Abstract
The aim of this study was to examine whether prolonged low-frequency force depression (PLFFD) that occurs in situ is the result of decreased myofibrillar Ca2+ sensitivity and/or reduced sarcoplasmic reticulum (SR) Ca2+ release. Intact rat gastrocnemius muscles were electrically stimulated via the sciatic nerve until force was reduced to ~50 % of the initial and dissected 30 min following the cessation of stimulation. Skinned fibre and whole muscle analyses were performed in the superficial region composed exclusively of type IIB fibres. Fatiguing stimulation significantly reduced the ratio of force at low frequency to that at high frequency to 65 % in skinned fibres (1 vs. 50 Hz) and 73 % in whole muscles (20 vs. 100 Hz). In order to evaluate changes in myofibrillar Ca2+ sensitivity and ryanodine receptor caffeine sensitivity, skinned fibres were activated in Ca2+- and caffeine-containing solutions, respectively. Skinned fibres from fatigued muscles displayed decreased caffeine sensitivity together with increased myofibrillar Ca2+ sensitivity. Treatment with 2,2′-dithiodipyridine and reduced glutathione induced a smaller increase in myofibrillar Ca2+sensitivity in fatigued than in rested fibres. In fatigued muscles, S-glutathionylation of troponin I was increased and submaximal SR Ca2+ release, induced by 4-chloro-m-cresol, was decreased. These findings suggest that in the early stage of PLFFD that occurs in fast-twitch muscles of exercising animals and humans, S-glutathionylation of troponin I may attenuate PLFFD by increasing myofibrillar Ca2+ sensitivity and that under such a circumstance, PLFFD may be ascribable to failure of SR Ca2+ release.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repetitive or sustained contraction of skeletal muscle leads to a progressive loss in the ability to produce a desired force, known as muscle fatigue. The mechanisms underlying muscle fatigue are multi-factorial and may have their origins anywhere from the central nervous system to the cellular level cross-bridge cycling. During the study of muscle fatigue, Edwards et al. (1977) observed a greater loss of force at low compared to high stimulation frequencies after a period of exercise in humans. This phenomenon was subsequently named ‘prolonged low-frequency force depression (PLFFD) (Bruton et al. 2008), since the depressed force at low frequencies is long-lasting, in some instances taking hours or even days for full recovery.
PLFFD may be functionally important as the motor discharge rate during voluntary contraction rarely exceeds 30 Hz (Grimby and Hannerz 1977), a firing frequency below which PLFFD is apparent. Since the discovery by Edwards et al. (1977), it has been shown that PLFFD occur in various human muscles not only after fatiguing activities but also during normal daily activities (Iguchi et al. 2008; Polkey and Moxham 2001; Westerblad et al. 2000). In spite of its importance and more than 30 years of investigation, the precise mechanism(s) of PLFFD remains incompletely understood. The fact that PLFFD is manifested also in isolated muscles makes it most likely that PLFFD is not primarily due to the impaired neural activation but is correlated with factors in the muscle cell themselves (Balog 2010; Keeton and Binder-Macleod 2006). With regard to events occurring within the muscle fibres, decreased myofibrillar Ca2+ sensitivity and/or reduced Ca2+ release via the ryanodine receptor (RyR), a Ca2+ release channel of the sarcoplasmic reticulum (SR), can in theory mediate a greater depression in force at low frequencies with little influence at high frequencies (for review, see Allen et al. 2008).
One of methods used to elucidate the mechanistic basis of PLFFD is an approach in which isolated whole muscles or single fibres (i.e., intact or skinned fibres) are electrically stimulated and/or treated with specified solutions (Bruton et al. 2008; Verburg et al. 2006, 2009). Using isolated intact fibres stimulated in vitro, Bruton et al. (2008) have demonstrated that PLFFD is associated with both decreased myofibrillar Ca2+ sensitivity and reduced Ca2+ release, but their contributions to PLFFD differ depending on differences in metabolism of reactive oxygen species (ROS), production of which is accelerated by vigorous muscle activity. In fibres from wild-type mouse muscles with low superoxide dismutase (SOD) activity, a force decrease induced by fatiguing stimulation was correlated with reduced Ca2+ release, whereas fibres from rat and SOD overexpressing mouse muscles, which have higher SOD activity than wild-type mouse muscles, exhibited decreased myofibrillar Ca2+ sensitivity (Bruton et al. 2008). These findings suggest that superoxide has noticeable effects on SR Ca2+ release, while hydrogen peroxide (H2O2) and/or hydroxyl radicals affect the myofibrillar Ca2+ sensitivity (Lamb and Westerblad 2011). In skinned fibres, exogenously applied H2O2 decreases the myofibrillar Ca2+ sensitivity only when myoglobin is present (Murphy et al. 2008), indicating that decreased myofibrillar Ca2+ sensitivity is due to hydroxyl radicals that are produced from H2O2 via the Fenton reaction.
The use of isolated muscle preparations makes it possible to control the extent of muscle fatigue, monitor the myoplasmic Ca2+ concentration [(Ca2+)] and force output simultaneously, and examine the impact of specific metabolites. However, as pointed out by Allen et al. (2011), such approaches (simplified models), although mechanistically powerful, have numerous disadvantages, including low temperature during muscle contraction, elimination of hypoxia, no blood flow and no interactions between neighboring cells. Of these, the issues with regard to hypoxia and blood flow would mainly affect accumulation of ROS in the myoplasm. In order to avoid hypoxia in isolated muscle fibres without intact blood supply, most of simplified models have employed well-oxygenated muscle preparations that are bubbled with 95 % O2 (Bruton et al. 2008; Cheng et al. 2013; Dutka et al. 2012; Mishima et al. 2005; Reardon and Allen 2009). Given that superoxide are generated in active muscles, particularly with supra-physiological oxygen tensions (Jamieson et al. 1986), it is reasonable to assume that during muscle contractions, isolated muscles undergo more severe oxidative stress than muscles in vivo do. This assumption is reinforced by recent observations by Dutka et al. (2012) who found that in vitro stimulation resulted in larger amounts of oxidized cysteine residues than in situ stimulation, implying that previous findings on PLFFD obtained from simplified models are not necessarily true for muscles of exercising animals and humans.
The purpose of this study was to examine whether PLFFD that occurs in situ is the result of the aforementioned two mechanisms (i.e., functional impairment of the contractile apparatus and the RyR). We tested the hypothesis that myofibrillar Ca2+ sensitivity would not be greatly affected by fatiguing muscle contraction, possibly because of the relatively low levels of ROS, and thus PLFFD would relate mainly to impaired RyR function. To this end, skinned fibre and whole muscle experiments were carried out in the superficial regions of rat fast-twitch gastrocnemius muscles that were fatigued by an in situ model developed in our laboratory (Mishima et al. 2008). Considering that S-glutathionylation (S-glut) of fast troponin I isoform (TnIf) can make the contractile apparatus more sensitive to Ca2+ (Mollica et al. 2012; Murphy et al. 2008), the experiment on myofibrillar Ca2+ sensitivity focused on the impact of S-glut. In the experiment on SR Ca2+ release, responses of the RyR to Ca2+ releasing agents were examined in order to disclose whether fatiguing contraction leads to perturbations in the RyR function. The present results reveal that in the early stage of PLFFD, S-glut of TnIf occurring in fast-twitch muscles attenuates PLFFD by increasing myofibrillar Ca2+ sensitivity and that under such a circumstance, PLFFD is ascribable to reductions in submaximal SR Ca2+ release.
Methods
Animals
All procedures were approved by the Animal Care Committee of Hiroshima University. Eight to nine-week old male Wistar rats (n = 9) were used in this study. The animals were fed on water and food ad libitum and housed in a thermally controlled room at 20–24 °C with a 12-h light/dark cycle. At the end of experiments, the rats were euthanized with an overdose of pentobarbital sodium (200 mg/kg body wt) followed by cervical dislocation.
Fatiguing stimulation
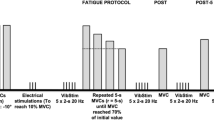
Intact gastrocnemius muscles from the right legs were electrically stimulated in situ as described in detail previously (Mishima et al. 2008). The muscles from contralateral (left) legs were used as controls. To prevent the extensor digitorum longus, tibialis anterior, plantaris and soleus muscles from contributing to force production, tenotomy of these muscles in both legs was performed under anesthesia using an intraperitoneal injection of pentobarbital sodium (50 mg/kg body wt). The animal was then placed in a supine position and the right hindlimb was attached to a homemade foot holder connected with an isometric transducer. The sciatic nerve of the right hindlimb was electrically stimulated (70 Hz, 0.35-s train, every 3 s) until force was reduced to ~50 % of the initial. Muscles were allowed to rest for 30 min after the end of fatiguing stimulation. Isometric force of intact gastrocnemius muscles from both legs was then measured at various frequencies (1–100 Hz, 1.5-s train) via stimulation of the sciatic nerve. Force responses were recorded on a personal computer and analyzed using LabChart software (version 7, ADInstruments, Japan). The ratio of force at low frequency to that at high frequency (low/high force ratio) has been used as an indicator of PLFFD (Bruton et al. 2008; Edwards et al. 1977). In the present study, 20 and 100 Hz were chosen as low and high frequencies of stimulation, respectively, since in force-stimulation frequency curve, force at 20 Hz was on the steep part of the curve whereas force at 100 Hz was on the plateau of the curve (data not shown).
Control and stimulated gastrocnemius muscles were excised immediately after measures of isometric force. The superficial regions of medial and lateral heads in the gastrocnemius muscles were used for skinned fibre and whole muscle analyses, respectively. In preliminary experiment, we found that our in situ model for muscle fatigue evoked persistent PLFFD for up to 8 h after the cessation of fatiguing stimulation. In order to compare the present results with those reported by Bruton et al. (2008), as with them, we employed the muscles that received 30 min of recovery.
Skinned fibre experiments
Skinned fibre preparations
Mechanically skinned fibres were prepared according to Lamb and Stephenson (1990). A part (medial head) of excised gastrocnemius muscle was pinned out at resting length under paraffin oil and kept cool on an ice pack. Single muscle fibres were dissected under a stereo-microscope and mechanically skinned by rolling back the sarcolemma with fine forceps. One or two skinned fibres were obtained from one whole muscle. A segment of the skinned fibre was then connected to a force transducer (Muscle tester, SI, Germany), stretched to 1.2 times resting length and transferred to a bath containing 2 ml of the K-hexamethylethylene-diaminetetraacetic acid (HDTA) solution (see below). All skinned fibres (n = 27) were initially subjected to measurement of action potential-induced force responses, followed by subsequent measurements of either myofibrillar Ca2+ sensitivity (n = 13) or caffeine threshold (n = 14). The contractile properties of skinned fibre were measured at room temperature (24–26 °C). Although the whole gastrocnemius muscle was placed in cold paraffin oil immediately after its excision, it took several minutes (5–30 min) to start measures with skinned fibres. In our preliminary experiment, we ascertained the time-dependent effect of immersion of whole muscles in cold paraffin oil and observed that stimulation-elicited changes in protein modifications in whole muscles and contractile properties of skinned fibres persisted at least for up to 60 min of immersion.
Basic solutions
All solutions was prepared as described in detail elsewhere (Bortolotto et al. 2001; Verburg et al. 2009), consisted of (in mM) 36 Na+, 126 K+, 90 N-2-hydroxyethylpiperazine-N′′-2-ethanesulfonic acid (HEPES), 8 ATPtotal and 10 creatine phosphate, and had a pH of 7.09–7.11 at 25 °C. The free Mg2+ concentration was set at 1.0 mM except in the release solution. The K-HDTA solution additionally contained 0.05 mM EGTA and 50 mM HDTA to give 10−6.9 M free Ca2+ (Table 1). The maximum Ca2+ activation solution was similar to the K-HDTA solution, but with all HDTA replaced by 49.5 mM Ca-EGTA and 0.5 mM free EGTA whereas the relaxation solution contained 50 mM free EGTA.
Action potential stimulation
The skinned fibre was placed in a small chamber containing approximately 300 μl of the K-HDTA solution and centered between two platinum electrodes placed in parallel. The fibre was stimulated with 1-ms pulses at a field strength of 80 V cm−1 with single pulse (for twitch), five pulses at 5 Hz, 10 pulses at 20 Hz, 20 pulses at 50 Hz and 30 pulses at 80 Hz. Because parvalbumin, a myoplasmic Ca2+-binding protein, had been washed from the skinned fibres (Verburg et al. 2006), maximal tetanic force of skinned fibre was reached at 50 Hz and the force response of 1 Hz (twitch) was relatively larger compared to that of intact fibres. In skinned fibre experiment, we chose 1 and 50 Hz as low and high frequencies of stimulation, respectively, since under control conditions, the low/high force ratio at 1 and 50 Hz in skinned fibres approximated that at 20 and 100 Hz in whole muscles (Figs. 1 ,2).
Effects of fatiguing stimulation on isometric force in whole gastrocnemius muscle. a Typical example of force output during fatiguing stimulation. A dash line indicates 50 % of the initial force. b Typical example of isometric force at 20 and 100 Hz after fatiguing stimulation. c Mean (+SD) of ratio of force at 20 Hz to that at 100 Hz. a P < 0.05, versus control
DTDP + GSH treatment
The effects of S-glut on myofibrillar Ca2+ sensitivity were assessed according to Mollica et al. (2012). A 100 mM stock of reduced glutathione (GSH) was made in the K-HDTA solution and diluted 20-fold in the relaxation solution to give a final concentration of 5 mM (GSH solution). A 100 mM stock of 2,2′-dithiodipyridine (DTDP) was made in pure ethanol and diluted 1000-fold in the relaxation solution to 100 μM (DTDP solution). The skinned fibre segment was immersed sequentially in the DTDP solution for 5 min and then the GSH solution for 2 min (DTDP + GSH treatment).
Myofibrillar Ca2+ sensitivity
The maximum Ca2+ activation and relaxation solutions were mixed in appropriate ratio with free [Ca2+] in the range of >10−9–10−4.7 M. The free [Ca2+] in the mixed solution was calculated according to the affinity constants given by Moisescu and Thieleczek (1978). The contractile apparatus was directly activated by exposing the skinned fibre segment to a sequence of solutions at progressively higher free [Ca2+] and then the fibre was fully relaxed in the relaxation solution. In each fibre, the [Ca2+] staircase procedure was repeated twice before and twice after DTDP + GSH treatment. The individual values were based on the average of two measures. Force produced at each [Ca2+] under a given condition was expressed as a percentage of the Ca2+-activated maximum force generated in the same condition and analyzed by individually fitting Hill curve. The [Ca2+]50 [(Ca2+) at half-maximum force] and the Δ[Ca2+]50 [difference in (Ca2+)50 between DTDP + GSH treatment and non-treatment] were compared between control and stimulated fibres.
Caffeine threshold
A caffeine threshold (Caff-th) at maximal SR Ca2+ load was measured using the procedure described by Bortolotto et al. (2001). The solutions (Caff-th solution) used to determine Caff-th were made from the K-HDTA solution, to which 0.45 mM EGTA and different concentrations of caffeine in the range of 2.0–9.0 mM were added (Table 1). In order to examine the Caff-th, the fibres were subjected to the full release-wash-load-wash-release cycle (step i-v). First, the SR of skinned fibre was emptied of Ca2+ in the release solution with 30 mM caffeine (step i). After washing the fibre in the wash solution (step ii), the SR was loaded to maximal capacity by incubating the fibre for 3 min in the load solution (step iii); in our preliminary experiment, maximal SR Ca2+ loading was found to be achieved by 3-min loading in both control and stimulated fibres. Subsequently, the fibre was washed again (step iv) and then exposed to the Caff-th solutions with a given caffeine concentration (step v). In step v, the fibre was immersed to increasing caffeine concentrations every time the cycle progressed. At the end of experiments, the fibre was maximally activated in the maximum Ca2+ activation solution and then relaxed in the relaxation solution. In this study, the Caff-th was defined as a caffeine concentration ([Caff-th]10) that produces a response elicited by Ca2+ release from the SR corresponding to 10 % of the maximum Ca2+-activated force according to Bortolotto et al. (2001).
Whole muscle experiments
Western blotting of S-glutathionylation of fast troponin I isoform
Small muscle pieces were powdered under liquid N2 and homogenized on ice in nine volumes (mass/vol.) of a solution composed of (in mM) 100 KCl, 20 Tris/HCl (pH 7.4) and 5 N-ethylmaleimide. The resultant homogenate was diluted in non-reducing buffer consisting of 125 mM Tris/HCl (pH 6.8), 10 % (vol./vol.) glycerol, 4 % SDS (mass/vol.), 0.01 % (mass/vol.) bromophenol blue and 5 mM N-ethylmaleimide. Forty μg of protein was applied to a 15 % (mass/vol.) polyacrylamide gel and SDS-PAGE was run at 100 V for 4 h in a cold room. The separated proteins were then wet-transferred to nitrocellulose for 90 min at 100 V. Membranes were incubated overnight at room temperature with anti-GSH (1:1000 dilution; monoclonal mouse, 101-A, Virogen, USA). After repeated washes in phosphate-buffered saline with 0.01 % Tween, they were incubated for 1 h at room temperature with anti-mouse IgG (1:2500 dilution; polyclonal rabbit, P0260 DakoCytomation, Denmark) and StrepTactin-HRP (1:5000 dilution; 161-0380, Bio-Rad, USA). Equal loading of TnIf was monitored with reprobing anti-TnI (1:1000 dilution; polyclonal rabbit, 4002, Cell Signaling Technology, USA). All antibodies were diluted in 1 % bovine serum albumin in phosphate-buffered saline with 0.01 % Tween. Chemiluminescent substrate (GE Healthcare, USA) was applied to the membranes. Immunoreactive bands were visualized and evaluated using infrared fluorescence (Lumicube, Liponics, Japan) and Image J (NIH, USA), respectively. The amounts of GSH contained in TnIf were evaluated by dividing the band density of anti-GSH signal by that of a corresponding TnIf signal. Protein concentration was determined by the Bradford assay using bovine serum albumin as a standard (Bradford 1976).
Electrophoresis of myosin light chain
Skinned fibre experiments indicated increased myofibrillar Ca2+ sensitivity in stimulated fibres (see 'Results'). It has been accepted that a regulatory fast myosin light chain (LC2f) is phosphorylated after a tetanus (Danieli-Betto et al. 2000) and that LC2f phosphorylation elicits an appreciable increase in myofibrillar Ca2+ sensitivity (Sweeney et al. 1993). In order to examine whether LC2f phosphorylation occurs in the fatigued muscles that received 30 min of recovery, two-dimensional electrophoresis was performed according to Wada and Pette (1993). Small muscle pieces were homogenized on ice in 40 volumes (mass/vol.) of a solution containing 5 M urea, 2.04 M thiourea, 10 mM sodium pyrophosphate, 0.13 % (vol./vol.) 2-mercaptoethanol. The gels were stained with Coomassie blue R and the intensity of the spots was evaluated using Image J. The amount of phosphorylated LC2f was expressed as a percentage of total LC2f.
SR Ca2+ release rate
Microsomes were prepared according to the procedure outlined by Lees and Williams (2004) with minor modifications. Small muscle pieces were homogenized on ice in 5 volumes (mass/vol.) of a homogenization buffer consisting of (in mM) 20 HEPES (pH 7.4), 1 EDTA, 0.2 phenylmethanesulphonyl fluoride, 0.83 benzamide, 0.0014 pepstain, 0.0022 leupeptin. Homogenates were centrifuged at 5000×g for 15 min at 4 °C. The supernatant fraction was further centrifuged at 10,000×g for 15 min at 4 °C and 600 mM (final concentration) KCl was then added to the resulting supernatant. Samples were incubated at 4 °C for 1 h with gentle shaking and then centrifuged at 50,000 × g for 60 min. The resultant pellet was suspended in the homogenization buffer containing 300 mM sucrose and 150 mM KCl. SR Ca2+ release rates were measured using the Ca2+ fluorescent dye indo-1 according to the procedure described in detail by Mishima et al. (2008). In brief, 5 μl of microsome was mixed with 1 ml of the assay mixture. Ca2+ uptake was initiated by adding 1 mM MgATP and allowed to continue until little or no change in the [Ca2+] was observed. Ca2+ release was then initiated by the addition of 4-chloro-m-cresol (CMC). The CMC dependence of Ca2+ release was evaluated by varying the CMC concentration ([CMC]) in the range of 4–10 mM. SR Ca2+release rates were determined using the peak first derivative of [Ca2+] versus time curve and normalized by a protein concentration in the microsome.The [CMC]50 was defined as a [CMC] required for half-maximum Ca2+ release rate and analyzed by individually fitting Hill curve. The [Ca2+] was monitored fluorometrically using a fluorometer (CAF-110, Nihon-Bunkko, Japan) and was computed using the ratiometric method of Grynkiewicz et al. (1985).
Statistical analysis
Statistical analyses were conducted with SigmaPlot statistical software (version 12, Systat Software, USA). All data are presented as mean ± SD, with n denoting the number of samples examined. Significance of differences between control and stimulated muscles was determined with a paired and an unpaired, two-tailed t test in whole muscle and skinned fibre experiments, respectively. For multiple comparisons, a one-way analysis of variance with Holm-Sidak post hoc test was performed. The acceptable level of significance was set to P < 0.05.
Results
Force output in whole muscles
Intact gastrocnemius muscles were fatigued by repeated tetanic stimulation until force was reduced to ~50 % (50.7 ± 1.2 %) of the initial, which required 266 ± 36 s (Fig. 1a). There was no significant difference in force at 100 Hz between control and stimulated muscles (3.81 ± 0.52 N for control vs. 3.36 ± 0.59 N for stimulated), whereas detectable reductions in force at 20 Hz were found in stimulated muscles (Fig. 1b); (1.86 ± 0.34 N for control vs. 1.17 ± 0.23 N for stimulated), indicating that force at high frequency but not at low frequency was restored during 30 min of recovery. As consequence, the low/high force ratio (20 vs. 100 Hz) in stimulated muscles declined to 73.3 % of the control value (Fig. 1c).
Force output in skinned fibres
As described above, we sought here to assess the mechanisms behind PLFFD using both whole muscles and skinned fibres and thus it was necessary to ascertain whether fatiguing stimulation was capable to evoke PLFFD at skinned fibre levels. Similar to what was shown for whole muscles, a significant reduction (35.2 %) in the low/high force ratio (1 vs. 50 Hz) was also observed in stimulated skinned fibres (Fig. 2b). These results indicate that our in situ model for muscle fatigue brought about PLFFD both in whole muscles and skinned fibres.
Myofibrillar Ca2+ sensitivity and effects of DTDP + GSH treatment
Our in situ model induced significant decreases in [Ca2+]50 (i.e., increased myofibrillar Ca2+ sensitivity) of stimulated fibres (Fig. 3; 2.54 ± 0.25 μM for control vs. 1.98 ± 0.41 μM for stimulated). Recently, DTDP + GSH treatment of skinned fibres, leading to S-glut of TnIf, has been shown to result in pronounced increases in myofibrillar Ca2+ sensitivity (Mollica et al. 2012). On the basis of this finding, it is conceivable that Δ[Ca2+]50 can be used as an indicator of the relative amount of S-glut which is present before DTDP + GSH treatment. With DTDP + GSH treatment, [Ca2+]50 was decreased in both control and stimulated fibres (Figs. 3b, 3d), which is in line with previous studies. However, the extent of changes (Δ[Ca2+]50) differed, with control fibres being 91 % greater than stimulated fibres (Fig. 3f; 1.08 ± 0.10 μM for control vs. 0.57 ± 0.20 μM for stimulated), implying that the TnIf in the stimulated fibres was already more S-glutathionylated before the DTDP + GSH treatment than was the TnIf in the control fibres. After DTDP + GSH treatment, the [Ca2+]50 in the stimulated and control fibres was increased to the same final level (Fig. 3f; 1.46 ± 0.10 μM for control vs. 1.46 ± 0.32 μM for stimulated). Whole muscle analyses using Western blot and two-dimensional electrophoresis revealed that fatiguing stimulation resulted in a 43 % increase in the level of S-glut of TnIf (Fig. 4) and no change in phosphorylated LC2f (Fig. 5), respectively. These results are strongly suggestive that the increased myofibrillar Ca2+ sensitivity found in stimulated fibres would be due to S-glut of TnIf.
Effects of fatiguing stimulation and DTDP + GSH treatment on myofibrillar Ca2+ sensitivity in skinned fibre. a and c typical example of force output in control (a) and stimulated (c) fibres exposed to solutions at progressively higher free [Ca2+] [small ticks in order (μM [Ca2+]): 0.40, 0.63. 1.00, 1.56, 2.51, 3.98, 19.95, < 0.001]. Horizontal arrows mark force levels achieved at 2.51 μM Ca2+. b and d Hill fits to force-[Ca2+] data in control (b) and stimulated (d) fibres before (filled circles) and after (open circles) DTDP + GSH treatment. The [Ca2+] was expressed as a base 10 logarithmic scale. E, mean (+SD) of [Ca2+]50 for Hill fits in B and D. a P < 0.05, versus control before DTDP + GSH treatment. b P < 0.05, versus before treatment. f, mean (+SD) of Δ[Ca2+]50. a P < 0.05, versus control. GSH, reduced glutathione; DTDP, 2,2′-dithiodipyridine; [Ca2+]50, Ca2+ concentration required for half-maximum force; Δ[Ca2+]50, difference in [Ca2+]50 between non-treatment and DTDP + GSH treatment
Effects of fatiguing stimulation on S-glutathionylation of fast troponin I (TnIf) in whole muscle. a upper panel: Western blotting of reduced glutathione (GSH). The bands at ~43 kDa and ~23 kDa correspond to actin and TnIf, respectively. Positions of molecular weight markers are indicated on the right. Lower panel: reprobe of same membrane for TnIf. b Mean (+SD) of GSH content contained in TnIf. The GSH content is expressed as a percentage of the control muscle. a P < 0.05, versus control
Effects of fatiguing stimulation on phosphorylation state of regulatory fast myosin light chain (LC2f) in whole muscle. a two-demensional electrophoreses of LC. Only the LC regions are shown. b Mean (+SD) of proportion of phosphorylated LC2f. The content of phosphorylated LC2f was expressed as a percentage of total LC2f. P-LC2, phosphorylated LC2f; LC1f and LC3f, alkali fast myosin light chain; α-Tm, β-Tm, α and β subunits of tropomyosin; IEF, isoelectric focusing
SR Ca2+ release in microsomes and skinned fibres
Impaired SR Ca2+ release has been regarded as another candidate responsible for PLFFD (Bruton et al. 2008). In whole muscle experiment, there was a trend toward a lower maximum Ca2+ release rate with CMC in stimulated than in control muscles, but these differences did not reach significant level (Fig. 6a). On the other hand, as judged by an increase in [CMC]50 in stimulated muscles (Fig. 6b; 4.58 ± 0.45 mM for control vs. 5.19 ± 0.49 mM for stimulated), the submaximal Ca2+ release rate was significantly decreased by fatiguing stimulation. This is in accordance with the results from skinned fibre experiment showing that fatiguing stimulation evoked a pronounced increase in [Caff-th]10 (Fig. 7b; 3.64 ± 1.62 mM for control vs. 7.42 ± 1.67 mM for stimulated).
Effects of fatiguing stimulation on CMC-induced Ca2+ release in microsomes. a Hill fits for sarcoplasmic reticulum (SR) Ca2+ release rate-CMC concentration data. b, mean (+SD) of [CMC]50. a P < 0.05, versus control. CMC, 4-chloro-m-cresol; [CMC]50, CMC concentration required for half-maximum Ca2+ release
Effects of fatiguing stimulation on caffeine threshold (Caff-th) of ryanodine receptor in skinned fibre a typical example of force output in skinned fibres exposed to caffeine solutions. Skinned fibres were subjected to increasing concentrations of caffeine (2.0–9.0 mM) after the sarcoplasmic reticulum was maximally loaded with Ca2+. Numbers on the right of ‘Caff-th’ indicate caffeine concentrations (in mM) contained in solution Caff-th (see methods). b Mean (+SD) of [Caff-th]10. a P < 0.05, versus control. [Caff-th]10, caffeine concentration required for a response elicited by Ca2+ release from the SR corresponding to 10 % of the maximum Ca2+-activated force
Discussion
Decreased myofibrillar Ca2+ sensitivity and/or reduced SR Ca2+ release have been regarded as a significant cause of PLFFD (Place et al. 2010; Wada et al. 2013). A number of in vitro experiments on isolated muscle preparations have provided evidence in favor of this idea (Andrade et al. 1998; Bruton et al. 2008). It is equivocal, however, whether these two phenomena also occur in in vivo conditions. The present investigation shows, at least in our in situ model for muscle fatigue, that during recovery periods after vigorous contractions, (1) PLFFD stems primarily from reductions in submaximal Ca2+ release and (2) changes in myofibrillar Ca2+ sensitivity do not contribute to PLFFD. In order to explore the mechanisms underlying PLFFD, this study focused on changes that occur 30 min after fatiguing stimulation. Studies published to date involving muscle fatigue have demonstrated that metabolic changes (e.g., accumulation of inorganic phosphate, Mg2+ and ADP) have a depressive effect on force production immediately after vigorous contractions (Allen et al. 2008). Our results do not, of course, argue against the influence of these metabolic factors. In view of the suggestion that myofibrillar Ca2+ sensitivity fluctuates depending on the cellular redox state (see below), the relative contributions of altered SR Ca2+ release and myofibrillar Ca2+ sensitivity to PLFFD would be expected to vary with time during recovery periods. Therefore, the findings here document the situation prevailing 30 min after the fatiguing stimulation, namely in the early stage of PLFFD.
In our in situ model, all fibres comprised in gastrocnemius muscles were most likely recruited during fatiguing stimulation, because muscle contractions were induced via supra-maximal stimulation of the sciatic nerve. In a previous in situ study in which the superficial and deep regions of rat gastrocnemius muscles were separately contracted by the stimulation protocol similar to that used in this study, 4-min stimulation was shown to result in the much larger decline in tetanic force in the superficial than in the deep region (73 vs. 37 %) (de Ruiter et al. 1995). Considering this finding, stimulation-induced depressions in force output found in whole muscles would be caused primarily by fibres present in the superficial region. Due to quantitatively and/or qualitatively distinct properties in enzymes associated with energy and ROS metabolism, myofibrillar proteins, and Ca2+-cycling proteins, responses of muscle fibres to vigorous contractions differ among fibre types, i.e., fast types IIA, IIX, and IIB and slow type I (Lamb and Westerblad 2011; Pette and Vrbova 1992). The superficial region of gastrocnemius muscle is predominantly composed of type IIB fibres (~90 %) with small amounts of type IIX fibres (Pette and Staron 1990). It can be assumed, therefore, that the present results from skinned fibre analyses reflect changes in type IIB fibres that are affected by stimulation.
Previous in vitro studies have revealed that treatment of type II fibres with DTDP + GSH, that is, S-Glut treatment, causes a marked increase in myofibrillar Ca2+ sensitivity and that a target for S-Glut is a particular cysteine residue residing on TnIf (Mollica et al. 2012; Murphy et al. 2008). A recent study on human skeletal muscles (Mollica et al. 2012) has revealed that TnIf can be S-glutathionylated not only with in vitro treatment but also during in vivo muscle contraction (cycling exercise), which is also confirmed by this study. Although S-glut production results from ROS interacting with GSH, protein modifications due to ROS can also have the detrimental effects on myofibrillar Ca2+sensitivity, which are mediated through, e.g., disulfide bridge, sulphenation, sulphination or sulphonation of cysteine residues (Lamb and Westerblad 2011; Murphy et al. 2008). It appears likely that the overall effect of ROS on myofibrillar Ca2+sensitivity depends on the balance between the potentiating effect of S-glut and the deleterious effect of other reactions. Therefore, S-Glut of TnIf that occur during in vivo contraction may not necessarily evoke increased Ca2+ sensitivity of the contractile apparatus. To our knowledge, this investigation is the first to indicate that muscle contractions that result in PLFFD are capable of inducing S-Glut-related increases in myofibrillar Ca2+ sensitivity. This result is suggestive that S-Glut would fulfill a role in attenuating force deficit in the early stage of PLFFD that occurs in muscles of exercising animals and humans.
Inconsistent with our results, Bruton et al. (2008), analyzing isolated rat intact fibres, attributed PLFFD to decreased myofibrillar Ca2+ sensitivity. The conflicting results could be explained by different H2O2 concentrations between isolated and non-isolated muscles. Early studies to reveal the mechanisms underlying the effects of ROS on contractile function have shown that application of exogenous H2O2 to isolated intact fibres results in a transiently increased myofibrillar Ca2+ sensitivity, but longer exposures to larger concentrations of H2O2 cause a fall in the sensitivity (Andrade et al. 1998, 2001). On the basis of these results and the findings that H2O2 can react with GSH to generate thiyl radicals, which can S-glutathionylate cysteine residue, it is postulated that at the low H2O2 concentration, produced S-Glut leads to increased myofibrillar Ca2+ sensitivity, while at the high concentration, hydroxyl radicals generated from H2O2 bring about decreased sensitivity (Lamb and Westerblad 2011). The fact that superoxide are readily generated in well-oxygenated fibres bubbled with high O2 levels (Jamieson et al. 1986) makes it reasonable to speculate that the H2O2 levels are relatively higher in isolated fibres than in rat fibres in situ (see 'Introduction').
The sensitivities of the RyR to both caffeine and CMC, direct activators of the RyR, have been considered as an indicator of open probability of the Ca2+ channel (Choisy et al. 2001; Herrmann-Frank et al. 1996; Lamb et al. 2001). Thus, the present results indicate that the depressed open probability of the RyR in the submaximal phase is responsible for PLFFD, at least, in muscles stimulated by our in situ model. The ability of these two agents to induce Ca2+ release from the SR is dependent not only on the responsiveness of the RyR, but also on the amounts of Ca2+ in the SR lumen; the low levels of SR Ca2+ loading result in the decreased sensitivity of the RyR. The reduced SR Ca2+ loading will occur if Ca2+ leakage from the SR is increased and/or SR Ca2+-ATPase function is impaired. These changes appear to be elicited, in some instances, by vigorous muscle contractions (Bellinger et al. 2008; Mishima et al. 2008), although it is uncertain if this was the case in this study. Assuming that the SR in the stimulated fibres would be loaded with Ca2+ to the absolutely same level as in the control fibres, our observations suggest that the decreased sensitivity of the RyR is due to modifications of the RyR itself and/or ancillary proteins that are associated with the RyR and can regulate a RyR opening (Andersson et al. 2012; Bruton et al. 2010). Whatever the reason for the decreased sensitivity of the RyR, the PLFFD observed must be the result of reduced SR Ca2+ release, given that the Ca2+ sensitivity of the contractile apparatus was not decreased (Fig. 3). It has been proposed that, in addition to impaired RyR function and decreased SR Ca2+ loading, reduced SR Ca2+ release could also involve some unknown mechanism(s), such as dysfunction of the dihydropyridine receptor or dysfunctional coupling between the dihydropyridine receptor and the RyR (Allen et al. 2008; Lamb and Westerblad 2011).
Our results conflict with those of Bruton et al. (2008) who observed that PLFFD related to reduced Ca2+ release only in wild-type mouse muscles with low SOD activity, but not in rat and SOD overexpressing mouse muscles with high SOD activity. On the basis of these observations, they concluded that endogenously produced superoxide exerts the deleterious effects on SR Ca2+ release. The reason for these conflicting findings between their study and ours is unclear. From the reason mentioned above (supra-physiological vs. physiological oxygenation), the possibility seems remote that larger amounts of superoxide were generated during fatiguing stimulation in our preparations compared to those used by Bruton et al. (2008). The impacts of oxidative modifications on SR Ca2+ release have been addressed by several authors. Experiments on skinned fibres and isolated RyR have demonstrated that oxidation of the RyR potentiates caffeine-induced Ca2+ release and Ca2+-induced Ca2+ release, respectively (Marengo et al. 1998; Posterino et al. 2003). Moreover, oxidation has been observed to have no effect of the amount of Ca2+ released by action potential (Posterino et al. 2003). These findings indicate that the RyR is sensitized by oxidation, but muscle fatigue-related depressions in SR Ca2+ release are likely to occur through factors other than oxidation of proteins involved in Ca2+ release.
In summary, the current study using an in situ fatigue model advances our understanding of PLFFD. We show that in the early stage of PLFFD, S-glut of TnIf occurring in fast-twitch muscles attenuates PLFFD by increasing myofibrillar Ca2+ sensitivity and that under such a circumstance, PLFFD may be ascribable to failure of SR Ca2+ release. In further study on PLFFD, it would appear productive to elucidate (1) contributions of changes in (a) myofibrillar Ca2+ sensitivity and (b) SR Ca2+ release in different fibre types, (2) time-dependent changes in (a) and (b) during the recovery phase after various modes of contractions, and (3) the molecular processes of depressions in (a) and (b). These analyses will be important for developing novel therapeutic strategies for countering muscle fatigue.
References
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Allen DG, Clugston E, Petersen Y, Roder IV, Chapman B, Rudolf R (2011) Interactions between intracellular calcium and phosphate in intact mouse muscle during fatigue. J Appl Physiol 111:358–366
Andersson DC, Betzenhauser MJ, Reiken S, Umanskaya A, Shiomi T, Marks AR (2012) Stress-induced increase in skeletal muscle force requires protein kinase A phosphorylation of the ryanodine receptor. J Physiol 590:6381–6387
Andrade FH, Reid MB, Allen DG, Westerblad H (1998) Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509:565–575
Andrade FH, Reid MB, Westerblad H (2001) Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J 15:309–311
Balog EM (2010) Excitation-contraction coupling and minor triadic proteins in low-frequency fatigue. Exerc Sport Sci Rev 38:135–142
Bellinger AM, Reiken S, Dura M, Murphy PW, Deng S-X, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR (2008) Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Nat Acad Sci USA 105:2198–2202
Bortolotto SK, Stephenson DG, Stephenson GMM (2001) Caffeine thresholds for contraction in electrophoretically typed, mechanically skinned muscle fibres from SHR and WKY rats. Pflügers Arch 441:692–700
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang S-J, Katz A, Larsson N-G, Westerblad H (2008) Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol 586:175–184
Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, Zhang SJ, Wada M, Tavi P, Nedergaard J, Katz A, Westerblad H (2010) Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol 21:4275–4288
Cheng AJ, Place N, Bruton JD, Holmberg HC, Westerblad H (2013) Doublet discharge stimulation increases sarcoplasmic reticulum Ca2+ release and improves performance during fatiguing contractions in mouse muscle fibres. J Physiol 591:3739–3748
Choisy S, Divet A, Huchet-Cadiou C, Leoty C (2001) Sarcoplasmic reticulum Ca2+ content affects 4-CmC and caffeine contractures of rat skinned skeletal muscle fibers. Jpn J Physiol 51:661–669
Danieli-Betto D, Germinario E, Esposito A, Biral D, Betto R (2000) Effects of fatigue on sarcoplasmic reticulum and myofibrillar properties of rat single muscle fibers. J Appl Physiol 89:891–898
de Ruiter CJ, de Haan A, Sargeant AJ (1995) Physiological characteristics of two extreme muscle compartments in gastrocnemius medialis of the anaesthetized rat. Acta Physiol Scand 153:313–324
Dutka TL, Verburg E, Larkins N, Hortemo KH, Lunde PK, Sejersted OM, Lamb GD (2012) ROS-mediated decline in maximum Ca2+-activated force in rat skeletal muscle fibers following in vitro and in vivo stimulation. PLoS ONE 7:e35226
Edwards RHT, Hill DK, Jones DA, Merton PA (1977) Fatigue of long duration in human skeletal muscle after exercise. J Physiol 272:769–778
Grimby L, Hannerz J (1977) Firing rate and recruitment order of toe extensor motor units in different modes of voluntary contraction. J Physiol 264:865–879
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescent properties. J Biol Chem 260:3440–3450
Herrmann-Frank A, Richter M, Sardor S, Mohr U, Lehmann-Horn F (1996) 4-chloro-m-cresol, a potent and specific activator of the skeletal muscle ryanodine receptor. Biochim Biophys Acta 1289:31–40
Iguchi M, Baldwin K, Boeyink C, Engle C, Kehoe M, Ganju A, Messaros AJ, Shields RK (2008) Low frequency fatigue in human quadriceps is fatigue dependent and not task dependent. J Electromyogr Kinesiol 18:308–316
Jamieson D, Chance B, Cadenas E, Boveris A (1986) The relation of free radical production to hyperoxia. Annu Rev Physiol 48:703–719
Keeton RB, Binder-Macleod SA (2006) Low-frequency fatigue. Phys Ther 86:1146–1150
Lamb GD, Stephenson DG (1990) Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol 423:495–517
Lamb GD, Westerblad H (2011) Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589:2119–2127
Lamb GD, Cellini MA, Stephenson DG (2001) Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol 531:715–728
Lees SJ, Williams JH (2004) Skeletal muscle sarcoplasmic reticulum glycogen status influences Ca2+ uptake supported by endogenously synthesized ATP. Am J Physiol 286:C97–C104
Marengo JJ, Hidalgo C, Bull R (1998) Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J 74:1263–1277
Mishima T, Yamada T, Matsunaga S, Wada M (2005) N-acetylcysteine fails to modulate the in vitro function of sarcoplasmic reticulum of diaphragm in the final phase of fatigue. Acta Physiol Scand 184:195–202
Mishima T, Yamada T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M (2008) Time course of changes in in vitro sarcoplasmic reticulum Ca2+-handling and Na+-K+-ATPase activity during repetitive contraction. Pflügers Arch 456:601–609
Moisescu DG, Thieleczek R (1978) Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol 275:241–262
Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD (2012) S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol 590:1443–1463
Murphy RM, Dutka TL, Lamb GD (2008) Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol 586:2203–2216
Pette D, Staron RS (1990) Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116:1–76
Pette D, Vrbova G (1992) Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol 120:115–202
Place N, Yamada T, Bruton JD, Westerblad H (2010) Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol 110:1–15
Polkey MI, Moxham J (2001) Clinical aspects of respiratory muscle dysfunction in the critically ill. Chest 119:926–939
Posterino GS, Cellini MA, Lamb GD (2003) Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle. J Physiol 547:807–823
Reardon TF, Allen DG (2009) Time to fatigue is increased in mouse muscle at 37 °C; the role of iron and reactive oxygen species. J Physiol 587:4705–4716
Sweeney HL, Bowman BF, Stull JT (1993) Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol 264:C1085–C1095
Verburg E, Dutka TL, Lamb GD (2006) Long-lasting muscle fatigue: partial disruption of excitation-contraction coupling by the elevated cytosolic [Ca2+] during contractions. Am J Physiol 290:C1199–C1208
Verburg E, Murphy RM, Richard I, Lamb GD (2009) Involvement of calpains in Ca2+-induced disruption of excitation-contraction coupling in mammalian skeletal muscle fibers. Am J Physiol 296:C1115–C1122
Wada M, Pette D (1993) Relationships between alkali light-chain complement and myosin heavy-chain isoforms in single fast-twitch fibers of rat and rabbit. Eur J Biochem 214:157–161
Wada M, Kuratani M, Kanzaki K (2013) Calcium kinetics of sarcoplasmic reticulum and muscle fatigue. J Phys Fitness Sports Med 2:169–178
Westerblad H, Bruton JD, Allen DG, Lännergren J (2000) Functional significance of Ca2+ in long-lasting fatigue of skeletal muscle. Eur J Appl Physiol 83:166–174
Acknowledgments
We thank Prof. G. D. Lamb, Dept. of Zoology, La Trobe Univ., for the excellent technical advice for skinned fibre experiment. Grants This study was supported by Grants-in-Aid for Scientific Research of Japan (No. 24500788; M. Wada).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, D., Kanzaki, K., Kuratani, M. et al. Contribution of impaired myofibril and ryanodine receptor function to prolonged low-frequency force depression after in situ stimulation in rat skeletal muscle. J Muscle Res Cell Motil 36, 275–286 (2015). https://doi.org/10.1007/s10974-015-9409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-015-9409-1