Abstract
Understanding the role of feedback structure (endogenous processes) and exogenous (climatic and environmental) factors in shaping the dynamics of natural populations is a central challenge within the field of population ecology. We attempted to explain the numerical fluctuations of two sympatric rodent species in agro-ecosystems of central Argentina using Royama’s theoretical framework for analyzing the dynamics of populations influenced by exogenous climatic forces. We found that both rodent species show a first-order negative feedback structure, suggesting that these populations are regulated by intra-specific competition (limited by food, space, or enemy-free space). In Akodon azarae endogenous structure seems to be very strongly influenced by human land-use represented by annual minimum normalized difference vegetation index (NDVI), with spring and summer rainfall having little influence upon carrying capacity. Calomys venustus’ population dynamics, on the other hand, seem to be more affected by local climate, also with spring and summer rainfall influencing the carrying capacity of the environment, but combined with spring mean temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wide spectrum of population dynamics observed in small mammal fluctuations has intrigued ecologists for a long time (Elton 1924). Most studies focusing of rodent dynamics in the Northern Hemisphere have emphasized the role of endogenous processes as the most important factors driving numerical oscillations (Ostfeld et al. 1993; Hörnfeldt 1994; Stenseth et al. 1996). In contrast, most studies of rodent dynamics from the Southern Hemisphere have emphasized the role of exogenous (climate) factors in driving the observed fluctuations (Pearson 1975; Jaksic and Lima 2003). Recently, ecologists have begun to understand how both feedback structures and exogenous factors interact in shaping the dynamics of small mammal populations (Leirs et al. 1997; Lima et al. 1999, 2001, 2002a, b, 2006, 2008, Murúa et al. 2003a, b).
Rodent populations from agro-ecosystems in central Argentina exhibit important inter-annual fluctuations in density (Crespo 1966; Crespo et al. 1970) that seem to be driven by factors such as climate, agricultural practices, and the interaction of these with intra and inter-specific competition of rodents (Kravetz and Polop 1983; Castellarini et al. 2002). For example, Akodon azarae (Fisher 1829) and Calomys venustus (Thomas 1894) are two of the most abundant small rodents inhabiting the agro-ecosystems of the Pampean region in Argentina. They are usually found in relatively stable habitats with high vegetation cover including crop field edges, roadsides, and railway banks (borders), and remnant areas of native vegetation (Mills et al. 1991; Priotto and Polop 1997). Although seasonal changes in Argentinean rodent species are quite well studied, there have been no previous attempts to study long-term inter-annual population changes and to deduce the relative importance of endogenous and exogenous processes in shaping the dynamics of small rodents in agro-ecosystems. Therefore the objective of this study was to analyze the effects of endogenous feedback structure and climatic factors on the dynamics of A. azarae and C. venustus in agro-ecosystems of central Argentina, using simple and appropriate population dynamics models.
Materials and methods
Study site
The study was carried out in the rural area of Chucul (Fig. 1), south-west of Córdoba, Argentina (33°01′34′′S; 64°11′21′′W). The area is a typical undulating pampean plain (600–900 m a.s.l.). The climate of the region is temperate with an average annual temperature of 23°C in January and 6°C in July. Annual rainfall is high, especially in summer, averaging 800 mm. The natural transitional landscape of woodland (dominated by Prosopis alba, P. nigra, P. caldenia, Celtis tala, Acacia caven and Geoffroea decorticans) and pampean natural grassland (Stipa spp.) remains in patches between crop fields. The vegetation has undergone marked alterations as a result of agriculture and cattle farming. Currently, the landscape mainly consists of individual crop fields surrounded by wire fences with borders dominated by weed species. The study was conducted on a railway bank with an average width of 50 m. The low train frequencies (<10 per year) allow the development of natural pastures and weeds interspersed with bushes. Despite the influence of nearby crop fields, the vegetation bears some resemblance to native vegetation. In this barely disturbed habitat rodent species such as A. azarae and C. venustus reach high population densities.
Small mammal data
We used mark–recapture data from small-mammal live-trapping conducted on four consecutive nights each month from January 1990 to June 2007 in a 6 × 10 grid (0.30 ha). Stations in the grid were separated by 10 m. One Sherman-type live trap baited with a mixture of peanut butter and cow fat was placed in each station. Small mammals captured were marked with a distinct numerical code, consisting of little cuts of the ears, and standard data on species, sex, reproductive condition, length, and weight were also recorded. Population abundance was estimated from the minimum number of animals known to be alive (MNKA). In this study we used peak annual MNKA obtained after the reproductive season of each year.
Mouse life history
Both species are omnivorous, but A. azarae (25–30 g) consumes higher proportions of arthropods whereas C. venustus (55 g) eats higher proportions of leaves and seeds (Bilenca et al. 1992; Castellarini et al. 1998). Populations of both species show a strong seasonal variation in abundance, with a minimum in spring and peaks in autumn (C. venustus) or late autumn–early winter (A. azarae) followed by a dramatic fall (Mills et al. 1991; Castellarini and Polop 2002). Reproduction is also seasonal; the breeding season may last from September–October to April–June (Mills et al. 1992; Polop et al. 2005).
Climatic and NDVI data
Data series of monthly temperature (minimum, median, and maximum) and rainfall were provided by the agro-meteorological laboratory from Río Cuarto National University (Argentina). We used seasonal estimates of these variables for the analyses performed in this study.
Normalized difference vegetation index (NDVI) is a measure of the presence and condition of green vegetation and is calculated as a normalized ratio of the red and near infrared bands (Lillesand and Kiefer 1994). NDVI values range from −1 to 1, with bare soil having values near 0, and high values indicating increasing green biomass and photosynthetic activity. NDVI data series for this study was obtained from the global inventory modeling and mapping studies (GIMMS) AVHRR 8 km, bimonthly (1981–2006) data set, available at http://glcf.umiacs.umd.edu/data/gimms/ (Tucker et al. 2005).
Diagnosis
Population dynamics are the result of the combined effects of feedback structure (ecological interactions within and between populations), limiting factors (food limitation by plants, or predator limitation through competition for enemy-free space), climatic influences (rainfall), and stochastic forces. To understand how these factors may determine rodent population fluctuations, we modeled both system-intrinsic processes (both within the population and between various trophic levels) and exogenous influences as a general model based in the R-function (Berryman 1999). The R-function represents the realized per-capita population growth rates that reflect the processes of individual survival and reproduction (Berryman 1999). Defining R t = log (N t ) − log (N t−1), we can express the R-function as (sensu Berryman 1999):
This model represents the basic feedback structure and integrates the stochastic and climatic forces that drive population dynamics in nature. Our first step was to estimate the order of the dynamic process (Royama 1977), i.e., how many time lags (N t−i ) should be included in the model for representation of the feedback structure. To estimate the order of the process we used the partial rate correlation (PRCF i ) between R t and ln (N t−i) = X t−i , after the effects of shorter lags were removed. We wrote Eq. 1 in logarithms to calculate the partial correlations.
where R t , the realized per-capita rate of change, is calculated from the data. We used the Population Analysis System, Single-Species Time-Series Analysis, to calculate PRCF t−d (PAS can be downloaded from http://classes.entom.wsu.edu/pas/). For statistical convenience we assumed a log–linear relationship between R t and lagged population density (Royama 1977).
Models of population dynamics
We used the non-linear logistic population model of discrete time proposed by Royama (1992), derived from the logistic equation of Ricker (1954). This model represents a randomly distributed population competing for a common resource which is consistent with scramble competition (Johst et al. 2008).
where R t is the realized logarithmic per-capita population growth rate, b is a positive constant representing the maximum finite reproductive rate, Xt−1 is the logarithmic population density in t−1, C is a constant representing competition and resource depletion, and a indicates the effect of interference on each individual as density increases (Royama 1992); a > 1 indicates that interference intensifies with density and a < 1 indicates habituation to interference. Therefore, density in each time period is obtained:
To model the effects of climate on the endogenous feedback structure of rodent populations we added extra terms to Eq. 3 representing vertical and/or lateral effects. Thus, the equation becomes:
where, d and e are the coefficients that account for lateral and vertical effects of climate, respectively.
We fitted Eq. 5 using the nls library in the program R by means of non-linear regression analyses. After models were fitted to the data, we calculated the Akaike information criterion corrected for small-sample bias (AIC c ), differences in AIC c (Δ i ), likelihood, Akaike weights (w i ), evidence ratios (w i /w j ), and log (likelihood) for each model (Burnham and Anderson 2004). Models with the lowest AIC c values were selected to draw inferences and run deterministic predictions. We used total trajectory and one-step-ahead deterministic predictions to simulate the dynamic behavior of the fitted models and correlations between observed and predicted values of population abundance to assess the performance of each of them.
Results
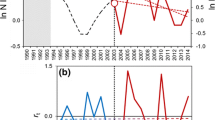
Population dynamics of the two rodent species studied here showed irregular numerical fluctuations (Fig. 2a, b), with years of high abundance and a sudden decrease during the period 1992–1997 for A. azarae (Fig. 2b). The maximum abundance of A. azarae was observed in 1991 and that of C. venustus (Fig. 2a) in 2004. In addition, the PRCF strongly suggested first-order dynamics for both species (Fig. 2c, d), which was also evident from the observed R-functions (Fig. 2e, f). For C. venustus, however, a second lag also seemed to contribute in some measure to the determination of R (Fig. 2c). The time series plot of A. azarae (Fig. 2b) seems to exhibit a discontinuity (a shift in dynamics) between 1992 and 1997, which is even more evident from R-function (Fig. 2f). This plot seems to show two R-functions, one at high population abundance and the other at low population abundance, suggesting a strong vertical perturbation effect.
Peak annual abundance (MNKA), partial rate correlation function (PRCF), and relationship between the logarithmic annual per-capita rate of change (R t = ln N t − ln N t−1) and population abundance (N t−1) expressed as MNKA (R-function) for C. venustus ( a, c, e) and A. azarae (b, d, f) during 1990–2007. Grey points are those we removed from the A. azarae R-function to fit models. Curves are the non-linear Ricker logistic model (equivalent to Eq. 3) fitted to the data (continuous line) using the complete series (dashed lines) removing grey points from the A. azarae series
Population dynamics models fitted for C. venustus and A. azarae, values of estimated parameters, coefficient of determination (R 2), AIC c , ΔAIC c , likelihood, Akaike weights (w i ), evidence ratios (w i /w j ) and log (likelihood) for each model are shown in Tables 1 and 2, respectively. The maximum finite reproductive rate was fixed at 1.7 (the maximum observed R in the series) for C. venustus in order to obtain convergence of the models, but this was not necessary for A. azarae.
Our analyses indicated that population abundance of the previous year explained 49 and 47% of the variation in per-capita growth rates of C. venustus (Table 1) and A. azarae (Table 2), respectively. All models show a nonlinear (a ≠ 1) negative first-order feedback structure (i.e., R is a nonlinear decreasing function of population abundance in a previous time), but in C. venustus, a < 1 and in A. azarae, a > 1. Exogenous effects improved the explained variance of the first-order feedback models by an average of 10% for both species, ranging from 1 to 22% and from 0 to 31% for C. venustus and A. azarae, respectively (Tables 1 and 2; Tables A1 and A2 in the electronic supplementary material, ESM). If we remove the points (corresponding to the abundances of 1992, 1993, 1995, and 1996) from the R-function (see gray dots in Fig. 2f), model fits improved substantially. The dynamics of A. azarae seemed to be mainly affected by its abundance (model A.2, R 2 = 0.87, ΔAIC c = 0; Table 2), although spring rainfall (acting both in an additive and/or non-additive manner) and summer and spring rainfall together also seemed to affect A. azarae’s population dynamics (ΔAIC c < 2, Table 2; Table A3 in ESM).
The best model for C. venustus, according to AIC c values, is the one including spring mean temperature as an additive positive effect (model A). However, we could not discriminate it from the next four models (ΔAIC c ≤ 2, Table 1) which indicated that summer rainfall alone (model C) and summer and spring rainfall together also seemed to be positively involved in the dynamics of C. venustus. On the other hand, model B, which explained 71% of the variability in R, combines a lateral effect of summer rainfall with a vertical effect of spring mean temperature. However, simulations of model E, which included lateral effects of summer and spring rainfall and spring mean temperature seemed to better predict the observed fluctuations of the C. venustus population than the simulations of model B (correlations between observed and predicted values of density were 0.70 and 0.66 for total trajectory predictions and 0.56 and 0.62 for one-step-ahead predictions for model E and B, respectively, Fig. 3a, b).
Comparisons between observed rodent abundances (points) and deterministic predictions (lines) for the best models for C. venustus and A. azarae. Runs began with an initial population abundance (MNKA) of 14 individuals for C. venustus and 29 for A. azarae (continuous line). Total trajectory and (dashed lines) one-step-ahead prediction. a Model B and c Model E for C. venustus. b Model A.1 and d Model B.1 for A. azarae
Dynamics of A. azarae seemed to be mainly affected by its abundance and the vertical effects of annual minimum NDVI (model A.1, R 2 = 0.76, ΔAIC c = 0), although summer and spring rainfall together also seemed to have an effect in A. azarae’s population dynamics (model B.1, ΔAIC c > 2, Table 2). When simulating the dynamics of model A.1 and B.1, both of them seem to capture the structure of the observed dynamics effectively (correlations between observed and predicted values of density were: 0.73 and 0.76 for total trajectory predictions, and 0.80 and 0.82 for one-step-ahead predictions for models A.1 and B.1, respectively (Fig. 3c, d). These models including the NDVI were able to simulate the collapse observed during the period 1992–1997.
Discussion
The population dynamics of both rodent species studied here showed irregular numerical fluctuations which seem to be described by a negative first-order feedback structure, suggesting that both species are regulated by intra-specific competition. However, endogenous structure seems to be stronger in A. azarae than in C. venustus population dynamics. Mechanisms such as territoriality, aggressive behavior, social and spatial organization observed in some small mammal species, may regulate population dynamics operating as density-dependent factors on maturation and recruitment (Stenseth et al. 1996; Prévot-Julliard et al. 1999; Lima et al. 2001, 2002b). In C. venustus, climate effects seem to be related to requirements for reproduction in the breeding season (rain and warm temperatures), probably reflecting vegetation growth and ripening, which offer cover and food. Meanwhile, in A. azarae climate seems to play a secondary role and the main exogenous perturbation seems to be driven by human land-use, as apparent from the vertical effect of annual minimum NDVI.
Regarding the collapse in A. azarae’s fluctuations, we detected some important environmental changes (from 1992 to 1996), mainly related to reductions in plant cover, that may have affected A. azarae’s population dynamics but not C. venustus’s (or at least not in a noticeable manner in the R-function). For example, we recorded that both in 1995 and in 1996 the area where the grid was placed or its surroundings were burnt. On the other hand, we detected a regional significant increase in peanut-sown area (Secretaría de Agricultura, Ganadería y Alimentación, Córdoba government: http://www.cba.gov.ar). This regional change in cover was also observed in NDVI minimum values which showed a considerable decrease during the period 1992–1996 (Fig. 4). In fact, several studies have indicated that A. azarae shows a strong habitat selection for highly covered and less disturbed habitats such as crop field edges and roadsides (Hodara et al. 2000a; Busch et al. 2001). This marked preference is also evident in microhabitat use, both within borders and in crop fields (Bilenca and Kravetz 1998). J. Priotto (personal communication) has, moreover, observed that captures of A. azarae decrease considerably in burnt areas, whereas Calomys species are captured normally. Furthermore, considering that A. azarae and C. venustus have distinct patterns of activity and diet, burning may have different effects on these species, despite their inhabiting the same place (Priotto and Polop 1997; Castellarini and Polop 2002). On the other hand, the regional increase in peanut-sown area and the subsequent reduction in NDVI (mainly in autumn and winter), may have had a substantial effect on A. azarae, because when this crop is harvested (autumn) soil is left almost completely bare and A. azarae is known to use crop fields during the breeding season (Bilenca and Kravetz 1998).
A first-order negative feedback structure seemed to capture the essential features of A. azarae dynamics, suggesting intra-specific competition as the main regulating mechanism. This is consistent with many studies that point out the importance of spacing behavior and intra-specific competition in this rodent. For example, some authors have found that during the breeding season, females of A. azarae are strongly territorial and aggressive (Bonaventura et al. 1992; Hodara et al. 2000a; Suárez and Kravetz 2001). Although it seems likely that intra-specific competition may be the regulating factor in A. azarae’s population dynamics, it is not clear, however, which is the limiting resource. This rodent species seems to respond positively to both food (Cittadino et al. 1994) and shelter addition (Hodara et al. 2000b). In particular, plant cover seems to be a very important resource for A. azarae (Ellis et al. 1997; Hodara et al. 2000a; Busch et al. 2001), mainly for sexually active females, which select microhabitats with a greater amount of green cover and higher densities of insects (Bilenca et al. 1992; Bilenca and Kravetz 1998). Cover also seems to be an important resource for A. azarae during winter (non-breeding season), when individuals of both sexes select highly covered areas in borders (Bilenca and Kravetz 1998). Thus, it seems likely that A. azarae is limited by areas with high plant cover, which provides refuge from predators and high-quality food (mainly insects).
Population fluctuations of C. venustus seemed to be the result of the endogenous feedback structure combined with lateral positive effects of spring and summer rainfall and spring mean temperature. Females of this rodent species show strong territorial behavior (Priotto et al. 2002), which become more pronounced during the breeding season (Priotto and Polop 2003). This is consistent with the lateral effects of the spring–summer rainfall and spring temperature observed in this species. Local weather (rainfall and temperature) seems to be a proxy for the limiting resources of C. venustus, probably reflecting an increase in food availability. In fact, C. venustus is mainly a folivorous–granivorous small rodent (Castellarini et al. 1998), with higher consumption of leaves in spring and grain in summer. Recent studies of small rodents have showed that the effect of rainfall (or temperature) on primary productivity can be easily incorporated as a lateral perturbation effect (Royama 1992) using simple population models (Lima et al. 2006, 2008). The same approach has been used to predict the dynamics of large mammal populations influenced by climatic variability (Berryman and Lima 2006; Lima and Berryman 2006).
In conclusion, the numerical fluctuations of the two rodent species studied here show first-order feedback structures suggesting that both populations are regulated by intra-specific competition (Johst et al. 2008). However, for the insectivorous rodent A. azarae, plant cover and human-induced changes in land-use seem to represent the main exogenous perturbations. On the other hand, the herbivorous rodent C. venustus seems to be more affected by rainfall and temperature, probably because of their effects on primary productivity. Our results show that simple, theory-based models, for example those provided by Royama (1992), are also useful in explaining and predicting the dynamics of populations inhabiting highly variable environments such as agro-ecosystems of central Argentina.
References
Berryman AA (1999) Principles of population dynamics and their application. Stanley Thornes, Cheltenham
Berryman AA, Lima M (2006) Deciphering the effects of climate on animal populations: diagnostic analysis provides new interpretation of Soay sheep dynamics. Am Nat 168:784–795. doi:10.1086/508670
Bilenca DN, Kravetz FO (1998) Seasonal variation in microhabitat use and feeding habits of the pampas mouse Akodon azarae in agroecosystems of central Argentina. Acta Theriol (Warsz) 43:195–203
Bilenca DN, Kravetz FO, Zuleta GA (1992) Food habits of Akodon azarae and Calomys laucha (Cricetidae, Rodentia) in agroecosystems of central Argentina. Mammalia 56:371–383
Bonaventura SM, Kravetz FO, Suárez OV (1992) The relationship between food availability, space and territoriality in Akodon azarae (Rodentia, Cricetidae). Mammalia 56:407–416
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. SMR 33:261–304
Busch M, Miño MH, Dadon JR, Hodara K (2001) Habitat selection by Akodon azarae and Calomys laucha (Rodentia, Muridae) in pampean agroecosystems. Mammalia 65:29–48
Castellarini F, Polop J (2002) Effects of extra food on population fluctuation pattern of the muroid rodent Calomys venustus. Aust Ecol 27:273–283. doi:10.1046/j.1442-9993.2002.01178.x
Castellarini F, Agnelli HL, Polop JJ (1998) Study on the diet and feeding preferences of Calomys venustus (Rodentia, Muridae). Mastozool Neotrop 5:5–11
Castellarini F, Provensal C, Polop J (2002) Effect of weather variables on the population fluctuation of muroid Calomys venustus in central Argentina. Acta Oecol 23:385–391. doi:10.1016/S1146-609X(02)01171-2
Cittadino EA, De Carli P, Busch M, Kravetz FO (1994) Effects of food supplementation on rodents in winter. J Mammal 75:446–453. doi:10.2307/1382566
Crespo JA (1966) Ecología de una comunidad de roedores silvestres en el Partido de Rojas, Provincia de Buenos Aires. Rev Mus Arg Cs Nat Ecol 1:73–134
Crespo J, Sabattini M, Piantanida J, Villafañe G (1970) Estudios ecológicos sobre roedores silvestres: Observaciones sobre densidad, reproducción y estructura de comunidades silvestres del sur de Córdoba. Monografía de la Secretaría de Estado de Salud Pública. Ministerio de Bienestar Social. Buenos Aires. Argentina pp 1–45
Ellis BA, Mills JN, Childs JE, Muzzini MC, Mckee KT Jr, Enria DA, Glass GE (1997) Structure and floristics of habitats associated with five rodents species in an agroecosystem in Central Argentina. J Zool (Lond) 243:437–460
Elton C (1924) Fluctuations in the numbers of animals. Br J Exp Biol 2:119–163
Hodara K, Busch M, Kittlein M, Kravetz FO (2000a) Density-dependent habitat selection between maize cropfields and their borders in two rodent species (Akodon azarae and Calomys laucha) of Pampean agroecosystems. Evol Ecol 14:571–593. doi:10.1023/A:1010823128530
Hodara K, Busch M, Kravetz FO (2000b) Effects of shelter addition on Akodon azarae and Calomys laucha (Rodentia, Muridae) in agroecosystems of central Argentina during winter. Mammalia 64:295–306
Hörnfeldt B (1994) Delayed density dependence as a determinant of vole cycles. Ecology 75:791–806. doi:10.2307/1941735
Jaksic FM, Lima M (2003) Myths and facts on ratadas: Bamboo blooms, rainfall peaks and rodent outbreaks in South America. Aust Ecol 28:237–251. doi:10.1046/j.1442-9993.2003.01271.x
Johst K, Berryman AA, Lima M (2008) From individual interactions to population dynamics: individual resource partitioning simulation exposes the causes of nonlinear intra-specific competition. Popul Ecol 50:79–90. doi:10.1007/s10144-007-0061-5
Kravetz FO, Polop JJ (1983) Comunidades de roedores en agroecosistemas del Departamento de Río Cuarto. Ecosur Argent 10:1–18
Leirs H, Stenseth NC, Nichols JD, Hines JE, Verhagen R, Verheyen W (1997) Stochastic seasonality and nonlinear density-dependence factors regulate population size in an African rodent. Nature 389:176–180. doi:10.1038/38271
Lillesand TM, Kiefer RW (1994) Remote sensing and image interpretation, 3rd edn. Wiley, New York
Lima M, Berryman AA (2006) Predicting nonlinear and non-additive effects of climate: the Alpine ibex revisited. Clim Res 32:129–135. doi:10.3354/cr032129
Lima M, Keymer JE, Jaksic FM (1999) El Niño-Southern Oscillation-Driven rainfall variability and delayed density dependence cause rodent outbreaks in western South America: linking demography and population dynamics. Am Nat 153:476–491. doi:10.1086/303191
Lima M, Julliard R, Stenseth NC, Jaksic FM (2001) Demographic dynamics of a neotropical small rodent (Phyllotis darwini): feedback structure, predation and climatic factors. J Anim Ecol 70:761–775. doi:10.1046/j.0021-8790.2001.00536.x
Lima M, Stenseth NC, Jaksic FM (2002a) Population dynamics of a South American rodent: Seasonal structure interacting with climate, density dependence and predator effects. Proc R Soc Lond B Biol Sci 269:2579–2586. doi:10.1098/rspb.2002.2142
Lima M, Merrit JF, Bozinovic F (2002b) Numerical fluctuations in the northern short-tailed shrew: evidence of non-linear feedback signatures on population dynamics and demography. J Anim Ecol 71:159–172. doi:10.1046/j.1365-2656.2002.00597.x
Lima M, Previtali MA, Meserve PL (2006) Climate and small rodent dynamics in semi-arid Chile: the role of lateral and vertical perturbations and intra-specific processes. Clim Res 30:125–132. doi:10.3354/cr030125
Lima M, Morgan Ernest SK, Brown JH, Belgrano A, Stenseth NC (2008) Chihuahuan desert kangaroo rats: nonlinear effects of population dynamics, competition, and rainfall. Ecology 89:2594–2603. doi:10.1890/07-1246.1
Mills JN, Ellis BA, Mckee KT, Maiztegui JI, Childs JE (1991) Habitat associations and relative densities of rodent populations in cultivated areas of central Argentina. J Mammal 72:470–479. doi:10.2307/1382129
Mills JN, Ellis BA, Mckee KT, Maiztegui JI, Childs JE (1992) Reproductive characteristics of rodent assemblages in cultivated regions of central Argentina. J Mammal 73:515–526. doi:10.2307/1382017
Murúa R, González LA, Lima M (2003a) Population dynamics of rice rats (a Hantavirus reservoir) in southern Chile: feedback structure and non-linear effects of climatic oscillations. Oikos 102:137–145. doi:10.1034/j.1600-0706.2003.12226.x
Murúa R, González LA, Lima M (2003b) Second-order feedback and climatic effects determine the dynamics of a small rodent population in a temperate forest of South America. Popul Ecol 45:19–24
Ostfeld RS, Canham CD, Pugh SR (1993) Intrinsic density-dependent regulation of vole populations. Nature 366:259–261. doi:10.1038/366259a0
Pearson OP (1975) An outbreak of mice in the coastal desert of Perú. Mammalia 39:375–386
Polop JJ, Provensal MC, Dauría P (2005) Reproductive characteristics of free-living Calomys venustus (Rodentia, Muridae). Acta Theriol (Warsz) 50:357–366
Prévot-Julliard A-C, Henttonen H, Yoccoz NG, Stenseth NC (1999) Delayed maturation in female bank voles: optimal decision or social constraint? J Anim Ecol 68:684–697. doi:10.1046/j.1365-2656.1999.00307.x
Priotto JW, Polop J (1997) Space and time use in syntopic populations of Akodon azarae and Calomys venustus (Rodentia, Muridae). Z Saugetierkunde 62:30–36
Priotto J, Polop J (2003) Effect of overwintering adults on juvenile survival of Calomys venustus (Muridae: Sigmodontinae). Aust Ecol 28:281–286. doi:10.1046/j.1442-9993.2003.01288.x
Priotto J, Steinmann A, Polop J (2002) Factors affecting home range size and overlap in Calomys venustus (Muridae: Sigmodontinae) in Argentine agroecosystems. Mamm Biol 67:97–104. doi:10.1078/1616-5047-00014
Ricker WE (1954) Stock and recruitment. J Fish Res Board Can 5:559–623
Royama T (1977) Population persistence and density dependence. Ecol Monogr 47:1–35. doi:10.2307/1942222
Royama T (1992) Analytical population dynamics. Chapman and Hall, London
Stenseth NC, Bjørnstad ON, Falck W (1996) Is spacing behaviour coupled with predator causing the microtine density cycle? A synthesis of current process-oriented and pattern-oriented studies. Proc R Soc Lond B Biol Sci 263:1423–1435. doi:10.1098/rspb.1996.0208
Suárez OV, Kravetz FO (2001) Male-female interaction during breeding and non-breeding seasons in Akodon azarae (Rodentia, Muridae). Iheringia. Ser Zool 91:171–176
Tucker CJ, Pinzon JE, Brown ME, Slayback DA, Pak EW, Mahoney R, Vermote EF, El Saleous N (2005) An extended AVHRR 8-km NDVI dataset compatible with MODIS and SPOT vegetation NDVI data. Int J Remote Sens 26:4485–4498. doi:10.1080/01431160500168686
Acknowledgments
We thank Marcos P. Torres for collaboration in the field work. This research was made possible by grants from the Secretaría de Ciencia y Técnica (SECYT), Universidad Nacional de Río Cuarto, Fondo para la Investigación Científica y Tecnológica (FONCYT) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). This article was written as result of an internship of V.A. funded by FONDECYT-FONDAP 15001 (Program 2) at the Center of Advanced Studies in Biology and Ecology (CASEB), Pontificia Universidad Católica de Chile, Santiago, Chile. V.A. thanks the host institution for good working facilities and financial assistance. The research on live animals was performed in a humane manner and was approved by national and international norms (http://www.sarem.org.ar).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andreo, V., Lima, M., Provensal, C. et al. Population dynamics of two rodent species in agro-ecosystems of central Argentina: intra-specific competition, land-use, and climate effects. Popul Ecol 51, 297–306 (2009). https://doi.org/10.1007/s10144-008-0123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-008-0123-3