002) The human footprint and the last of the wild. Bioscience 52:891
Simone I, Cagnacci F, Provensal MC, Polop JJ (2010) Environmental determinants of the small mammal assemblage in an agroecosystem of central Argentina: the role of Calomys musculinus. Mamm Biol 75:496–509
Smith AC, Fahrig L, Francis CM (2011) Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography 34:103–113
Sommaro L, Gomez MD, Bonatto F, Steinmann A, Chiappero M, Priotto J (2010) Corn mice (Calomys musculinus) movement in linear habitats of agricultural ecosystems. J Mammal 91:668–673
Stanton RL, Morrissey CA, Clark RG (2018) Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agric Ecosyst Environ 254:244–254
Thies C, Steffan-dewenter I, Tscharntke T (2003) Effects of landscape context on herbivory and parasitism at different spatial scales. Oikos 1:18–25
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol Lett 8:857–874
Tyre AJ, Tenhumberg B, Field SA, Niejalke D, Parris K, Possingham HP (2003) Improving precision and reducing bias in biological surveys: estimating false-negative error rates. Ecol Appl 13:1790–1801
Viglizzo EF, Roberto ZE, Lértora F, López Gay E, Bernardos JN (1997) Climate and land-use change in field-crop ecosystems of Argentina. Agric Ecosyst Environ 66:61–79
Weibull A, Östman Ö, Granqvist A (2003) Species richness in agroecosystems: the effect of landscape, habitat and farm management. Biodivers Conserv 12:1335–1355
Wiens JA (2002) Central concepts and issues of landscape ecology. In: Gutzwiller KJ (ed) Applying landscape ecology in biological conservation. Springer, New York, pp 3–21
Zingg S, Grenz J, Humbert JY (2018) Landscape-scale effects of land unse intensity on birds and butterflies. Agric Ecosyst Environ 267:119–128
Zipkin EF, Dewan A, Andrew Royle J (2009) Impacts of forest fragmentation on species richness: a hierarchical approach to community modelling. J Appl Ecol 46:815–822
Zollner PA (2000) Comparing the landscape level perceptual abilities of forest sciurids in fragmented agricultural landscapes. Landscape Ecol 15:523–533
Acknowledgements
We are thankful to José A. Coda, Facundo Contreras and Javier Escudero for fieldwork assistance and to Andrea Goijman for the help in statistical analyses. We are thankful to Veronica Andreo for help with correcting the English and 2 Reviewers and the Editor for helpful comments on earlier versions.
Data Availability
The datasets generated and/or analyzed during the current study are available in the Open Science Framework (https://osf.io/) repository.
Funding
This work was supported by Consejo Nacional de Investigación Científica y Técnica (CONICET) [PIP CONICET No. 11220150100034] and Universidad Nacional de Río Cuarto, Córdoba, Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Serafini, V.N., Priotto, J.W. & Gomez, M.D. Effects of agroecosystem landscape complexity on small mammals: a multi-species approach at different spatial scales. Landscape Ecol 34, 1117–1129 (2019). https://doi.org/10.1007/s10980-019-00825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00825-8