Abstract
Laboratory rats are sedentary if housed in conditions where activity is limited. Changes in muscle characteristics with chronic inactivity were investigated by comparing sedentary rats with rats undertaking voluntary wheel running for either 6 or 12 weeks. EDL (type II fibers) and soleus (SOL) muscles (predominantly type I fibers) were examined. When measured within 1–2 h post-running, calcium sensitivity of the contractile apparatus was increased, but only in type II fibers. This increase disappeared when fibers were treated with DTT, indicative of oxidative regulation of the contractile apparatus, and was absent in fibers from rats that had ceased running 24 h prior to experiments. Specific force production was ~ 10 to 25% lower in muscle fibers of sedentary compared to active rats, and excitability of skinned fibers was decreased. Muscle glycogen content was ~ 30% lower and glycogen synthase content ~ 50% higher in SOL of sedentary rats, and in EDL glycogenin was 30% lower. Na+, K+-ATPase α1 subunit density was ~ 20% lower in both EDL and SOL in sedentary rats, and GAPDH content in SOL ~ 35% higher. There were no changes in content of the calcium handling proteins calsequestrin and SERCA, but the content of CSQ-like protein was increased in active rats (by ~ 20% in EDL and 60% in SOL). These findings show that voluntary exercise elicits an acute oxidation-induced increase in Ca2+ sensitivity in type II fibers, and also that there are substantial changes in skeletal muscle characteristics and biochemical processes in sedentary rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The beneficial effects of exercise training on skeletal muscle function are clearly established and well recognised, but the adverse effects of sedentary behaviour are less clear cut and are not necessarily simply the converse of undertaking specific exercise training. Most research on the effects of inactivity on muscle function has focussed on the results of models involving almost complete muscle disuse, such as with hind-limb suspension, immobilisation or spinal cord transection in rat and mouse (Steffen and Musacchia 1984; Fitts et al. 1986; Desplanches et al. 1987; Thomason et al. 1987; Talmadge et al. 2002; Kim and Thompson 2013), or bedrest, unilateral limb suspension or cast immobilisation in humans (Larsson et al. 1996; Widrick et al. 2002; Adams et al. 2003; Trappe et al. 2004; Salanova et al. 2013; Brocca et al. 2015; Lamboley et al. 2016; Perry et al. 2016). However, the changes in muscle function occurring with such profound inactivity are likely greater and possibly different from those arising simply from sedentary behaviour.
The laboratory rat is a widely-used model for examining physiological function and biochemical processes in skeletal muscle. However, such rats are typically confined in small cages and display quite sedentary behaviour, and it is unknown whether the lack of normal activity adversely affects or otherwise modifies their skeletal muscle properties. This study compared the skeletal muscle properties of rats confined to normal cages with those of rats undertaking voluntary wheel running of 1–4 km per night for 6 or 12 weeks. Previous studies examining the effects of wheel running exercise on skeletal muscle function have examined changes in fiber cross-sectional area, fiber type and membrane conductance but otherwise have been focussed primarily on changes at the whole muscle level (e.g. muscle mass and force) (Ishihara et al. 1998; Kariya et al. 2004; Gallo et al. 2006; Broch-Lips et al. 2011), and also on particular biochemical aspects, such as citrate synthase activity and GLUT4 levels (Rodnick et al. 1992; Henriksen and Halseth 1995; Kriketos et al. 1995; Rockl et al. 2007).
The current study used mechanically skinned muscle fibers to examine differences between sedentary and active rats in physiological performance at the single muscle fiber level, including calcium sensitivity of the contractile apparatus, maximum Ca2+-activated specific force, and depolarisation-induced force responses. In addition, whole muscle homogenates, containing the total cellular constituents, were examined to determine a large range of parameters, including myosin heavy chain composition, glycogen level, and the relative contents of a range of key proteins involved in calcium handling, glycogen formation and breakdown, aerobic and glycolytic pathways, and sodium–potassium pumping. Together these findings identify a number of important acute and chronic alterations occurring in skeletal muscle fibers of active versus sedentary rats, highlighting the importance of adequate activity for normal muscle composition and function.
Materials and methods
General chemicals and solutions
All chemicals were from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Two heavily Ca2+-buffered solutions were used for examining the properties of the contractile apparatus; ‘relaxing solution’, containing (in mM): 126 K+, 36 Na+, 1 free Mg2+ (10.3 total Mg2+), 90 HEPES, 50 EGTA, 8 ATP, 10 creatine phosphate, pH 7.10, pCa (= − log10[Ca2+]) > 9, and an osmolality of 295 ± 10 mosmol/kg H2O, and ‘maximal Ca2+-activating solution’ which was very similar but with 50 mM CaEGTA (at pCa ~ 4.7) instead of EGTA, and with 8.1 total Mg2+ to keep the free [Mg2+] at 1 mM. For assessing fiber type, a strontium-based solution at pSr 5.2 was made similarly to ‘maximal Ca2+-activating solution’ but with Sr2+ instead of Ca2+ and mixing this with ‘relaxing solution’ a ratio of 1:7. K-HDTA solution was used to examine excitation–contraction coupling and SR properties; it was similar to relaxing solution but with HDTA replacing EGTA, and contained (in mM): 126 K+, 36 Na+, 1 free Mg2+ (8.5 total Mg2+), 90 HEPES, 50 HDTA, 0.05 EGTA, 8 ATP, 10 creatine phosphate, pH 7.10, with the free [Ca2+] weakly buffered at pCa ~ 7.1, and an osmolality of 295 ± 10 mosmol/kg H2O. Additionally, a Na-HDTA solution was made similarly to the K-HDTA solution by substituting all K+ with Na+, which was achieved by adjusting pH with NaOH instead of KOH. Na-HDTA solution was used to depolarize the transverse-tubules in skinned muscle fibers.
Animals and the voluntary running protocol
Three groups of male Sprague–Dawley rats (nine in each, n = 4 SED and n = 5 RUN per group, see descriptions below, animals obtained from Monash University Animal Services, Clayton, Victoria) were used in this study, with the rats in each group being individually housed either in standard rat cages (sedentary, SED) or in cages with free access to a running wheel (Rebbeck et al. 2014) (Lafayette Instrument, Model 80859L, Lafayette, IN, USA), the latter resulting in rats voluntarily running 1–4 km per 24 h (running was observed to take place only at night). All rats were obtained at 12 weeks of age, and sacrificed by overdose of isoflurane at the same final age (24 week-old), with approval from the La Trobe University Ethics Committee. The five RUN rats in Group 1 performed voluntary wheel running for 6 weeks (from 18 to 24 weeks of age), whereas the five RUN rats in each of Groups 2 and 3 did wheel running for 12 weeks (from 12 to 24 weeks of age). The RUN rats in Groups 1 and 2 were sacrificed within 1–2 h after their (nightly) running, whereas the RUN rats in Group 3 were ‘rested’ for 1 day after the 12 weeks running by transferring them back to a standard rat cage for 24 h before sacrifice. The four randomly selected litter mates of each RUN group were kept in standard cages for the entire period (SED rats).
Physiological measurements were made in skinned muscle fibers from the extensor digitorum longus (EDL) muscles of all three groups, but from the soleus (SOL) muscles only of Groups 1 and 2. Biochemical parameters were measured in muscles of all three groups; for most parameters the findings were very similar for the muscles of both 12 week running groups (Groups 2 and 3, RUN and RUN-REST), and these are shown as pooled data in Table 1.
Single muscle fiber dissection and sample preparation
After rats were sacrificed, both EDL muscles and both SOL muscles were dissected from each animal and weighed, one being used for homogenate preparation and the other for obtaining single fiber segments. Muscle homogenates were prepared in relaxing solution to give a concentration of 50 µg wet weight tissue per µl solution. Where required, 0.14 µg/µl amylase was added to degrade glycogen granules. In both cases homogenate samples were further diluted with relaxing solution to ~ 2.5 µg/µl wet weight muscle and then 3 × SDS loading buffer was added (1:2 vol/vol) for western blotting, as described before (Xu et al. 2015). To obtain single muscle fiber segments, EDL or SOL muscles were pinned at resting length in room temperature paraffin oil on a Sylgaard layer and kept cool on an ice pack (~ 10 °C). Single muscle fiber segments were dissected under a light microscope and the sarcolemma removed by mechanically skinning the fiber segment using fine forceps, as described previously (Murphy et al. 2009b). The fiber type of each skinned fiber was ascertained by exposing it to the solution at pSr 5.2; rat type II fibers produce very little force at pSr 5.2, whereas type I fibers produce > 80% of the maximum force (Bortolotto et al. 2000; Murphy et al. 2009a).
Measurement of contractile apparatus parameters
Fiber cross-sectional area (in mm2) was calculated by measuring the fiber diameter in three different places along the length of the fiber, as described previously (Xu et al. 2017), and specific force (in mN/mm2) calculated by normalising the maximum Ca2+-activated force of the fiber by its cross-sectional area. The fiber segment was mounted onto a force transducer (AME801, SensoNor, Horten, Norway) at its resting length (i.e. length just less than that eliciting measurable passive force), and the sarcomere length measured by laser diffraction in a subset of the fibers as described previously (Stephenson and Williams 1981). The resting sarcomere length was the same in SED and RUN rats for both type I and type II fibers (all ~ 2.57 ± 0.05, n = 20–21 for each group).
The fiber was then stretched to 120% of its resting length and transferred into a Perspex bath containing 2 ml of relaxing solution for 2 min, and then activated in a sequence of solutions with progressively higher levels of free [Ca2+] (pCa ~ 9 to 4.7), with maximum force produced at pCa 4.7 (Trinh and Lamb 2006). Force was recorded with a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, Australia). The fiber was then moved back into relaxing solution where it fully relaxed again. Such force–pCa staircases were repeated for 2–3 times for each fiber. The fiber was then treated with 10 mM DTT (in relaxing solution) for 10 min, and the calcium sensitivity (force–pCa curve) tested again with two repetitions of the force–pCa staircase, and this was then repeated following a second DTT treatment. The force at each [Ca2+] within a given sequence was expressed as a percentage of the corresponding maximum force, and the data fitted with a Hill curve, using GraphPad Prism 6 software, to ascertain values of pCa50 (pCa at half of the maximum force production) and the Hill coefficient (h). As reported previously (Posterino and Lamb 2003; Mollica et al. 2012), there was a small reduction in maximum force and in Ca2+-sensitivity with each repetition of the force–pCa staircase. The magnitude of this small progressive was ascertained in each fiber individually, and the progressive decline in pCa50 occurring with successive repetition (~ 0.002 pCa units per repetition) was removed from the data shown in Figs. 1D and 2D.
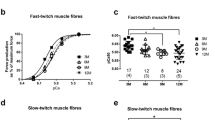
Calcium sensitivity of type II muscle fibers of sedentary and active rats. a Force responses in skinned EDL fiber from sedentary rat (SED) to solutions of progressively higher free [Ca2+] (pCa > 9, 6.4, 6.2, 6.0, 5.88, 5.7, 5.4, 4.7; arrows labelled 1–8, respectively) before and after two treatments with reducing agent DTT (see “Materials and methods”; response in each condition examined twice successively, only one shown after each DTT treatment). b Force responses in EDL fiber from RUN rat. c force–pCa curves before and after DTT for fibers in a and b from SED (red) or RUN (blue) animals. d pCa50 values (i.e. pCa giving at half maximum force) for all type II fibers from SED (Redl et al. 2007), RUN (both 6 and 12 week running; blue) and RUN-REST rats (green), both before and after DTT treatment. Bars indicate mean ± SD. *Significant difference following DTT treatment (P < 0.05, paired t test). #pCa50 value before DTT treatment in RUN rats is significantly different from other two groups (P < 0.05, One-way ANOVA). Number of fibers indicated, with number of rats shown in brackets. (Color figure online)
Calcium sensitivity of type I soleus muscle fibers of sedentary and active rats. a Force responses in skinned fiber from RUN rat, presented as in Fig. 1. At the end, the fiber was exposed to a strontium-containing solution (at pSr 5.2), eliciting a large force response, indicating the fiber was type I (see “Materials and methods”). b Force–pCa curves before and after DTT treatment for representative fibers from SED (red) and RUN (blue). c pCa50 values for all type I fibers from SED and RUN. Number of fibers indicated, with number of rats shown in brackets. (Color figure online)
Muscle myosin heavy chain (MHC) composition assay
Myosin heavy chain (MHC) composition was determined by separating denatured muscle homogenates by SDS–PAGE so as to distinguish the four MHC isoforms, MHCIIa, MHCIIx, MHCIIb and MHCI (Xu et al. 2017). The separating gel consisted of 32% v/v glycerol, 8% w/v acrylamide with a 50:1 ratio of acrylamide to N′-ethylenebisacrylamide (Wyckelsma et al.), 0.2 M Tris-HCl (pH 8.8), 0.1 M glycine, 0.4% w/v SDS, 0.1% w/v ammonium persulfate, and 0.05% v/v N,N,N′,N′-tetramethylethylenediamine (TEMED). The stacking gel comprised 32% v/v glycerol, 4% w/v acrylamide, and Bis at the same ratio (50:1) to acrylamide, 70 mM Tris–HCl (pH 6.7), 4 mM EDTA, 0.4% w/v SDS, 0.1% w/v ammonium persulfate, and 0.05% v/v TEMED. The gel was run using two different buffers, the lower running buffer consisting of 0.05 M Tris (base), 75 mM glycine, and 0.05% w/v SDS, and the upper running buffer, which was at 6x the concentration of the lower running buffer and had β-ME added (final concentration: 0.12% v/v). The gel underwent electrophoresis at 150 V at 4 °C for 24 h, and immediately after running, Coomassie brilliant blue G250 was used to stain the gel and visualize the MHC bands. The images were collected by G:BOX Chemi (Syngene, USA) and the densitometry analysis was conducted using Quantity One (Bio-Rad, USA).
Glycogen assay
After muscles were homogenized in relaxing solution to give a 50 µg/µl wet weight tissue as mentioned above, 4 M potassium hydroxide was added to 50 µl of each homogenate 2:1 (vol/vol, KOH/homogenate), and samples were heated at 70 °C for 30 min. After heating, the pH was brought to 4.5, which is the optimal pH for amyloglucosidase, using glacial acetic acid (Ajax Finechem, Sydney, NSW, Australia). Four units of amyloglucosidase (A1602, Sigma-Aldrich) were added, and samples incubated at 55 °C for 2 h. Then PGO enzyme (P7119, Sigma-Aldrich) was prepared by dissolving one capsule in 1 ml o-dianisidine/dihydrochloride (D3252, Sigma-Aldrich) and made to 50 ml volume with ddH2O as described before (Xu et al. 2016). 240 µl of prepared PGO enzyme was added into each sample tube and glucose standard solution (calibration range 7–500 µg/ml glucose) and a blank (ddH2O). Glucose standards were prepared by dissolving a powder stock of glucose (Ajax Finechem) in ddH2O. After 30 min, absorbance of samples and standards on 96-well plate were read on a UV–VIS spectrophotometer (SpectraMax M5, Molecular Devices, Broadmeadows, VIC, Australia) at 450 nm and the glucose content of samples determined relative to the glucose calibration curve. All samples were measured in triplicate on the spectrophotometer and each sample was prepared on 2–3 independent occasions.
Western blotting and antibody information
Proteins in denatured muscle samples were separated on 4–15% Criterion Stain Free gels (Bio-Rad, Hercules, CA, USA) at 200 V for 1 h. On every gel a graded set of 4–5 amounts of a standard muscle homogenate was run alongside the samples under investigation, so as to enable generation of a signal calibration curve for each protein of interest for that gel. The total protein in each lane on the gel was imaged after UV activation on a Stain Free imager (Bio-Rad). Proteins on the gel were transferred to nitrocellulose membrane (100 V, 30 min). After transfer, the membrane was treated with Miser antibody extender solution NC (Thermo Scientific) for 10 min and then washed and placed into blocking solution (5% skim milk powder diluted in tris-buffered saline with Tween; TBST). The membrane was then treated with the primary antibody (see below) and incubated overnight at 4 °C and a further 2–3 h at room temperature. The membrane was then washed with blocking solution and exposed to the secondary antibody diluted in blocking solution. After the membrane was washed with TBST, the protein bands were visualised using West Femto chemiluminescent substrate (Thermo Scientific). Images were collected, and densitometry was performed using Chemidoc MP system and Image Lab version 5.2 (Bio-Rad) (Xu et al. 2015). Western blot signals of given proteins in a sample were expressed relative to their respective 4–5 point calibration curve and were then normalised to the total protein in that lane, also expressed relative to its calibration curve (Murphy et al. 2012). All data were then expressed relative to the average of the SED samples on the given gel.
The following antibodies were used: polyclonal rabbit anti Cytochrome C oxidase IV (COXIV) (Cell Signalling #4844), monoclonal mouse anti GAPDH (Abcam, #ab8245), monoclonal mouse anti-calsequestrin1 (CSQ1) (Abcam, #ab2824), polyclonal rabbit anti- calsequestrin2 (CSQ2) (Abcam, #3516), monoclonal mouse anti-sarcoplasmic reticulum calcium-ATPase1 (SERCA1) (Developmental Studies Hybridoma Bank (DSHB), #CaF2-5D2), polyclonal rabbit anti SERCA2 (Badrilla, #A010-20), rabbit monoclonal anti-glycogen synthase (GS) (Epitpmics, #1720-1), anti-glycogen branching enzyme (Heinemeier et al. 2007), anti-glycogen debranching enzyme (GDE), anti-glycogen phosphorylase (GP), and anti-glycogenin, all raised in rabbit (Parker et al. 2007; Ryu et al. 2009), mouse monoclonal anti-glucose transporter 4 (GLUT4) (Cell Signaling, #2213). For Na+, K+-ATPase (NKA), there are two main subunits the catalytic subunit NKAα, and the regulatory subunit NKAβ, and two isoforms (α1/α2, β1/β2) for each of them. Antibody for NKAα1 was polyclonal from rabbit (Cell Signaling, #3010), whereas the NKAα2 antibody was rabbit polyclonal (Merck Millpore, #07-674). NKAβ1 antibody was mouse monoclonal (Santa Cruz, #C464.8) and NKAβ2 antibody was rabbit polyclonal (Merck Millpore, #06-171).
Statistical analysis
All results are presented as individual data points or as mean values ± standard deviation (SD), and the data in Table 1 is the mean ± standard error of mean (SEM). Data were analysed using paired or unpaired one- or two-sided t tests or by one-way ANOVA, as indicated. Statistical significance level was set at P < 0.05. All statistical analysis was undertaken by using Prism Version 6 (GraphPad, La Jolla, CA, USA).
Results
The body mass of rats after 12 weeks of wheel running was on average almost 10% lower than in their sedentary litter mates, though the difference did not reach the significance level (P = 0.055) (Table 1). Although the masses of the EDL and soleus muscles were not significantly different between the running and sedentary rats, the muscle to body mass ratio of both muscles was ~ 13% higher in the running rats (Table 1). The rats did virtually all of the wheel running at night but the activity was sporadic rather than continuous; the 12 weeks wheel running rats ran a total of ~ 1 to 4 km per 24 h (2.1 ±0.9 km) and those doing 6 weeks wheel running ran ~ 0.5 to 2 km per 24 h (1.2 ±0.6 km). The shorter running distance in the 6 week running group was likely because they were 18 weeks old rather than 12 weeks old when given access to the running wheel, given that previous research found that running distance was progressively reduced with increased age or age at onset of wheel running (Mondon et al. 1985; Kariya et al. 2004; Garvey et al. 2015). The total distances run by the rats in the present study would be classed as ‘low’ in comparison to previous studies with younger rats (see Rodnick et al. 1989, 1992; Broch-Lips et al. 2011), with the rats being active but not exercising intensively.
Effect of wheel running on Ca2+-sensitivity of the contractile apparatus
The Ca2+-sensitivity of muscle fibers from SED and RUN rats was examined by activating each skinned fiber in a series of solutions with free [Ca2+] heavily-buffered at successively higher levels (pCa ~ 9 to 4.7), eliciting progressively greater levels of force (e.g. Fig. 1) (see “Materials and methods”). This force–pCa staircase was repeated twice in succession before and after each of two treatments with the reducing agent DTT. Force produced at each pCa was expressed relative to the maximum force for that staircase, and plotted against pCa to generate a force–pCa curve for each individual case (e.g. Figs. 1c, 2b). The Ca2+-sensitivity in type II fibers before any DTT treatment was significantly higher (by ~ 0.04 pCa units) in the exercising rats compared to the sedentary rats (compare pCa50 values (pCa eliciting 50% maximum force) for RUN (blue squares) versus SED (red squares) in Fig. 1d); this increase was similar in rats performing either 6 or 12 weeks voluntary wheel running and those results are shown pooled together. The Hill coefficient (steepness of force–pCa relationship) was not significantly different between the active and sedentary fibers and was not affected by the DTT treatment. Importantly, the increase in Ca2+-sensitivity was completely reversed by the first DTT treatment of the fibers, such that the pCa50 in the RUN fibers decreased to a level similar to that in SED fibers after the DTT treatment (compare blue triangles with red triangles in Fig. 1d). Subjecting the fibers to a second DTT treatment had no further effect on Ca2+-sensitivity in either RUN or SED fibers (e.g. Fig. 1a, b), showing that the first treatment was sufficient for maximal effect. The reversing effect of the first DTT treatment demonstrated that the increase in Ca2+-sensitivity in the type II fibers in the wheel running rats was the result of a reversible oxidation-related process. There was no correlation between the daily running distance of individual rats and the size of the oxidation-induced increase in Ca2+-sensitivity [linear regression results for 6 weeks running (R2 = 0.04), and 12 weeks running (R2 = 0.004)]. Importantly, when wheel running rats were rested for one day before being sacrificed, the Ca2+-sensitivity in the type II fibers was not detectably different from that in the sedentary rats, and DTT treatment had no greater effect (compare data for RUN-REST rats, green squares and triangles, with that of the SED rats, red symbols, in Fig. 1d). This shows that the oxidation-induced increase in Ca2+-sensitivity in the type II fibers occurring in the exercising rats completely reversed in-vivo with 24 h rest.
In contrast to the findings in the type II fibers, there was no significant difference between the exercising and sedentary rats in the Ca2+-sensitivity of type I fibers (Fig. 2c, blue squares versus red squares), and DTT treatment had little if any effect on Ca2+-sensitivity in either case (Fig. 2b, c). The Hill coefficient of the force–pCa fits in the type I fibers also were no different between the exercising and sedentary rats and were unaltered by the DTT treatment (e.g. see steepness of curves in Fig. 2b).
Specific force and depolarisation-induced force responses in fibers of RUN and SED rats
Specific force in each individual skinned fiber was assessed as the maximum Ca2+-activated force (to a solution at pCa 4.7) normalised by the fiber cross-sectional area (see Material and Methods). Figure 3 presents the specific force values measured in all type I (SOL) and type II (EDL) fibers from the rats that performed 6 or 12 weeks wheel running and from their sedentary litter mates. Relative to the sedentary rats, specific force in fibers of the active rats was on average ~ 8% higher in type II fibers (SED 230.8 ±24.4, RUN 248.5 ±31.2, Fig. 3a) and ~ 36% in type I fibres (SED 176.2 ±30.8, RUN 239 ± 35.2, Fig. 3b). The results were similar regardless of whether the rats performed either 6 or 12 weeks running or were rested for one day following the running before examining the fibers (Fig. 3).
Specific force in type II (a) and type I (b) fibers from sedentary and active rats. Different symbols used for data from rats of three experimental groups (see “Materials and methods”) where RUN cohort did wheel running for 6 weeks (open symbols), 12 weeks (closed symbols) or 12 weeks with 1 day rest (open-cross symbols). *RUN significantly different from SED (P < 0.05, unpaired t test). Number of fibers shown, with number of animals in brackets
Depolarisation-induced force responses were also examined in skinned fibers from the EDL and SOL muscles of the active and sedentary rats (e.g. Fig. 4). When a muscle fiber is mechanically skinned the transverse (t-) system seals off, and if the skinned fiber segment is placed in the standard potassium-based HDTA solution it can become polarised again, because the action of the Na–K pump in the t-system membrane establishes a high sodium ion concentration and a low potassium ion concentration within the t-system. If the t-system is sufficiently well polarized, the voltage-sensors (dihydropyridine receptors) in the t-system return to an activatable state (Chua and Dulhunty 1988), and if the skinned fiber is subsequently transferred into the zero potassium Na-HDTA solution, the t-system is rapidly depolarized, activating the voltage-sensors, which in turn trigger Ca2+ release from the SR and a resultant force response (Lamb 2002). Thus, the magnitude of the depolarization-induced response in a skinned fiber (relative to the maximum Ca2+-activated force in that fiber) can be used as a measure of the polarization of the t-system membrane and excitability of that fiber. We have previously shown that maximal depolarization-induced responses can readily be elicited in skinned type II fibers from EDL muscle of sedentary rats, but that the responses in type I fibers from SOL muscle were much poorer [(Lamb and Stephenson 1994; Xu et al. 2017) for rats of 20 weeks and older]. It was found here that the depolarization-induced responses in the SOL type I fibers were considerably enhanced in the wheel running rats compared to their sedentary litter mates (Fig. 4a–c), with the improvement being similar irrespective of whether the rats did 6 or 12 weeks of wheel running exercise (Fig. 4c). These data indicate that the resting polarization and excitability of the t-system in the type I skinned fibers was substantially increased in the wheel running rats. The responsiveness of the skinned type II (EDL) fibers was equally high for the fibers of both sedentary and exercising rats (Fig. 4d), indicating that the t-system was well polarized in the skinned fibers in both groups of rats.
Depolarisation-induced force responses (Depol) in skinned muscle fibers. Examples of force responses in type I soleus fiber from SED (a) and RUN (b). c Maximum size of depolarization-induced force response, expressed relative to maximum Ca2+-activated force in same fiber, for all SOL type I fibers examined [6 weeks running (open symbols), 12 weeks running (closed symbols)]. D Data for type II fibers from EDL muscle. *Significantly different from SED (P < 0.05, unpaired t test). Number of fibers shown, with number of animals in brackets
Myosin heavy chain composition and glycogen content of EDL and SOL muscles in sedentary and active rats
The MHC isoforms in the EDL and SOL muscles of the active and sedentary rats were separated by SDS–PAGE (Fig. 5). The predominant MHC isoforms in rat EDL muscle are the fast isoforms, MHCIIa, MHCIIx and MHCIIb, whereas SOL muscle has predominantly the slow MHCI isoform and a small proportion of the fast MHCIIa isoform. It was found that there was an increase in the proportion of the MHCIIa isoform in the EDL muscle of the rats that did 12 weeks of wheel running relative to that in their sedentary counterparts (~ 6% of total MHC in SED versus ~ 14% in RUN) (Fig. 5c), whereas the percentages of MHCIIa in SOL muscle was not significantly changed (Fig. 5d). The results are broadly similar to those of a previous study on wheel running rats, which found the MHCIIa proportion to be ~ 8 and 11% in EDL muscle and ~ 14 and 17% in SOL muscle in sedentary and running rats respectively (Gallo et al. 2006).
Myosin heavy chain (MHC) composition in muscles of SED and RUN rats. a Representative example of SDS–PAGE of MHC isoforms in EDL muscle from SED and RUN. b Representative gel images for SOL muscle. Percentage of MHC isoforms in EDL (c) and SOL muscles (d) for SED (grey bar) and RUN (black bar). Value for each individual muscle sample derived as average of measurements on 3–4 independent gels. *Significant difference from SED (P < 0.05, unpaired t test). n = 8 muscles for SED and n = 10 for RUN, with each muscle from different rat. Data for 12 weeks running rats
Muscle glycogen content of SOL muscle was ~ 25% higher in the wheel running rats comparing to their sedentary litter mates (Fig. 6). This difference in SOL glycogen content between running and sedentary rats was similar in rats sacrificed 1–2 h after the exercise and those rested for 24 h before sacrifice, and also in rats doing either 6 or 12 weeks of wheel running. In contrast, glycogen content in EDL muscles was not significantly different between the active and sedentary rats (Fig. 6).
Muscle glycogen content in sedentary and active rats. Glycogen content in muscle homogenates of EDL and SOL muscle from 12 week running (closed symbols) and 12 week running-1 day rest rats (open symbols). Each muscle sample examined 2–3 times and measured in triplicate. *Significant difference from SOL SED (P < 0.05, unpaired t test)
Relative amounts of key proteins in EDL and SOL muscles in active and sedentary rats
Whole muscle homogenates of the EDL and SOL muscle from all SED and RUN rats were analysed by western blot to determine the relative amount of specified proteins. The data obtained from rats performing 12 weeks running were similar irrespective of whether the muscles were examined soon after, or 24 h after, cessation of running (e.g. Figs. 7, 8), and the data are shown pooled together in Table 2. Very similar trends were also seen in the muscle of the rats performing 6 weeks running (not shown). The proteins are categorised into four different groups, those being metabolic enzymes, Ca2+-related proteins, glycogen-related enzymes, and Na+, K+-ATPase (NKA) proteins. It is important to note that the samples were not fractionated in any way, and that quantification of all western blot signals utilised calibration standards run on the same gel (see “Materials and methods”) so as to take account of the relevant signal detection limits and sensitivity for that protein on that gel, and all values were expressed relative to total protein measured in the given sample lane, not to some arbitrary protein assumed to be unchanged (see methodology review by Murphy and Lamb (Murphy and Lamb 2013)).
GAPDH in EDL and SOL muscles of SED and RUN rats. Representative western blots of GAPDH (at ~ 38 kDa, upper row in each panel, with MHC band on Stain Free gel for that lane shown beneath each) in two EDL (a) and two SOL (b) muscles, with data values for all muscles shown beneath (see “Materials and methods”). Each data point is average for 3–4 repeats of given sample run on separate gels. *Significant difference from SED (P < 0.05, unpaired t test). n = 8 muscles for SED and n = 10 of RUN, each muscle from a different rat. Calibration samples run on each gel not shown
Calsequestrin and CSQ-like proteins in EDL and SOL muscles of SED and RUN rats. Representative western blots for CSQ1 (at ~ 63 kDa) and CSQ-like (~ 130 to 180 kDa) proteins in two examples of EDL and SOL muscles; lower panels present values found across all samples examined. Horizontal bars show mean value ± SD. Each data point is average for 3–4 repeats of given sample run on separate gels. *Significant difference from SED (P < 0.05, unpaired t test). Calibration samples run on each gel not shown
Metabolic enzymes
COXIV (~ 17 kDa) and GAPDH (~ 38 kDa) were examined as representative enzymes indicating the aerobic and glycolytic abilities, respectively, of the skeletal muscles, and analysed with one-tailed t tests as they were predicted to increase and decrease, respectively in RUN compared with SED. The relative amount of COXIV increased in EDL (p = 0.035) but not SOL (Table 2), whereas GAPDH decreased in RUN versus SED in SOL (Fig. 6b), but not in EDL (Fig. 6a) (Table 2).
Ca2+-related proteins
The relative amounts of the SR Ca2+ storage protein, calsequestrin (CSQ), and the sarcoplasmic reticulum calcium-ATPase (SERCA), were also examined. There are two main isoforms of CSQ in rat skeletal muscle, CSQ1 (~ 63 kDa) and CSQ2 (55 kDa), with CSQ1 being the sole or predominant isoform present in type II fibers and expressed at lower abundance in type I fibers, and CSQ2 (~ 55 kDa) being predominantly expressed in type I fibers with little or no expression in type II fibers (Murphy et al. 2009a). CSQ1 levels were not significantly different between SED and RUN in either EDL or SOL (Fig. 7a, b; Table 2), and likewise, the relative amount of CSQ2 in SOL muscle was no different between SED and RUN (Table 2).
CSQ-like proteins are a series of higher molecular weight proteins at ~ 130 to 180 kDa detected by CSQ antibodies (Cala et al. 1990). The distribution of CSQ-like proteins in different muscle types is similar to the corresponding CSQ protein (Murphy et al. 2009a), but the function of these CSQ-like proteins are not understood. In both EDL and SOL muscles the amount of the CSQ-like proteins was found to be increased in RUN compared with SED rats (Fig. 8c, d; Table 2).
SERCA1 (~ 110 kDa) is the predominant or exclusive isoform SERCA present in rat type II fibers, and SERCA2 (~ 100 kDa) is the predominant or exclusive SERCA isoform present in rat type I fibers (Murphy et al. 2009a). The amounts of SERCA1 in EDL and SERCA2 in SOL were not significantly different between SED and RUN (Table 2).
Glycogen metabolic enzymes
Glycogen-related proteins can be categorised into two groups, glycogen anabolic enzymes and catabolic enzymes, according to their functions. In terms of anabolic enzymes, there are two main enzymes, glycogen synthase (GS, ~ 85 kDa) and glycogen branching enzyme (GBE, ~ 70 kDa). Similarly, for catabolic enzymes there are also two main enzymes involved, glycogen phosphorylase [GP, ~ 95 kDa, only EDL examined, as GP amount is very low in SOL muscle (Murphy et al. 2012)] and glycogen debranching enzyme (GDE, ~ 165 kDa). In addition, glycogenin (~ 37 kDa), the core protein of glycogen granules, and glucose transporter 4 (GLUT4, ~ 46 kDa), the predominant transporter of glucose into skeletal muscles, were also examined as critical proteins in glycogen metabolism. It was found that the relative amounts of both glycogen anabolic and catabolic enzymes in EDL and SOL muscles did not differ significantly between RUN and SED except for the amount of GS in SOL muscle, which was decreased ~ 34% in RUN compared to SED and glycogenin, which was increased ~ 35% in EDL muscles in RUN compared to SED (Table 2). GLUT4, analysed using a one-tailed t-test as an increase was predicted in the RUN group, increased in EDL (p = 0.04) but not SOL muscles (Table 2).
Na+, K+-ATPase in EDL and SOL muscles of SED and RUN
The Na+, K+-ATPase (NKA) is present in the sarcolemma and the membrane of t-tubules in order to maintain the sodium and potassium ion gradients. The NKA complex is comprised of the catalytic subunit NKAα and the regulatory subunit NKAβ. There are two main isoforms of NKAα in skeletal muscles, the ubiquitous isoform NKAα1 (~ 100 kDa) and the skeletal muscle-specific isoform NKAα2 (~ 100 kDa), and also two main isoforms of NKAβ, with NKAβ1 being the predominant isoform in type I fibers and NKAβ2 in type II fibers (Wyckelsma et al. 2015). The amount of NKAα1 was ~ 21% higher in EDL and ~ 18% higher in SOL in RUN versus SED (Table 2), whereas the amount of NKAα2 was not significantly different in either muscle (Table 2). The amounts of NKAβ2 in EDL and NKAβ1 in SOL muscle also were not significantly different between SED and RUN (Table 2).
Discussion
Calcium sensitivity increase in type II fibers is an acute effect of wheel running
The Ca2+-sensitivity in the type II fibers was increased in rats examined within ~ 1 to 2 h after wheel running activity, and this effect was reversed by treatment with the reducing agent DTT (Fig. 1) demonstrating that it was due to an ‘oxidation-related’ modification of the contractile apparatus. No such effect was seen in the type I fibers (Fig. 2). This increase in Ca2+-sensitivity was almost certainly due to S-glutathionylation of Cys134 on fast troponin I in the type II fibers (Mollica et al. 2012; Dutka et al. 2017), which has been shown to increase sensitivity in type II fibers only (type I fibers lack this cysteine residue) and to occur in exercising humans (Mollica et al. 2012) and in rat muscle stimulated intensively in-vitro (Watanabe et al. 2015). This increase in Ca2+-sensitivity would be expected to appreciably improve force production at submaximal cytosolic calcium levels (see force–pCa curves in Fig. 1c) and hence help counter to some extent the deleterious changes responsible for muscle fatigue (see Allen et al. 2008); the fact that this effect persists for at least 1–2 h after exercising suggests that it might also be factor in the beneficial effects of ‘warming-up’ exercise. S-glutathionylation of cysteine residues can occur by interaction of reactive oxygen or nitrogen species with the glutathione present endogenously in muscle fibers. We recently showed that S-nitrosylation of the same cysteine residue (Cys134 on troponin I) results in a decrease in Ca2+-sensitivity, in a competitive manner with S-glutationylation (Dutka et al. 2017). Muscle activity can increase both reactive oxygen and reactive nitrogen species (Powers and Jackson 2008) and DTT reverses both types of modification. Consequently, it is uncertain whether the wheel running activity here resulted from both S-glutathionylation and S-nitrosylation modifications or only S-glutathionylation, although it is clear that the net effect of all such modifications was an increase Ca2+-sensitivity. It was further found that the increase in Ca2+-sensitivity was lost if the rats stopped wheel running for 24 h (Fig. 1d), indicating that the oxidation-induced increase in sensitivity was an acute effect of the exercise that largely reversed under the reducing intracellular conditions prevailing inside non-exercising muscle. DTT treatment caused a small decrease in calcium sensitivity in the type II fibers even in the sedentary rats (~ 0.01 pCa units versus ~ 0.04 pCa units in RUN rats) (Fig. 1d). This is consistent with the small level of Sglutathionylation of troponin Cys134 seen in the muscle of even non-exercising rats (e.g. see Figs. 4 and 9 in Mollica et al. 2012).
Specific force in single skeletal muscle fibers
Ca2+-activated specific force in single fibers in the wheel running rats was significantly greater than in the matching sedentary rats, the increase being ~ 36% in type I SOL fibers and ~ 8% in type II EDL fibers (Fig. 3). Changes in specific force at the single fiber level have not previously been examined in a comparable animal model. In studies on whole muscle properties, no significant change in soleus muscle tetanic force per gram weight was seen in wheel running rats compared to their sedentary counterparts (Gallo et al. 2006; Broch-Lips et al. 2011). The seeming difference from the present findings might be the result of the different measure of specific force (Ca2+-activated force per unit cross-sectional area in individual fibers versus tetanic force per gram weight in whole muscle) or influenced by age and growth given that both the whole muscle studies examined rats that commenced running at only 3 to 4 weeks and examined the muscles at 12 to 16 weeks final age. A study examining tetanic force in single fibers from wheel running C57BL/10 mice relative to sedentary counterparts at 20 weeks final age, found ~ 39% increase in SOL muscle fibers and little or no change in EDL fibers (Hayes and Williams 1996), which is broadly similar to the changes in specific force found here. The molecular basis of the increased specific force seen here is not known, but it might be due to an increase in myosin and/or actin density, given that studies on prolonged bed-rest in humans found a 15–40% decrease in Ca2+-activated specific force at the single fiber level that was seemingly attributable to a ~ 30 to 40% decrease in myosin/myofibrillar protein content (Larsson et al. 1996; Brocca et al. 2015). Regardless of the exact molecular basis, the substantially lower specific force seen here in the sedentary rats indicates decreased muscle fiber quality, not just quantity, and this must be expected to adversely affect muscle performance in such animals.
Depolarization-induced responses
Depolarization-induced responses in skinned fibers from SOL muscle were substantially improved in the wheel running rats (Fig. 4). Such force responses are highly dependent on the t-system being properly polarized at rest in the skinned fibers, and are decreased if the resting t-system potential is less polarized than normal, as this causes inactivation of the voltage-sensors in the t-system membrane (Chua and Dulhunty 1988; Lamb and Stephenson 1990; Pedersen et al. 2004). The total Na+, K+ ATPase (NKA) activity in the t-system membrane is a key factor governing the resting polarization of the t-system. The density of NKAα2, the α subunit of the muscle-specific isoform of NKA, was not significantly changed in the wheel running rats, but the density of NKAα1, the ubiquitous isoform of NKA, was ~ 20% greater in both EDL and SOL (Table 2), and the overall increase in NKA density likely contributed to the improved responses in the SOL fibers. (Although the densities of the NKA β subunits were not significantly increased in the wheel running rats, the mean changes seen were broadly consistent, being intermediate between the changes in the two variants of the α subunit; see Table 2). This increase in NKA density is consistent with the increase in ouabain binding sites in soleus muscle reported previously in wheel running rats (Broch-Lips et al. 2011). That study also found that the membrane conductance of the soleus muscle fibers was decreased ~ 30% in the wheel running rats and an increased robustness of excitability in muscles from active rats, likely due to changes in membrane permeabilities to chloride and potassium, and such changes could also have altered the resting potential in the skinned SOL fibers examined here, leading to the improved responses to depolarization.
Metabolic enzymes
In SOL muscle, the amount of GAPDH decreased ~ 25% in RUN compared with SED rats (Table 2). GAPDH is a critical glycolytic enzyme that is widely considered as a ‘house-keeping’ protein, because it is highly and ubiquitously expressed (Tristan et al. 2011). The present results indicate that it is not appropriate to use GAPDH for normalising protein amounts in western blotting, as it is significantly affected by the level of muscle activity, and as we have also shown in humans, with age and muscle fiber type (Wyckelsma et al. 2016). This is in line with work suggesting it is not a good housekeeping gene for mRNA work (Murphy et al. 2003). The decreased GAPDH in SOL muscle in RUN suggests that the predominantly oxidative SOL muscle became less glycolytic with the increased activity, although this was not detected as any shift in MHC isoform. This was perhaps not surprising, as SOL muscle only contained MHC I and MHC II isoforms, which are both oxidative in nature and so they would rely less on flux through the glycolytic pathway as fuel is able to enter the Krebs cycle for oxidative phosphorylation and more efficient production of ATP. It was further found that the amount of the mitochondrial marker, COXIV, was increased in EDL muscles (Table 2), supporting the small shift seen to more oxidative MHC IIa containing fibres in that same muscle (Fig. 5). A previous study examining 2–4 weeks of wheel running in female Sprague–Dawley rats reported an ~ 50% increase in citrate synthase activity, an effective marker of mitochondrial capacity (Larsen et al. 2012) in the fast-twitch plantaris muscle (Henriksen and Halseth 1995). Overall, the findings support that the 12 weeks wheel running resulted in a metabolic shift in the muscles.
Energy storage aspects and glycogen metabolism
Glycogen content in SOL muscle was increased ~ 25% in RUN rats, but unchanged in EDL muscle (Fig. 6). A previous study reported that 5-weeks of wheel running in young (100 g) rats did not alter glycogen levels in either SOL and fast-twitch epitrochlearis muscles (Rodnick et al. 1992). In older (16 month) rats, glycogen increased ~ 50% in the gastrocnemius muscle following 8 weeks of wheel running, albeit with an average of only ~ 83 m/day (Garvey et al. 2015). The increased glycogen content in SOL muscle of RUN rats supports an increased capacity for endurance performance, which is expected with the increased activity in the animals. A possible reason for glycogen being increased in SOL muscle but unchanged in EDL muscle in the exercising rats is that EDL muscle is a fast-twitch muscle, and its high bursting force and low endurance properties require fast access to glycogen rather than a necessarily high content glycogen. Indeed glycogen granules in EDL muscle fibers are likely less branched, due to a fourfold lower content of GBE that forms the 1,6-glycosidic bonds than in SOL muscle, allowing faster utilisation (Murphy et al. 2012; Xu et al. 2015). In order to examine the capacity for glycogen utilisation, we measured the various enzymes essential for glycogen metabolism, the anabolic enzymes (GS and GBE) and catabolic enzymes (GDE and GP), as well as the core protein glycogenin and the muscle-specific glucose transporter GLUT4. Not surprisingly, the voluntary running did not affect the amount of glycogen catabolic enzymes in either EDL or SOL muscle (Table 2), as voluntary wheel running did not place a stress on the muscle to provide glycogen breakdown more quickly, but rather the muscles required a sustained ability to utilise glycogen, which likely could be accommodated for by the GP and GDE already present in the muscles. Whilst GBE was unchanged, the GS content in SOL muscle was decreased in RUN rats (Table 2). GBE is a cytosolic protein (Murphy et al. 2012) and seemingly able to move within the cytoplasm as required by the muscle. GS, on the other hand, is highly regulated through various phosphorylation states (Prats et al. 2009, 2011) and over half the pool is bound tightly within skeletal muscle (Murphy et al. 2012). A combination of tight regulation and an increased glycogen content might be responsible for the downregulation of the GS protein in SOL from RUN rats.
GLUT4 protein was increased 26% in EDL from RUN rats (Table 2). It is clear that GLUT4 protein abundance and glucose uptake rates are highly dependent on exercise level (Ren et al. 1994; Christ-Roberts et al. 2004). In female Sprague–Dawley rats, 2–4 weeks of wheel running resulted in ~ 50% increase in GLUT4 in the fast-twitch plantaris muscle (Henriksen and Halseth 1995). This robust adaptation was accompanied by an ~ 50% in citrate synthase activity, as discussed above. The core glycogen protein, glycogenin was increased 35% (Table 2). Together, these data suggest an increased capacity for glycogen synthesis in EDL muscle, but this was not observed. Given the abundance of the various glycogen associated enzymes in SOL and EDL muscles from rats, we have previously hypothesised that glycogen granules in those muscles have different structures and overall granule size (Murphy et al. 2012; Xu et al. 2015). We further suggest here, that the increased glycogenin abundance with unchanged glycogen content seen in RUN EDL muscle indicates that the overall number of glycogen granules increased but not the overall size of the granules. This was not measured, and could be assessed using electron microscopy (Nielsen et al. 2011) but that was outside the scope of the current study. In contrast, the unchanged glycogenin amount with increased glycogen content seen in RUN SOL muscle suggests that the increased glycogen content with voluntary running was not newly generated granules but instead the result of more tiers being added to the existing granules.
Calcium storage and transporting proteins unchanged with chronic inactivity
Finally, it was found that CSQ and SERCA levels in EDL or SOL muscles did not differ between SED and RUN rats (Fig. 8; Table 2). These proteins are critical for maintaining calcium homeostasis in, and overall function of skeletal muscle cells (Ebashi 1972). As such, perhaps it was not surprisingly that voluntary wheel running had no effect on their overall abundances. In contrast, the amount of CSQ-like proteins increased in both the EDL muscle (by ~ 18%) and SOL muscle (by ~ 61%) in the RUN rats (Fig. 8; Table 2). The specific function of these CSQ-like proteins is not known. CSQ-like protein levels were found to increase with age in both mice (Chevessier et al. 2004) and humans (Lamboley et al. 2015). It has been suggested that CSQ-like proteins are closely associated with calcium homeostasis in skeletal muscle (Culligan et al. 2002), possibly being post translationally modified isoforms of CSQ (Cala et al. 1990; Murray et al. 1998; Froemming et al. 2000; Froemming and Ohlendieck 2001). However, it appears that the CSQ-like proteins do not bind appreciable amounts of calcium (Murphy et al. 2009a), arguing against them having a major direct effect on calcium storage or cycling. Thus, it remains unclear whether the increase in amounts of CSQ-like proteins seen here is functionally important.
Conclusion
Overall, this study has provided a comprehensive assessment of both the physiological and biochemical characteristics of rat fast- and slow-twitch muscle in animals that were or were not provided free access to a running wheel. Some beneficial effects of oxidation were seen with exercise, as the increased calcium sensitivity of fast-twitch fibers meant that the fibers produced ~ 20 to 30% more force at the same cytosolic [Ca2+]. Given the similarities in mammalian contractile apparatus properties, these findings could explain how warming-up prior to exercise in humans can actually improve performance in fast-twitch muscle fibers, which presumably could be very beneficial in some types of intense brief exercise, such as sprinting.
Other adaptations, including altered MHC composition, mitochondrial and glycolytic enzymes, glycogen content and associated proteins and the ubiquitous Na+-K+ATPase α1, all support robust muscle plasticity and even voluntary running over a 6–12 week period was sufficient to alter the biochemical profile of some muscles. These findings suggest that experimental data obtained from relatively sedentary animal models should be used with caution, because the sedentary behaviour appears to alter many of muscle parameters both physiologically and biochemically.
References
Adams GR, Caiozzo VJ, Baldwin KM (2003) Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol (1985) 95:2185–2201
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM (2000) MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol 279:C1564-1577
Brocca L, Longa E, Cannavino J, Seynnes O, de Vito G, McPhee J, Narici M, Pellegrino MA, Bottinelli R (2015) Human skeletal muscle fibre contractile properties and proteomic profile: adaptations to 3 weeks of unilateral lower limb suspension and active recovery. J Physiol 593:5361–5385
Broch-Lips M, de Paoli F, Pedersen TH, Overgaard K, Nielsen OB (2011) Effects of 8 wk of voluntary unloaded wheel running on K+ tolerance and excitability of soleus muscles in rat. J Appl Physiol (1985) 111:212–220
Cala SE, Scott BT, Jones LR (1990) Intralumenal sarcoplasmic reticulum Ca(2+)-binding proteins. Semin Cell Biol 1:265–275
Chevessier F, Marty I, Paturneau-Jouas M, Hantai D, Verdiere-Sahuque M (2004) Tubular aggregates are from whole sarcoplasmic reticulum origin: alterations in calcium binding protein expression in mouse skeletal muscle during aging. Neuromuscul Disord 14:208–216
Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Kashyap S, Mandarino LJ (2004) Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metab Clin Exp 53:1233–1242
Chua M, Dulhunty AF (1988) Inactivation of excitation-contraction coupling in rat extensor digitorum longus and soleus muscles. J Gen Physiol 91:737–757
Culligan K, Banville N, Dowling P, Ohlendieck K (2002) Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle. J Appl Physiol 92:435–445
Desplanches D, Mayet MH, Sempore B, Flandrois R (1987) Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol (1985) 63:558–563
Dutka TL, Mollica JP, Lamboley CR, Weerakkody VC, Greening DW, Posterino GS, Murphy RM, Lamb GD (2017) S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am J Physiol Cell Physiol 312:C316-c327
Ebashi S (1972) Calcium ions and muscle contraction. Nature 240:217–218
Fitts RH, Metzger JM, Riley DA, Unsworth BR (1986) Models of disuse: a comparison of hindlimb suspension and immobilization. J Appl Physiol (1985) 60:1946–1953
Froemming GR, Ohlendieck K (2001) The role of ion-regulatory membrane proteins of excitation-contraction coupling and relaxation in inherited muscle diseases. Front Biosci 6:D65–D74
Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K (2000) Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers. Biochim Biophys Acta 1466:151–168
Gallo M, Gordon T, Syrotuik D, Shu Y, Tyreman N, MacLean I, Kenwell Z, Putman CT (2006) Effects of long-term creatine feeding and running on isometric functional measures and myosin heavy chain content of rat skeletal muscles. Pflugers Arch 452:744–755
Garvey SM, Russ DW, Skelding MB, Dugle JE, Edens NK (2015) Molecular and metabolomic effects of voluntary running wheel activity on skeletal muscle in late middle-aged rats. Physiol Rep 3:E12319
Hayes A, Williams DA (1996) Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol (1985) 80:670–679
Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M (2007) Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol 102:573–581
Henriksen EJ, Halseth AE (1995) Adaptive responses of GLUT-4 and citrate synthase in fast-twitch muscle of voluntary running rats. Am J Physiol 268:R130-134
Ishihara A, Roy RR, Ohira Y, Ibata Y, Edgerton VR (1998) Hypertrophy of rat plantaris muscle fibers after voluntary running with increasing loads. J Appl Physiol (1985) 84:2183–2189
Kariya F, Yamauchi H, Kobayashi K, Narusawa M, Nakahara Y (2004) Effects of prolonged voluntary wheel-running on muscle structure and function in rat skeletal muscle. Eur J Appl Physiol 92:90–97
Kim JH, Thompson LV (2013) Inactivity, age, and exercise: single-muscle fiber power generation. J Appl Physiol (1985) 114:90–98
Kriketos AD, Pan DA, Sutton JR, Hoh JF, Baur LA, Cooney GJ, Jenkins AB, Storlien LH (1995) Relationships between muscle membrane lipids, fiber type, and enzyme activities in sedentary and exercised rats. Am J Physiol Regul Integr Comp Physiol 269:R1154–R1162
Lamb GD (2002) Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil 23:81–91
Lamb GD, Stephenson DG (1990) Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol 423:495–517
Lamb GD, Stephenson DG (1994) Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol 478(Pt 2):331–339
Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD (2015) Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593:2499–2514
Lamboley CR, Wyckelsma VL, Perry BD, McKenna MJ, Lamb GD (2016) Effect of 23-day muscle disuse on sarcoplasmic reticulum Ca2+ properties and contractility in human type I and type II skeletal muscle fibers. J Appl Physiol (1985) 121:483–492
Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590:3349–3360
Larsson L, Li X, Berg HE, Frontera WR (1996) Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch 432:320–328
Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD (2012) S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol 590:1443–1463
Mondon CE, Dolkas CB, Sims C, Reaven GM (1985) Spontaneous running activity in male rats: effect of age. J Appl Physiol (1985) 58:1553–1557
Murphy RM, Lamb GD (2013) Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol Lond 591:5823–5831
Murphy RM, Watt KK, Cameron-Smith D, Gibbons CJ, Snow RJ (2003) Effects of creatine supplementation on housekeeping genes in human skeletal muscle using real-time RT-PCR. Physiol Genom 12:163–174
Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD (2009a) Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol 587:443–460
Murphy RM, Mollica JP, Lamb GD (2009b) Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp Cell Res 315:1015–1028
Murphy RM, Xu H, Latchman H, Larkins NT, Gooley PR, Stapleton DI (2012) Single fiber analyses of glycogen-related proteins reveal their differential association with glycogen in rat skeletal muscle. Am J Physiol Cell Physiol 303:C1146–C1155
Murray BE, Froemming GR, Maguire PB, Ohlendieck K (1998) Excitation-contraction-relaxation cycle: role of Ca2+-regulatory membrane proteins in normal, stimulated and pathological skeletal muscle (review). Int J Mol Med 1:677–687
Nielsen J, Holmberg HC, Schroder HD, Saltin B, Ortenblad N (2011) Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J Physiol 589:2871–2885
Parker GJ, Koay A, Gilbert-Wilson R, Waddington LJ, Stapleton D (2007) AMP-activated protein kinase does not associate with glycogen alpha-particles from rat liver. Biochem Biophys Res Commun 362:811–815
Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG (2004) Intracellular acidosis enhances the excitability of working muscle. Science 305:1144–1147
Perry BD, Wyckelsma VL, Murphy RM, Steward CH, Anderson M, Levinger I, Petersen AC, McKenna MJ (2016) Dissociation between short-term unloading and resistance training effects on skeletal muscle Na+,K+-ATPase, muscle function, and fatigue in humans. J Appl Physiol (1985) 121:1074–1086
Posterino GS, Lamb GD (2003) Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J Physiol 551:219–237
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Prats C, Helge JW, Nordby P, Qvortrup K, Ploug T, Dela F, Wojtaszewski JF (2009) Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization. J Biol Chem 284:15692–15700
Prats C, Gomez-Cabello A, Hansen AV (2011) Intracellular compartmentalization of skeletal muscle glycogen metabolism and insulin signalling. Exp Physiol 96:385–390
Rebbeck RT, Karunasekara Y, Board PG, Beard NA, Casarotto MG, Dulhunty AF (2014) Skeletal muscle excitation-contraction coupling: who are the dancing partners? Int J Biochem Cell Biol 48:28–38
Redl C, Gfoehler M, Pandy MG (2007) Sensitivity of muscle force estimates to variations in muscle-tendon properties. Hum Mov Sci 26:306–319
Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO (1994) Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269:14396–14401
Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ (2007) Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56:2062–2069
Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE (1989) Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol (1985) 66:1250–1257
Rodnick KJ, Henriksen EJ, James DE, Holloszy JO (1992) Exercise training, glucose transporters, and glucose transport in rat skeletal muscles. Am J Physiol 262:C9-14
Ryu JH, Drain J, Kim JH, McGee S, Gray-Weale A, Waddington L, Parker GJ, Hargreaves M, Yoo SH, Stapleton D (2009) Comparative structural analyses of purified glycogen particles from rat liver, human skeletal muscle and commercial preparations. Int J Biol Macromol 45:478–482
Salanova M, Schiffl G, Gutsmann M, Felsenberg D, Furlan S, Volpe P, Clarke A, Blottner D (2013) Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol 1:514–526
Steffen JM, Musacchia XJ (1984) Effect of hypokinesia and hypodynamia on protein, RNA, and DNA in rat hindlimb muscles. Am J Physiol 247:R728-732
Stephenson DG, Williams DA (1981) Calcium-activated force responses in fast-twitch and slow-twitch skinned muscle-fibers of the rat at different temperatures. J Physiol Lond 317:281–302
Talmadge RJ, Roy RR, Caiozzo VJ, Edgerton VR (2002) Mechanical properties of rat soleus after long-term spinal cord transection. J Appl Physiol (1985) 93:1487–1497
Thomason DB, Herrick RE, Surdyka D, Baldwin KM (1987) Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol (1985) 63:130–137
Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P (2004) Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557:501–513
Trinh HH, Lamb GD (2006) Matching of sarcoplasmic reticulum and contractile properties in rat fast- and slow-twitch muscle fibres. Clin Exp Pharmacol Physiol 33:591–600
Tristan C, Shahani N, Sedlak TW, Sawa A (2011) The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal 23:317–323
Watanabe D, Kanzaki K, Kuratani M, Matsunaga S, Yanaka N, Wada M (2015) Contribution of impaired myofibril and ryanodine receptor function to prolonged low-frequency force depression after in situ stimulation in rat skeletal muscle. J Muscle Res Cell Motil 36:275–286
Widrick JJ, Trappe SW, Romatowski JG, Riley DA, Costill DL, Fitts RH (2002) Unilateral lower limb suspension does not mimic bed rest or spaceflight effects on human muscle fiber function. J Appl Physiol 93:354–360
Wyckelsma VL, McKenna MJ, Serpiello FR, Lamboley CR, Aughey RJ, Stepto NK, Bishop DJ, Murphy RM (2015) Single-fiber expression and fiber-specific adaptability to short-term intense exercise training of Na+-K+-ATPase alpha- and beta-isoforms in human skeletal muscle. J Appl Physiol (1985) 118:699–706
Wyckelsma VL, McKenna MJ, Levinger I, Petersen AC, Lamboley CR, Murphy RM (2016) Cell specific differences in the protein abundances of GAPDH and Na(+),K(+)-ATPase in skeletal muscle from aged individuals. Exp Gerontol 75:8–15
Xu H, Stapleton D, Murphy RM (2015) Rat skeletal muscle glycogen degradation pathways reveal differential association of glycogen-related proteins with glycogen granules. J Physiol Biochem 71:267–280
Xu H, Frankenberg NT, Lamb GD, Gooley PR, Stapleton DI, Murphy RM (2016) When phosphorylated at Thr148, the beta2-subunit of AMP-activated kinase does not associate with glycogen in skeletal muscle. Am J Physiol Cell Physiol 311:C35-42
Xu H, Lamb GD, Murphy RM (2017) Changes in contractile and metabolic parameters of skeletal muscle as rats age from 3 to 12 months. J Muscle Res Cell Motil. https://doi.org/10.1007/s10974-017-9484-6
Acknowledgements
This was supported by a National Health and Medical Research Council (Australia) Grant (APP1085331) to GDL and RMM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Ren, X., Lamb, G.D. et al. Physiological and biochemical characteristics of skeletal muscles in sedentary and active rats. J Muscle Res Cell Motil 39, 1–16 (2018). https://doi.org/10.1007/s10974-018-9493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-018-9493-0